Manuscript accepted on :02-Feb-2023
Published online on: 01-03-2023
Plagiarism Check: Yes
Reviewed by: Dr. Maysaa Kadhim Al-Malkey
Second Review by: Dr. Nagham Aljamali
Final Approval by: Dr. Patorn Piromchai
Fifteen Aprila Fajrin1,2* , Dina Permatasari2
, Dina Permatasari2 , Devira Asdar2
, Devira Asdar2 and Ika Puspita Dewi1,2
and Ika Puspita Dewi1,2
1Department of Clinical and Community Pharmacy, Faculty of Pharmacy, Universitas Jember, East Java 68121, Indonesia
2Preclinical Pharmacology Research Group Faculty of Pharmacy, Universitas Jember, East Java 68121, Indonesia.
Corresponding Author E-mail: fifteen.farmasi@unej.ac.id
DOI : https://dx.doi.org/10.13005/bpj/2596
Abstract
Red ginger contains 6-shogaol, which has a neuroprotective effect and is crucial in several brain diseases, including Alzheimer’s. This study investigated the neuroprotective effect of red ginger extract (RGE) containing 6-shogaol on scopolamine-induced memory loss in mice. Male Balb/C mice (n = 30; 6–7 weeks old) were divided into six groups: normal, Alzheimer, drug control (donepezil), and RGE (100, 200, and 400 mg/kg BW). In the acute experiment, the mice were treated 60 minutes before the test, followed by 1 mg/kg of scopolamine 30 minutes later. Thirty minutes later, the mice were placed individually in a Y-maze to observe spontaneous alteration activity (SAA). In the chronic experiment, the mice were treated once daily for seven days. On days 8–14, the mice were administered scopolamine and treatment. SAA was observed every 3–4 days. On day 15, malondialdehyde (MDA) and acetylcholinesterase (AChE) levels in the serum and brain were determined. RGE treatment 400 mg/kg BW, containing 1.664 µg of 6-shogaol per 100 mg of ethanol RGE, reduced memory loss better than the other two doses. RGE successfully decreased MDA and increased AChE in the serum and brain. RGE also showed effectively to improve memory in Alzheimer’s disease.
Keywords
Alzheimer; memory loss; neuroprotective; red ginger extract; 6-shogaol
Download this article as:| Copy the following to cite this article: Fajrin F. A, Permatasari D, Asdar D, Dewi I. P. Neuroprotective Activity of Ethanolic Extract of Red Ginger Containing 6-Shogaol on Scopolamine-Induced Memory Impairment in Alzheimer's Mice. Biomed Pharmacol J 2023;16(1). |
| Copy the following to cite this URL: Fajrin F. A, Permatasari D, Asdar D, Dewi I. P. Neuroprotective Activity of Ethanolic Extract of Red Ginger Containing 6-Shogaol on Scopolamine-Induced Memory Impairment in Alzheimer's Mice. Biomed Pharmacol J 2023;16(1). Available from: https://bit.ly/3EL5tVE |
Introduction
Alzheimer’s disease, one of the neurodegenerative diseases, is a general manifestation of dementia.1 Usually, Alzheimer’s disease, which affects almost 40 billion people worldwide, is characterized by cognitive dysfunction and memory loss in the elderly.2 Disease progression is related to damage to the structure and function of the hippocampus and neocortex, which are responsible for cognitive and memory action.3
Several pathways contribute to the pathophysiology of Alzheimer’s disease, such as loss of synapses, deposition of β-amyloid plaque, neuron fibrillation changes, and hyperphosphorylation of tau protein.3 All changes in Alzheimer’s disease activate the N-methyl-D-aspartate receptor (NMDAR) and oxidative stress accumulation. However, β-amyloid induces exocytosis of NMDAR due to an increase in calcium influx and oxidative stress.4 NMDAR hyperactivity, intracellular calcium accumulation, and oxidative stress also contribute to tau protein dysfunction.5
The high concentration of acetylcholine found in synapses indicates the cholinergic system’s increased function in Alzheimer’s disease. Some drugs that block acetylcholinesterase (AChE) have the potential to reduce the loss of memory in Alzheimer’s disease. AChE inhibitors can enhance neural function by increasing acetylcholine (ACh) levels.6 Some of the drugs that work as acetylcholinesterase inhibitors, such as donepezil, rivastigmine, and tacrin, are used minimally due to their side effects and hepatotoxicity.7,8 Even though preclinical studies have shown positive outcomes, anti-Alzheimer drugs’ activity in clinical practice is still debated, and finding other substances with new targets is essential.9
Ginger has anti-inflammation, antioxidant, and anticancer effects, and 6-shogaol is one of the vital compounds present in ginger responsible for these effects.10,11 The amount of 6-shogaol in red ginger is higher than in other variants of ginger. Previous studies have shown that 6-shogaol has the potential as a neuroprotector due to its antioxidant and anti-inflammatory properties.12-14 In addition, 6-shogaol can minimize NMDAR expression and restore sciatic nerve damage in diabetic mice with neuropathy.15,16
This study tried to find the effect of red ginger extract (RGE) containing 6-shogaol as a neuroprotector on scopolamine-induced memory loss in mice. A previous study showed that scopolamine was a muscarinic acetylcholine receptor antagonist and widely used in Alzheimer’s models. We evaluated the effect of different RGE doses to potentially prevent and treat learning and memory deficiency in a mouse model of Alzheimer’s disease. We also examined whether RGE treatment improves scopolamine-induced memory impairment by conducting Y-maze tests in short- and long-term induction.
Materials and methods
This study was conducted in the Faculty of Pharmacy, University of Jember Indonesia. All experiment was completed after 12 months (2020 – middle 2022).
Extraction
The rhizomes of Zingiber officinale var Rubrum (red ginger) were harvested by the U.P.T. Laboratorium Herbal Medica Batu Malang, East Java, Indonesia (074/226A/102.7/2020) with aged 6–8 months. Plants were dried at room temperature. The rhizomes were ground, and the dried powder (0.5 kg) was macerated thrice for 24 h in 96% ethanol (1:5). The residue was evaporated using a rotary evaporator to obtain a thick extract; the yield was 20.134%.
Determination of 6-Shogaol in RGE using high-performance liquid chromatography
Analysis was performed using high-performance liquid chromatography (HPLC) using Cecil Adept CE4300 equipped with an autoinjector sampler programmed at 10 μL capacity per injection. Chromatographic separations were performed on an RPC-18 column (150 mm × 4.6 mm). The mobile phase (combination of acetonitrile and water) was seen in Table 1. The chromatographic run time was 400 min at 25°C, with a 1 mL/min flow rate. All chromatograms were monitored at 254 nm. The amount of 6-shogaol was counted using a calibration curve. This method was followed previously study by Jazokaite et al.17 with some modifications.
Table 1: Mobile-phase-exchange stage until 40 minutes.
| Time (min) | 0 | 5 | 25 | 28 | 30 | 35 | 37 | 40 |
| Water (%) | 100 | 90 | 50 | 0 | 0 | 30 | 100 | 100 |
| Acetonitrile (%) | 0 | 10 | 50 | 100 | 100 | 70 | 0 | 0 |
Determination of acetylcholinesterase (AChE) inhibitory activity
The AChE inhibitory activity of the extract was determined using Ellman’s method18 with some modifications. Briefly, AChE (type VI-S lyophilized powder, 518 U/mg solid, 844 U/mg protein) was diluted using 50 mM of Tris-HCl buffer (pH 8.0). The solution was mixed between 100 μL of 3 mM 5,5-Dithio-bis-(2-nitrobenzoic acid) (DTNB) in Tris-HCl buffer, 20 μL of 0.26 U/mL of AChE, and 40 μL of buffer (50 mM Tris, pH 8.0), 20 μL of samples (RGE in various concentrations (0–1000 μg/mL); 6-shogaol (0–500 μg/mL); and donepezil as a control (0–250 μg/mL) in buffer). Samples were incubated at 25°C for 15 min and the absorbances were determined at 412 nm using a microplate reader (ELx800; Dialab GmbH; Austria). The absorbance was measured every 5 min for 20 min to monitor the ACh hydrolysis. All reactions were performed in triplicate. The percentage of inhibition was calculated using the following formula:19
Inhibition (%) = [(E − S)/E] × 100, where E was the activity of the enzyme without the test compound and S was the activity of the enzyme with the test compound.
Animals and ethics statements
Thirty male Balb/C mice (20–30 g, 6–7 weeks old) were provided and certified by the Faculty of Pharmacy, University of Jember, Jember, Indonesia. All mice were maintained in standard-clean 30 × 20 cm2 cages with free access to pellet and water ad libitum. They lived at a temperature of 25°C ± 1°C in a 12 h/12 h light/dark cycle.
Before using animals, all tests involving animals were previously approved by the Ethical Committee of Medical Research, University of Jember (997/UN25.8/KEPK/DL/2020).
Scopolamine-induced acute memory loss
The 30 mice were randomly divided into six groups: normal control, Alzheimer’s (no treatment), drug control (donepezil), RGE 100 mg/kg body weight (BW), RGE 200 mg/kg BW, and RGE 400 mg/kg BW. After overnight fasting, each group was administered treatment as follows: normal group (1% sodium carboxymethyl cellulose), Alzheimer group (1% sodium carboxymethyl cellulose), drug control group (1 mg/kg BW of donepezil), RGE100 group (100 mg/kg BW of RGE), RGE200 group (200 mg/kg BW of RGE), and RGE400 group (400 mg/kg BW of RGE). After 1 h, 25 mice were intraperitoneally injected with 1 mg/kg BW of scopolamine in sterile normal saline; the normal control group received a normal saline injection. Finally, 30 min later, all mice were placed individually in a Y-maze to observe spontaneous alteration activity.
Scopolamine-induced long-term memory loss in Alzheimer’s disease
We randomly divided 30 mice into six groups, as described in Section 2.6. On days 8–14, 25 mice were intraperitoneally injected with 1 mg/kg BW of scopolamine in sterile normal saline 1 h after treatment; the normal control group received a normal saline injection. Finally, 30 min later, all mice were placed individually in a Y-maze to observe spontaneous alteration activity on days 0, 3, 7, 10, and 14.
Determination of spontaneous alteration activity
Spontaneous alternation activity was assessed using a single, dark acrylic Y-maze with each arm measuring 35 cm (length) × 6 cm (width) × 18 cm (height). Each mouse was placed individually at the end of one arm and allowed to move freely for 8 min. The behavior of each mouse was recorded using a webcam (Jete W7, Indonesia). The arms of the maze were cleaned between sessions using 70% ethanol. An entry was considered complete when a mouse’s entire body, including the tail, was entirely within the arm. The number of entries per arm was calculated during 8 min. The number of alterations was defined as the ability of a mouse to enter the three arms consecutively, for example, A-B-C, B-C-A, or C-A-B. The percentage of alterations of each mouse was calculated as (total number of arms entered − 2) × 100.20
Preparation of serum and brain homogenates
On day 15, all mice were euthanized. Their brain and blood were obtained for further tests. The blood was centrifuged at 4000 rpm for 15 min, and the serum was stored at –80°C for additional tests. The whole brain was quickly removed and cleaned using chilled normal saline on ice. The brain was weighed to determine its relative weight before further examination. A 200 mg homogenate of brain samples was homogenized using a micro pestle (Axygen; cat. #PES-15-B-SI; USA) on ice, and the homogenized tissue was centrifuged at 4000 rpm for 15 min at 4°C. Finally, the homogenate was placed at –80°C to measure various biochemical parameters.21
MDA assay in the serum and brain
Malondialdehyde (MDA) levels in the serum and brain were measured by the method of Ohakawa et al.22 The principle of this method was MDA reacted with thiobarbituric acid (TBA) to produce a colored complex, which was measured using a spectrophotometer at 532 nm (UVD-2950; Labomed, Inc., Los Angeles, CA, USA). The result was expressed in µM.
Protein in the serum and brain
Total protein in the serum and brain were calculated using protein kits (Fluitest® TP; Germany) according to the manufacturer’s instructions. The total protein concentration was analyzed at 546 nm using a spectrophotometer (UVD-2950; Labomed, Inc.). The result was expressed in g/L.
AChE in the serum and brain
According to the manufacturer’s instructions, the AChE concentrations in the serum and brain were assessed using ELISA kits (Bioassay Technology Laboratory, cat. #E1347Mo, China). AChE activity was measured by the method of den Blaauwen et al23 using a colorimetric reagent kit (Bioassay Technology Laboratory, cat. #E1347Mo, China) according to the kit’s instructions. Each assay was performed in duplicate, and the results were expressed as ng/mL.
Histopatology of the hippoccampus
The histopathologic assessment was performed on the brains of 2–3 mice randomly selected from each group. The brains were immediately fixed in 10% phosphate-buffered formaldehyde, embedded in paraffin using a tissue processor (Tissue-Tek VIP 5 Jr; TEC 5 EM J-2 5233; TEC 5 CM J-2 5234; Sakura Finetek Japan), and 5 µm longitudinal sections were cut. The sections were stained with hematoxylin and eosin (H&E) and examined using a microscope (Olympus CX33; Olympus, Japan).
Data analysis
All data were reported as the mean ± standard error of the mean (SEM). Statistically significant differences between groups were assessed using SPSS Programme version 20. Data were analyzed with one-way analysis of variance (ANOVA), followed by least significant difference (LSD) post hoc tests. A significant difference between groups was considered when p < 0.05.
Results and Discussion
6-shogaol concentration in RGE
The calibration curve was plotted from 6-shogaol in the range of 5-500 ppm and resulted in a 0.999 correlation coefficient which means linear. The linear regression was y = 6.2572x − 1.5962, where y is the response as the peak area and x is the concentration in ppm (Fig. 1C). From this equation, the 6-shogaol content was 4.1235% ± 0.1920% w/w mg in 100 mg of ethanol RGE. Figs 1A and 1B showed consecutively the chromatograms of 6-shogaol and the ethanol RGE.
 |
Figure 1: Chromatogram of 6-shogaol (A) and RGE (B) using HPLC in 254 nm. |
Determination of acetylcholinesterase inhibitory activity
AChE is an enzyme responsible for ACh metabolism. The AChE inhibitory activity of RGE and 6-shogaol was tested using donepezil as a drug control. Both RGE and 6-shogaol showed lower AChE inhibitory activity than donepezil, while 6-shogaol showed better AChE inhibitory activity than RGE. The median inhibitory concentration (IC50) indicated the potency of AChE inhibition (Table 2). A low IC50 indicated better AChE inhibitory activity.
Table 2: AChE inhibitory activity (IC50) of RGE and 6-shogaol.
| No. | Compound | n | IC50 ± SEM |
| 1 | Red ginger extract | 4 | 2938.55 ± 307.60a |
| 2 | 6-shogaol | 3 | 361.18 ± 5.91b |
| 3 | Donepezil | 4 | 5.38 ± 0.87c |
Data are expressed as the mean ± SEM. Statistical analysis used one-way ANOVA followed by the LSD test. Different subscript letters are considered significantly different between groups using a 95% confidence interval. AChE, acetylcholinesterase; IC50, median inhibitory concentration; RGE, red ginger extract; SEM, standard error of the mean; ANOVA, analysis of variance; LSD, least significant difference.
Spontaneous alteration activity after scopolamine-induced acute memory loss using a Y-maze
In the Y-maze test, the Alzheimer group showed a significantly decreased total number of entries per arm (16.0 ± 4.45), the total number of alterations (12.25 ± 4.55), and percentage alteration (38.49% ± 13.40%) compared to the normal control group (Table 3; p < 0.05). RGE treatment changed the behavior of mice after a single dose of scopolamine. The RGE400 group showed the maximum total number of entries per arm, total number of alterations, and percentage of alteration. These values were the same for the drug control group.
Table 3: Spontaneous alteration activity of each group after scopolamine-induced acute memory loss.
| Group | Treatment | Total number of entries per arm
(±SEM) |
Total number of alterations
(±SEM) |
Percentage alteration
(±SEM) |
| Normal | CMC-Na 1% | 46.5 ± 6.41a | 32.25 ± 4.55a | 61.02 ± 2.92a |
| Alzheimer | CMC-Na 1% | 16.0 ± 4.45b | 7.25 ± 3.64b | 38.49 ± 13.40b |
| Donepezil | 1 mg/kg BW | 41.5 ± 11.26a | 20.75 ± 4.97a | 64.22 ± 8.46a |
| RGE | 100 mg/kg BW | 23.5 ± 2.36b | 13.75 ± 3.20b | 61.80 ± 7.04a |
| RGE | 200 mg/kg BW | 32.75 ± 3.79a | 24.75 ± 3.75a | 80.02 ± 4.45a |
|
RGE |
400 mg/kg BW | 25.0 ± 5.64b | 18.25 ± 4.13b |
66.81 ± 3.15a |
Data are expressed as the mean ± SEM of 4–5 mice. Statistical analysis used one-way ANOVA followed by the LSD test. Different subscript letters are considered significantly different between groups using a 95% confidence interval. CMC-Na, sodium carboxymethyl cellulose; RGE, red ginger extract; BW, body weight; SEM, standard error of the mean; ANOVA, analysis of variance; LSD, least significant difference.
Spontaneous alteration activity after scopolamine-induced Alzheimer’s disease using a Y-maze
After 7 days of scopolamine administration, the memory behavior of mice changed. The number of entries per arm, the total number of alterations, and the percentage of alteration significantly decreased in the Alzheimer group day by day compared with the normal control group (Figure 2A–C and Table 4; p < 0.05). RGE administration (7 days before and 7 days during scopolamine administration) successfully reversed the alteration percentage as an effect of memory loss due to scopolamine (Figure 2C). On day 14, the RGE400 group showed the best improvement in scopolamine-induced memory loss by an increase in the total number of entries per arm (928.75 ± 4.66), the total number of alterations (20.75 ± 3.14), and percentage alteration (73.79% ± 9.06%) compared to the Alzheimer group (p < 0.05). This improvement was the same in the drug control group (p > 0.05), except for the total number of alterations, indicating that 400 mg/kg BW of RGE treatment leads to better activity than donepezil.
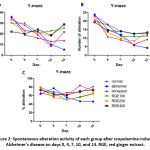 |
Figure 2: Spontaneous alteration activity of each group after scopolamine-induced Alzheimer’s disease on days 0, 4, 7, 10, and 14. RGE, red ginger extract. |
Table 4: Spontaneous alteration activity of each group after scopolamine-induced Alzheimer’s disease on day 14.
| Group | Treatment | Total number of entries per arm on day 14 (± SEM) | Total number of alterations on day 14 (± SEM) | Percentage alteration on day 14
(% ± SEM) |
| Normal | CMC-Na 1% | 21.75 ± 2.10a | 12.50 ± 1.19a | 71.04 ± 11.78a |
| Alzheimer | CMC-Na 1% | 5.25 ± 1.65b | 4.25 ± 1.93b | 26.78 ± 15.53b |
| Donepezil | 1 mg/kg BW | 13.75 ± 1.31c | 11.75 ± 2.32a | 81.25 ± 11.97a |
| RGE | 100 mg/kg BW | 25.25 ± 4.75 a | 16.25 ± 2.69a | 65.12 ± 12.01a |
| RGE | 200 mg/kg BW | 28.75 ± 2.84a | 16.00 ± 1.41a | 64.22 ± 4.31a |
| RGE | 400 mg/kg BW | 28.75 ± 4.66a | 20.75 ± 3.14c | 73.79 ± 9.06a |
Data are expressed as the mean ± SEM of 4–5 mice. Statistical analysis used one-way ANOVA followed by the LSD test. Different subscript letters considered the significant difference between groups using a 95% confidence interval. CMC-Na, sodium carboxymethyl cellulose; RGE, red ginger extract; BW, body weight; SEM, standard error of the mean; ANOVA, analysis of variance; LSD, least significant difference.
Determination of relative brain weight and protein concentration
Scopolamine induced a significant increase in protein levels in both serum and brain. Table 5 shows the effect of different doses of RGE on serum and brain protein levels. RGE in different doses and donepezil both resulted in a significant increase (p < 0.05) in serum and brain protein levels compared to the Alzheimer group.
Table 5: Effects of RGE administration on brain weight and protein levels during scopolamine-induced memory loss in Alzheimer’s disease.
| Group | Treatment | Brain weight
(g ± SEM) |
Relative brain weight
(% ± SEM) |
Brain protein
(g/dL ± SEM) |
Serum protein (g/dL ± SEM) |
| Normal | CMC-Na 1% | 0.36 ± 0.01 | 1.90 ± 0.13 | 1.43 ± 0.32a | 7.17 ± 0.18a |
| Alzheimer | CMC-Na 1% | 0.41 ± 0.02 | 1.76 ± 0.07 | 0.64 ± 0.04b | 5.39 ± 0.29b |
| Donepezil | 1 mg/kg BW | 0.34 ± 0.11 | 1.72 ± 0.06 | 1.22 ± 0.22a | 6.94 ± 0.65a |
| RGE | 100 mg/kg BW | 0.40 ± 0.02 | 1.46 ± 0.20 | 1.09 ± 0.26a | 6.16 ± 0.49a |
| RGE | 200 mg/kg BW | 0.43 ± 0.01 | 1.38 ± 0.05 | 1.60 ± 0.33a | 6.31 ± 0.29a |
| RGE | 400 mg/kg BW | 0.42 ± 0.01 | 1.52 ± 0.16 | 1.85 ± 0.23c | 7.22 ± 0.28a |
Data are expressed as the mean ± SEM of 4–5 mice. Statistical analysis used one-way ANOVA followed by the LSD test. Different subscript letters were considered significantly different between groups using a 95% confidence interval. CMC-Na, sodium carboxymethyl cellulose; RGE, red ginger extract; BW, body weight; SEM, standard error of the mean; ANOVA, analysis of variance; LSD, least significant difference.
Determination of MDA levels in the serum and brain
As seen in Table 6, the MDA concentration in the brain was seen lower than in serum. Scopolamine administration for 7 days increased MDA levels in both serum (29.99 ± 2.12 µM) and brain (19.03 ± 1.04 µM) in Alzheimer’s group compared to the normal control group (p < 0.05). It explained that scopolamine caused the accumulation of ROS and brought a higher level of MDA. Administration of RGE was successful to reduce MDA concentration in both the brain dan serum. The optimum dose of RGE which successfully reduced The MDA levels in the serum and brain was 400 mg/kg BW. This reduction was the same in the drug control group, donepezil (p > 0.05).
Table 6: Effects of RGE administration on MDA and protein levels during scopolamine-induced memory loss in Alzheimer’s disease.
| Group | Treatment | Serum MDA
(µM ± SEM) |
Brain MDA
(µM ± SEM) |
| Normal | CMC-Na 1% | 17.37 ± 0.59a | 11.68 ± 1.58a |
| Alzheimer | CMC-Na 1% | 29.99 ± 2.12b | 19.03 ± 1.04b |
| Donepezil | 1 mg/kg BW | 6.57 ± 2.04c | 4.16 ± 0.86c |
| RGE | 100 mg/kg BW | 20.13 ± 1.70a | 17.05 ± 1.66b |
| RGE | 200 mg/kg BW | 17.37 ± 1.36a | 15.37 ± 1.57b |
| RGE | 400 mg/kg BW | 9.32 ± 2.36c | 4.57 ± 2.40c |
Data are expressed as the mean ± SEM of 4-5 mice. Statistical analysis used one-way ANOVA followed by the LSD test. Different subscript letters were considered significantly different between groups using a 95% confidence interval. CMC-Na, sodium carboxymethyl cellulose; RGE, red ginger extract; MDA, malondialdehyde; BW, body weight; SEM, standard error of the mean; ANOVA, analysis of variance; LSD, least significant difference.
Determination of AChE levels in the serum and brain
Scopolamine injection significantly decreased AChE concentration in the brain (12.90 ± 0.78 ng/mL) and serum (12.80 ± 1.19 ng/mL) in Alzheimer’s group compared to the normal group (p < 0.05). The activity of RGE in decreasing AChE concentration depended on the dose. The RGE dose 200 mg/kg BW and 400 mg/kg BW groups showed the optimum dose to reduce AChE levels in the serum and brain compared to RGE dose 100 mg/kg BW. The potency of RGE to minimize AChE levels was the same as the drug control group after scopolamine administration (p < 0.05), as seen in Table 7.
Table 7: Effects of RGE administration on AChE levels during scopolamine-induced memory loss in Alzheimer’s disease.
| Group | Treatment | Serum AChE
(ng/mL ± SEM) |
Brain AChE
(ng/mL ± SEM) |
| Normal | CMC-Na 1% | 21.35 ± 2.08a | 17.15 ± 0.57a |
| Scopolamine | CMC-Na 1% | 12.80 ± 1.19b | 12.90 ± 0.78b |
| Donepezil | 1 mg/kg BW | 19.95 ± 2.65a | 18.10 ± 1.69a |
| RGE | 100 mg/kg BW | 18.95 ± 1.08a | 14.95 ± 1.03c |
| RGE | 200 mg/kg BW | 18.00 ± 1.41a | 18.35 ± 0.84a |
| RGE | 400 mg/kg BW | 19.55 ± 2.63a | 19.70 ± 1.63a |
Data are expressed as the mean ± SEM of 4-5 mice. Statistical analysis used one-way ANOVA followed by the LSD test. Different subscript letters considered the significant difference between groups using a 95% confidence interval. CMC-Na, sodium carboxymethyl cellulose; RGE, red ginger extract; AChE, acetylcholinesterase; BW, body weight; SEM, standard error of the mean; ANOVA, analysis of variance; LSD, least significant difference.
Histology of the hippocampus of mice with Alzheimer’s disease posttreatment
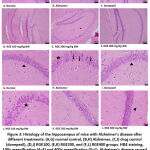
Repeated scopolamine administration resulted in disorganization, a decrease in thickness, as well as the density of pyramidal cells in cornu ammonis (CA)1 and CA3 subregions and granular cells of the dentate gyrus (DG) hippocampal subregions (Fig. 3B). RGE administration resolved the morphology of the hippocampus (Figs 3D–F; 3J–L). The neurons showed a uniform size and were well arranged. Each neuron also performed a rounded central vesicular nucleus with a prominent nucleolus in the RGE400 group (Fig. 3L). These effects were better than those in RGE100 and RGE200 groups (Figs. 3J–K). This morphology was also seen in the normal control (Fig. 3G) and drug control group (Fig. 3I).
Discussion
Scopolamine disturbs learning and memory functions and further leads to impairing the learning acquisition in both short- and long-term memories.24 Scopolamine also causes brain connectivity alterations, similar to those reported in Alzheimer’s disease.25 Furthermore, cholinergic neurons in the central nervous system were known to involve in mediating memories of both humans and animals in the short- and long-term.6,8 Many previous studies have found a relationship between the cholinergic system hypothesis and the function of learning, memory, and cognition. The disturbance of the cholinergic system causes a critical role in the early stage of Alzheimer’s disease.26-28
The spontaneous alternation activity score in the Y-maze test, that we used in our study, indicates the condition of working (short-term) memory.29 Scopolamine-induced reduction in the spontaneous alternation score increases after RGE treatment. RGE treatment improves the memory of mice with Alzheimer’s disease, as shown by the total number of entries per arm, the total number of alterations, and the percentage of alteration in this study.
We found that RGE treatment inhibits AChE from metabolizing ACh. An increase in ACh would restore the neurotransmitter homeostasis in the brain and improve learning and memory functions.27,30 Our result indicated a relationship between AChE levels in the serum and brain. This is interesting because our in vitro results implied that the activity of red ginger oil in inhibiting AChE is relatively weak compared to donepezil, while our in vivo results showed that the activity of RGE to block AChE is the same as that of donepezil. We think further studies are required because in vitro and in vivo tests use different approaches with different results.
In addition to increasing the function of the cholinergic system, oxidative stress also plays a major role in neurodegenerative disorders such as Alzheimer’s disease.31 Oxidative stress, or damage such as protein oxidation, lipid oxidation, DNA oxidation, and glycolysis, actively induces changes in learning and memory in Alzheimer’s disease.32 There are two possible conceptual approaches to the treatment of Alzheimer’s disease. The first is to prevent the onset of the disease, reduce the secondary pathologies or slow the progression of the disease, leading to the arrest or even repair of neuronal damage after the onset of the disease. The second is to provide symptomatic treatment to treat tertiary cognitive symptoms of the disease and to protect patients from further cognitive decline.33
Learning, memory, and cognition dysfunction induced by scopolamine administration in animal models is associated with changes in reactive oxygen species production and antioxidant enzyme expression.24,34,35 We found increased MDA levels in both the serum and brain of mice with Alzheimer’s disease. RGE treatment reduced these MDA levels and increased memory function, which was tested using the Y-maze. Elevated MDA levels have been found in the brain, cerebrospinal fluid, blood, and urine of Alzheimer’s disease patients.36 One limitation of this study was that we did not check any other antioxidant enzyme since this is the first report of RGE activity in Alzheimer’s disease.
RGE treatment at doses of 400 mg/kg BW had the best effect on memory function improvement through the AChE and MDA pathway. This dose of the ethanolic extract was equal to 16.49–24.74 mg of 6-shogaol. Another neuroprotector study on 6-shogaol also determined a similar result: 15–20 mg/kg BW of 6-shogaol could improve nerve function in mice.12,15,37
Conclusion
RGE treatment at a dose of 400 mg/kg BW is effective in reducing memory loss in Alzheimer’s disease by increasing the total number of entries per arm, the total number of alterations, and the percentage of alteration. RGE, which contains 6-shogaol inhibits AChE activity, increases protein levels, and reduces MDA levels in both serum and brain. RGE also improves the morphology of the hippocampus of Alzheimer’s mice.
Acknowledgment
The authors wish to thank Indriasih and Herdinik for their excellent technical assistance. This manuscript/data has not been submitted for possible publication in others journal.
Conflict of Interest
The authors declare no conflict of interest.
Funding Source
This work was partly supported by IsDB funding from the University of Jember No. 2620/UN25.3.1/LT/2020.
Reference
- DeTure M.A and Dickson D.W. The neuropathological diagnosis of Alzheimer’s disease. Molecular Neurodegeneration., 2019; 14(31): 32.
CrossRef - Mota S.I, Ferreira I.L and Rego A.C. Dysfunctional synapse in Alzheimer’s disease: a focus on NMDA receptors. Neuropharmacology., 2014; 76(A): 16-26.
CrossRef - Zhang Y, Li P, Feng J and Wu M. Dysfunction of NMDA receptors in Alzheimer’s disease. Neurol Sci., 2016; 37: 1039-1047.
CrossRef - Bezprozvanny I and Mattson M.P. Neuronal calcium mishandling and pathogenesis of Alzheimer’s disease. Trends Neurosci., 2008; 31: 454-463.
CrossRef - Kamat P.K, Rai S, Swarnkar S, Shukla R, Ali S, Najmi A.K and Nath C. Okadaic acid-induced tau phosphorylation in rat brain: role of NMDA receptor. Neuroscience., 2013; 238: 97-113.
CrossRef - García-Ayllón M.S, Small D.H, Avila J and Sáez-Valero J. Revisiting the role of acetylcholinesterase in Alzheimer’s disease: cross-talk with P-tau and β-amyloid. Front Mol Neurosci., 2011;4:22.
CrossRef - Akram M and Nawaz A. Effects of medicinal plants on Alzheimer disease and memory deficits. Neural Regen Res., 2017; 12: 660-670
CrossRef - Sharma K. Cholinesterase inhibitors as Alzheimer’s therapeutics (Review). Mol Med Rep., 2019; 20: 1479-1487.
CrossRef - Frozza R.L, Lourenco M.V and De Felice F.G. Challenges for Alzheimer’s disease therapy: insights from novel mechanism beyond memory defects. Front Neurosci., 2018; 12: 37.
CrossRef - Ali B.H, Blunden G, Tanira M.O and Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem Toxicol., 2008; 46: 409-420.
CrossRef - Semwal R.B, Semwal D.K, Combrinck S and Viljoen A.M. Gingerols and shogaols: important nutraceutical principles from ginger. Phytochemistry., 2015; 117: 554-568.
CrossRef - Ha S.K, Moon E, Ju M.S, Kim D.H, Ryu J.H, Oh M.S and Kim S.Y. 6-Shogaol, a ginger product, modulates neuroinflammation: A new approach to neuroprotection. Neuropharmacology., 2012; 63: 211-223.
CrossRef - Park G, Kim H.G, Ju M.S, Ha S.K, Park Y, Kim S.Y and Oh M.S. 6-Shogaol, an active compound of ginger, protects dopaminergic neurons in Parkinson’s disease models via anti-neuroinflammation. Acta Pharmacol Sin., 2013; 34: 1131-1139.
CrossRef - Moon M, Kim H.G, Choi J.G, Oh H, Lee P.K, Ha S.K, Kim S.Y, Park Y, Huh Y and Oh M.S. 6-Shogaol, an active constituent of ginger, attenuates neuroinflammation and cognitive deficits in animal models of dementia. Biochem Biophys Res Commun., 2014; 449: 8-13.
CrossRef - Fajrin F.A, Nugroho A.E, Nurrochmad A and Susilowati R. The improvement of pain behavior and sciatic nerves’ morphology in mice model of painful diabetic neuropathy upon administration of ginger (Zingiber officinale Roscoe.) extract and its pungent compound, 6-Shogaol. J Nat Sci Biol Med., 2019; 10: 149
CrossRef - Fajrin F.A, Nugroho A.E, Nurrochmad A and Susilowati R. Ginger extract and its compound, 6-shogaol, attenuates painful diabetic neuropathy in mice via reducing TRPV1 and NMDAR2B expressions in the spinal cord. J Ethnopharmacol., 2020; 249: 112396.
CrossRef - Jazokaite R, Marksa M, Zevzikoviene A and Zevzikovas A. Chromatographic analysis of 6-gingerol and 6-shogaol using TLC and HPLC methods. Sciencerise: Pharmaceutical Science., 2019; 2(18): 10-15.
CrossRef - Ellman G.L, Courtney K.D, Andres V and Featherstone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol., 1961: 7; 88-95.
CrossRef - Mathew M and Subramanian S. In vitro screening for anti-cholinesterase and antioxidant activity of methanolic extracts of ayurvedic medicinal plants used for cognitive disorders. PloS one., 2014; 9(1): 86804.
CrossRef - Lee G.Y, Lee C, Park G.H and Jang J.H. Amelioration of scopolamine-Induced Learning and Memory Impairment by α-Pinene in C57BL/6 Mice. Evid Based Complement Alternat Med., 2017; 2017: 4926815.
CrossRef - Rapaka D, Bitra V.R, Vishala T.C and Akula A. Vitis vinifera acts as anti-Alzheimer’s agent by modulating biochemical parameters implicated in cognition and memory. J Ayurveda Integr Med., 2019; 10: 241-247.
CrossRef - Ohakawa H, Oshishi N and Yagi K. Assay for lipid peroxidation in animal tissue by thiobarbituric acid reaction. Anal Biochem., 1979; 75: 351-358.
CrossRef - den Blaauwen D.H, Pope W.A and Tritschler W. Cholinesterase mit Butyrylthiocholin-iodid als Substrat: Referenzwerte in Abhangigkeit von Alter und Geschlecht unter Berucksichtigung hormonaler Einflusse und Schwangerschaft. J. Clin. Chem. Clin. Biochem., 1983; 21: 381-6.
CrossRef - Malekzadeh S, Edalatmanesh M.A, Mehrabani D and Shariati M, Drugs induced Alzheimer’s disease in animal model. GMJ., 2017; 6: 185-196.
- Bajo R, Pusil S, López M.E, Canuet L, Pereda E, Osipova D, Maestú F and Pekkonen E. Scopolamine effects on functional brain connectivity: a pharmacological model of Alzheimer’s disease. Sci Rep., 2015; 5: 9748.
CrossRef - Haam J and Yake J.L. Cholinergic modulation of the hippocampal region and memory function. J Neurochem., 2017; 142(Suppl 2): 111-21.
CrossRef - Hampel H, Mesulam M.M, Cuello A.C, Farlow M.R, Giacobini E, Grossberg G.T, Khachaturian A.S, Vergallo A, Cavedo E, Snyder P.J and Khachaturian Z.S. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain., 2018; 141(7): 1917-33.
CrossRef - Maurer S.V and Williams C.L. The cholinergic system modulates memory and hippocampal plasticity via its interactions with non-neuronal cells. Front. Immunol., 2017; 8: 1489
CrossRef - Miedel C.J, Patton J.M, Miedel A.N, Miedel E.S and Levenson J.M. Assessment of Spontaneous Alternation, Novel Object Recognition and Limb Clasping in Transgenic Mouse Models of Amyloid-β and Tau Neuropathology. J. Vis. Exp., 2017; 123: 55523.
CrossRef - Picciotto M.R, Higley M.J and Mineur Y.S. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron., 2012; 76(1): 116-29.
CrossRef - Simunkova M, Alwasel S.H, Alhazza I.M, Jomova K, Kollar V, Rusko M and Valko M. Management of oxidative stress and other pathologies in Alzheimer’s disease. Arch Toxicol., 2019; 93(9): 2491-2513.
CrossRef - Veurink G, Perry G and Singh S.K. Role of antioxidants and a nutrient rich diet in Alzheimer’s disease. Open Biol., 2020; 10: 200084.
CrossRef - Feng Y and Wang X. Antioxidant therapies for Alzheimer’s disease. Oxid Med Cell Longev., 2012; 2012: 472932.
- Haider S, Tabassum S and Perveen T. Scopolamine-induced greater alterations in neurochemical profile and increased oxidative stress demonstrated a better model of dementia: a comparative study. Brain Res Bull., 2016; 127: 234-247.
CrossRef - Wong-Guerra M, Jiménez-Martin J, Pardo-Andreu G.L, Fonseca-Fonseca L.A, Souza D.O, de Assis A.M, Ramirez-Sanchez J, Del Valle R.M and Nuñez-Figueredo Y. Mitochondrial involvement in memory impairment induced by scopolamine in rats. Neurol Res., 2017; 39: 649-659.
CrossRef - Arslan J, Jamshed H and Qureshi H. Early detection and prevention of alzheimer’s disease: role of oxidative markers and natural antioxidants. Front Aging Neurosci., 2020; 12: 231.
CrossRef - Sapkota A, Park S.J and Choi J.W. Neuroprotective effects of 6-shogaol and its metabolite, 6-Paradol, in a mouse model of multiple sclerosis. Biomol Ther (Seoul)., 2019; 27: 152-159.
CrossRef