Manuscript accepted on :15-12-2022
Published online on: 14-02-2023
Plagiarism Check: Yes
Reviewed by: Dr. Ramachandra Barik
Second Review by: Dr. Cecilia Jimeno
Final Approval by: Dr. Patorn Promchai
Hassan Khaled Nagi , Suzy Fawzy Michael
, Suzy Fawzy Michael , Hosam Ahmed Hamed*
, Hosam Ahmed Hamed* and Faten Farid Awadallah
and Faten Farid Awadallah
Critical Care Medicine, Faculty of Medicine, Cairo University, Cairo, Egypt
Corresponding Author E-mail: hosamahmed2205@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2598
Abstract
Background: One of the most frequent complications following heart surgery is atrial fibrillation (AF). The most popular diagnostic procedure for evaluating atrial function is echocardiography, however it has certain drawbacks. Originally, 2D echocardiography has been used to measure volumes to determine left atrial function. Objective: to examine the relationship between the development of post-operative AF following isolated CABG and preoperative evaluation of LA function using 2D echocardiography and left atrium 2D speckle tracking strain echocardiography. Patients and Methods: A set of 149 consecutive patients enrolled in a prospective observational study, they admitted to cardio-thoracic surgery department for elective isolated coronary artery bypass grafting surgery, during July 2018 to June 2019. 22 patients were excluded from the study due to bad image quality. Results: Readings of speckle tracking data showed significant less LA reservoir strain (OR 1.75, 95% CI: 0.65-4.69, P≤0001), LA conduit strains (OR 0.6, 95% CI: 0.22-1.62, P=0.31) and LA contractile strain (OR 0.65, 95% CI: 0.24-1.77, P=0.40) in POAF (+). Remaining parameters were non-significant. Also, Age (P=0.03), LA diameter (P=0.04), and LAVI (P=0.03) were the only factors that were identified as potential predictors of POAF in multivariate logistic regression analysis. Conclusion: we concluded that, age, LA size and LAVI are significantly associated with the occurrence of POAF in our patients.
Keywords
Atrial Fibrillation; Coronary artery bypass; Echocardiography; Left Atrial function
Download this article as:| Copy the following to cite this article: Nagi H. K, Michael S. F, Hamed H. A, Awadallah F. F. Left Atrial Function as a Predictor for Postoperative Atrial Fibrillation. Biomed Pharmacol J 2023;16(1). |
| Copy the following to cite this URL: Nagi H. K, Michael S. F, Hamed H. A, Awadallah F. F. Left Atrial Function as a Predictor for Postoperative Atrial Fibrillation. Biomed Pharmacol J 2023;16(1). Available from: https://bit.ly/3lAi5s3 |
Introduction
One of the most frequent complications following heart surgery is atrial fibrillation (AF). This results in lengthier hospital stays, higher hospital costs, an increased risk of thromboembolic events, and mortality.1 Despite the fact that 20–40% of individuals experience new-onset postoperative atrial fibrillation (NoPOAF) after coronary artery bypass graft (CABG) surgery 2, the underlying causes are not fully understood. However, it has historically been believed to be temporary and benign for the patient.3
According to recent data, POAF may be more malignant than previously believed, as evidenced by its correlation with follow-up mortality and morbidity.4, 5 Atrial fibrillation (AF), stroke, myocardial infarction, and heart failure have all been linked to enlarged left atrial (LA) size and LA dysfunction in the past.6 Additionally, this research points to a role for LA dysfunction brought on by oxidative stress, inflammation, and atrial fibrosis .7 In other words, preoperative LA dysfunction caused by acute functional depression may be the origin of POAF following coronary artery bypass grafting (CABG).8
Preoperative LA dysfunction may therefore become a key factor in identifying individuals who are at risk for POAF following CABG surgery as the measurement of LA function advances.8
Although echocardiography is the most popular diagnostic procedure for evaluating atrial function, the technology has several drawbacks. By evaluating volumes using 2D echocardiography, left atrial function has traditionally been evaluated. Doppler scans of the pulmonary vein and transmittal can also be used to evaluate it. The assessment of cardiac deformation with color tissue Doppler-derived strain has recently been included as an alternate technique.9
This technique has a few drawbacks, though, including poor reproducibility, angle dependence, artefacts in the signal, the fact that it only measures regional strain and doesn’t provide information on the curved area of the atrial roof, among others. It has been suggested to utilize speckle tracking echocardiography (STE) strain to get around these restrictions on measuring atrial function. We can assess both global and regional atrial strain using this method, which is angle-independent and does not arise from Doppler but rather from 2D echocardiography. The examination of regional atrial myocardial deformation indicated by a dimensionless parameter can be done using STE, a new technique for 2D echocardiography image analysis.9 This study intended to investigate the relationship between the development of post-operative AF after isolated CABG and the preoperative assessment of LA function using 2D echocardiography and left atrium 2D speckle tracking strain echocardiography.
Patient and Methods
A total of 149 consecutive patients were enrolled in a prospective observational study A who admitted to the cardio-thoracic surgery department for elective isolated coronary artery bypass grafting surgery, during July 2018 to June 2019. 22 patients were excluded from the study due to bad image quality.
Inclusion criteria
The patient must be older than 18 years old, have a preoperative sinus rhythm, and be having an elective, isolated CABG (with no other concurrent cardiac or extracardiac procedures).
Exclusion criteria
Prior sinus rhythm, hyperthyroidism or hypothyroidism, renal failure requiring hemodialysis, moderate to severe valvular heart disease, prior valve surgery, current use of antiarrhythmic medications, recent myocardial infarction within a month of surgery, and patient undergoing redo CABG are past rhythms that are not sinus.
Sample size
Based on the previous study in 2020, the sample size was calculated with a power of 80% at level of significance of 5%, using the software developed by Rollin Brant for the Estimation of Sample Size. 129 consecutive patients were allocated in the current study, 97 patients with POAF post-operative AF and 32 patients without POAF post-operative AF. 10
Patients were divided into of the following 2 groups:
Group (I) who developed post-operative AF (POAF)
Group (II) who did not develop post-operative AF.
All Patients and controls included in the study were subjected to the following:
Clinical history: clinical data were obtained pre-operatively regarding; age and gender, risk factors (DM, HTN, Smoking, obesity), previous history of myocardial infarction, medication in use including (B-blocker, ACE-I, diuretics, anti-platelet, statin, and nitrates) and pre-operative laboratory.
Clinical evaluation: including resting heart rate, class of heart failure (NYHA class), and chest pain Canadian class.
Coronary angiography: Four to twelve weeks before their scheduled operation, all patients had coronary angiography. A severe stenosis was deemed to exist when the lumen was reduced by 50%.11
Post-operative data: included bypass time and cross clamping time, number of bypass vessels, 3-incidence of myocardial infarction, inotropic support post-operative, post-operative laboratories and duration of hospital stay and mortality.
Statistical analysis
All data were compiled and analyzed using SPSS version 21 (SPSS Inc., Chicago, IL). Continuous variables are presented as means (±standard deviation [SD]), and categorical variables are presented using relative frequency distributions and percentages. Continuous variables were compared using Student’s t-test or the Mann-Whitney test, and categorical data were analyzed using the chi-square test, Yates’ continuity correction, Fisher’s exact test, and/or unadjusted odds ratios (ORs) as appropriate. Statistical significance established at p≤0.05. A 2-sided P≤0.05 considered statistically significant.
Ethical consideration
All the patients signed a written informed consent explaining the aims and the protocol of the study before inclusion and any study-related procedures. Approval of the study protocol was obtained from the Ethical Scientific Committee of Cairo University Hospital.
Results
A CONSORT flow chart of the study population shows that, a total of162 patients who attended to Cardio-Thoracic Surgery Department of Cairo University Hospitals, in the period from July 2018 to June 2019 for elective isolated coronary artery bypass grafting surgery. 20 patients were excluded from the study (5 patients declined consent and 15 patients did not meet the inclusion criteria. Of 149 patients, 20 patients were excluded from the study due to bad image quality, 129 patients were willing to participate in the study and consented for participation, 97 patients developed post-operative AF POAF (+ve) and 32 patients did not developed POAF (-ve), (Figure 1).
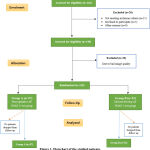 |
Figure 1: Flowchart of the studied patients. |
In the present prospective study, Patients were divided into who developed post-operative AF POAF (+ve) and Patients who did not developed POAF (-ve). In POAF (-ve) group, mean age was 57.9±9.2 years, 87.6%were males, 12.4% were females and mean BMI was 29.6±4.9. In POAF (+ve) group, mean age was 61.6±7.9 years, 81.3%were males, 18.8% were females and mean BMI was 29.1±5.9. POAF (+ve) group was significantly older than POAF (-ve) (P=0.045). There was no significant difference among patients regarding, Gender (P=0.36) and BMI (P=0.68). Also, In POAF (-ve) group, 70.1% of patients had DM, 72.2% had HTN, 48.5% were smokers, 42.3% had NYHA class one, 54.6% had Chest pain Canadian Class two and mean heart rate was 67.7. In POAF (+ve) group, 84.4% of patients had DM, 87.5% had HTN, 53.1% were smokers, 40.6% had NYHA class one, 46.9% had Chest pain Canadian Class one and mean heart rate was 67.6.There was no significant difference among patients regarding, DM (P=0.11), HTN (P=0.07), Smoking (P=0.64), NYHA class (P=0.55), Chest pain Canadian class (P=0.28) and heart rate on admission (P=0.98), (Table 1).
Table 1: Demographics and baseline risk factors in both groups.
| Post-operative AF | ||||||
| POAF (- ve) | POAF (+ve) | P value | ||||
| Gender n,% | Female | 12 | 12.4% | 6 | 18.8% | 0.36 |
| Male | 85 | 87.6% | 26 | 81.3% | ||
| Age years | 57.9 | ± 9.2 | 61.6 | ± 7.9 | 0.045* | |
| BMI | 29.6 | ± 4.9 | 29.1 | ± 5.9 | 0.68 | |
| DM n,% | No | 29 | 29.9% | 5 | 15.6% | 0.11 |
| Yes | 68 | 70.1% | 27 | 84.4% | ||
| HTN n, % | No | 27 | 27.8% | 4 | 12.5% | 0.07 |
| Yes | 70 | 72.2% | 28 | 87.5% | ||
| Smoking n,% | No | 50 | 51.5% | 15 | 46.9% | 0.64 |
| Yes | 47 | 48.5% | 17 | 53.1% | ||
| NYHA n,% | 0 | 36 | 37.1% | 9 | 28.1% | 0.55 |
| 1 | 41 | 42.3% | 13 | 40.6% | ||
| 2 | 19 | 19.6% | 9 | 28.1% | ||
| 3 | 1 | 1.0% | 1 | 3.1% | ||
| Chest pain Canadian CLASS n,% | 1 | 38 | 39.2% | 15 | 46.9% | 0.28 |
| 2 | 53 | 54.6% | 13 | 40.6% | ||
| 3 | 6 | 6.2% | 4 | 12.5% | ||
| Heart rate on admission | 67.7 | ±12.3 | 67.6 | ±13.2 | 0.98 | |
In POAF (-ve) group, 95.9% of patients were on Aspirin, 53.6% were on Nitrate, 55.7% were on ACEI, 88.7% were on B Blocker, 18.6% were on Diuretic and 94.8% were on Statin. In POAF (+ve) group, 93.8% of patients were on Aspirin, 50.0% were on Nitrate, 56.3% were on ACEI, 87.5% were on B Blocker, 25.0% were on Diuretic and 96.9% were on Statin. There was no significant difference among patients regarding, Aspirin (P=0.63), Nitrate (P=0.72), ACE-I (P=0.95), B Blocker (P=0.85), Diuretic (P=0.43) and Statin (P=0.62), (Table 2).
Table 2: Pre-operative medication in both groups.
| Medications | Post-operative AF | ||||
| POAF (- ve) | POAF (+ve) | P value | |||
| Aspirin | N | % Use | 2 | 6.3% | 0.63 |
| 93 | 95.9% | 30 | 93.8% | ||
| Nitrate | 52 | 53.6% | 16 | 50.0% | 0.72 |
| ACEI | 54 | 55.7% | 18 | 56.3% | 0.95 |
| B Blocker | 86 | 88.7% | 28 | 87.5% | 0.85 |
| Diuretic | 18 | 18.6% | 8 | 25.0% | 0.43 |
| Statin | 92 | 94.8% | 31 | 96.9% | 0.62 |
Regarding preoperative laboratory data, There were no significant difference between both groups as regard, hemoglobin (P=0.93), WBC (P=0.36), platelet (P=0.18), creatinine (p=0.47), alanine transaminase (p=0.21), sodium (p=0.74), potassium (p=0.56), magnesium (p=0.70), total cholesterol(p=0.84), creatinine kinase (p=0.13), alkaline phosphatase (P=0.51), HBA1C (P=0.09) and C reactive (P=0.19), (Table 3).
Table 3. Pre-operative and postoperative laboratory in both groups.
| Post-operative AF | |||||
| Pre-operative laboratory | POAF (-ve) | POAF (+ve) | P value | ||
| Hemoglobin | 13.62 | ±1.67 | 13.65 | ±1.60 | 0.93 |
| WBC | 7.77 | ±1.98 | 8.32 | ±2.31 | 0.19 |
| Platelet | 262.1 | ±72.5 | 242.7 | ±68.6 | 0.18 |
| CREAT | 0.94 | ±0.16 | 0.92 | ±0.15 | 0.47 |
| Alanine transaminase | 27.3 | ±15.2 | 31.3 | ±17.6 | 0.21 |
| Sodium | 136.1 | ±2.9 | 135.9 | ±3.1 | 0.74 |
| Potassium | 4.44 | ±0.39 | 4.39 | ±0.37 | 0.56 |
| Magnesium | 0.84 | ±0.15 | 0.83 | ±0.18 | 0.70 |
| Total cholesterol | 3.87 | ±0.99 | 3.83 | ±1.01 | 0.84 |
| Creat. Kinase | 89.5 | ±55.0 | 114.0 | ±83.9 | 0.13 |
| Alkaline phosphatase | 87.1 | ±33.4 | 91.8 | ±40.0 | 0.51 |
| HBa1c | 7.8 | ±1.8 | 8.4 | ±1.9 | 0.09 |
| C reactive protein | 11.57 | ±13.90 | 15.86 | ±22.10 | 0.19 |
| Post-operative laboratory | |||||
| Hemoglobin | 10.21 | ±1.02 | 10.16 | ±0.95 | 0.80 |
| WBC | 13.68 | ±3.57 | 14.50 | ±4.61 | 0.29 |
| Platelet | 196.10 | ±64.95 | 175.77 | ±48.52 | 0.10 |
| Creatinine | 0.90 | ±0.26 | 0.92 | ±0.22 | 0.64 |
| Alanine transaminase | 24.15 | ±13.99 | 78.74 | ±163.90 | 0.06 |
| Sodium | 137.68 | ±2.89 | 138.85 | ±3.25 | 0.06 |
| Potassium | 4.53 | ±0.27 | 4.59 | ±0.20 | 0.25 |
| Magnesium | 1.24 | ±0.23 | 1.31 | ±0.21 | 0.11 |
| Creatinine kinase | 638.47 | ±484.88 | 933.45 | ±1389.53 | 0.24 |
| Troponin | 2.59 | ±5.20 | 4.76 | ±6.88 | 0.10 |
| Alkaline phosphatase | 63.25 | ±18.44 | 65.20 | ±27.00 | 0.64 |
| C-reactive protein | 149.04 | ±37.78 | 148.51 | ±41.48 | 0.94 |
Readings of laboratory data post-operative were recorded. None of the laboratories finding showed significant difference between the 2 groups (Table 4).
Table 4: Shows operative and post-operative data in both groups
| Post-operative AF | ||||||
| POAF (-ve) | POAF (+ve) | P value | ||||
| NO. of bypass grafts | 1.0 | 7 | 7.2% | 3 | 9.4% | 0.44 |
| 2.0 | 30 | 30.9% | 13 | 40.6% | ||
| 3.0 | 44 | 45.4% | 14 | 43.8% | ||
| 4.0 | 16 | 16.5% | 2 | 6.3% | ||
| Cross clamp | 58.7 | ±30.7 | 68.7 | ±32.8 | 0.12 | |
| Bypass time | 76.6 | ±42.0 | 85.8 | ±39.4 | 0.28 | |
| IABP | 4 | 4.1% | 4 | 12.5% | 0.75 | |
| Patient on inotropic | 76 | 78.4% | 29 | 90.6% | 0.12 | |
| Post-operative MI | 15 | 15.5% | 10 | 31.3% | 0.05* | |
| Hospital stays | 9.1 | ±3.6 | 11.5 | ±4.3 | 0.002* | |
| On pump | 84 | 86.6% | 29 | 90.6% | 0.54 | |
| Off pump | 13 | 13.4% | 3 | 9.4% | 0.54 | |
| Mortality | 2 | 2.1% | 4 | 12.5% | 0.03* | |
In POAF (-ve) group, 45.4% of patients underwent three of bypass grafts, mean Cross clamp was 58.7, mean bypass time was 76.6, 4.1% had IABP, 78.4% were on inotropic, 15.5% had postoperative MI, mean hospital stay was 9.1 days, 86.6% were on pump and mortality rate was 2.1%. In POAF (+ve) group, 43.8% of patients underwent three of bypass grafts, mean cross-clamp was 68.7, mean bypass time was 85.8, 12.5% had IABP, 90.6% were on inotropic, 31.3% had postoperative MI, mean hospital stay was 11.5 days, 90.6% were on pump and mortality rate was 12.5%. The POAF (+ve) group had significant higher post-operative MI (P=0.05), longer hospital stays (P=0.002) and significant mortality (P=0.03). While none of the following showed significant difference; Number of bypass grafts (P=0.44), cross-clamp (P=0.12), Bypass time (P=0.28), IABP (P=0.08), on inotropic (P=0.12), and on pump (P =0.54), (Table 5).
Table 5: Preoperative 2D Echo and tissue Doppler data in both groups.
| Post-operative AF | |||||
| POAF (-ve) | POAF (+ve) | P value | |||
| Left ventricle end-diastolic diameter | 52.11 | ±7.17 | 52.62 | ±6.26 | 0.71 |
| Left ventricle end-diastolic volume | 95.77 | ±22.93 | 97.60 | ±28.32 | 0.71 |
| Left ventricle end-systolic diameter | 37.87 | ±6.84 | 38.14 | ±7.36 | 0.84 |
| Left ventricle end-systolic volume | 49.38 | 17.30 | 51.95 | 18.66 | 0.47 |
| Left ventricle ejection fraction | 50.25 | ±9.14 | 48.53 | ±8.01 | 0.34 |
| Left ventricle mass index | 97.11 | ±25.14 | 92.77 | ±16.82 | 0.36 |
| Left atrium diameter | 37.29 | ±4.35 | 40.09 | ±3.47 | <0.001* |
| Left atrium volume | 44.95 | ±16.45 | 44.47 | ±11.51 | 0.87 |
| Left atrium volume index (LAVI) | 26.51 | ±5.44 | 30.09 | ±5.74 | 0.001* |
| Mitral e velocity | 73.68 | ±20.88 | 81.71 | ±20.72 | 0.06 |
| Mitral a velocity | 76.08 | ±18.54 | 80.13 | ±17.88 | 0.28 |
| Mitral e/a ratio | 1.05 | ±0.54 | 1.06 | ±0.39 | 0.88 |
| Mitral e’ velocity Septal | 6.61 | ±1.91 | 6.78 | ±1.60 | 0.63 |
| Mitral e’ velocity lateral | 8.74 | ±2.75 | 8.78 | ±2.86 | 0.95 |
| Mitral e’ vel. (mean) | 7.66 | ±2.09 | 7.77 | ±1.96 | 0.80 |
| Mitral a’ vel. Septal | 8.55 | ±1.91 | 8.96 | ±2.01 | 0.30 |
| Mitral a’ vel. Lateral | 9.73 | ±2.56 | 10.80 | ±2.72 | 0.06 |
| Mitral a’ vel. (mean) | 9.11 | ±1.97 | 9.86 | ±2.16 | 0.06 |
| Mitral s’ vel. Septal | 6.87 | ±6.09 | 6.16 | ±1.39 | 0.51 |
| Mitral s’ vel lateral | 7.15 | ±2.13 | 7.48 | ±1.82 | 0.43 |
| Mitral s’ vel (mean) | 6.71 | ±1.62 | 6.80 | ±1.39 | 0.77 |
| Mitral e/e’ ratio | 9.96 | ±3.66 | 11.13 | ±4.15 | 0.13 |
| Mitral e deceleration time | 194.62 | ±45.88 | 180.16 | ±35.85 | 0.10 |
Readings of Echo and tissue Doppler pre-operative were recorded. There was significant longer LA diameter (P<0.001) and higher LAVI (P=0.001) in POAF (+ve), (Table 6).
Table 6: Speckle tracking data in both groups
| Post-operative AF | |||||
| POAF (-ve) | POAF (+ve) | P value | |||
| LV global strain | -11.56 | ±2.60 | -11.17 | ±2.78 | 0.47 |
| LA reservoir strain | 27.90 | ±7.42 | 22.31 | ±5.48 | <.0001* |
| LA Conduit strains | 15.05 | ±5.28 | 12.83 | ±4.35 | 0.03* |
| LA contractile strain | 12.86 | ±5.49 | 9.68 | ±4.33 | 0.003* |
| LA reservoir strain rate | 2.93 | ±1.26 | 2.61 | ±0.74 | 0.18 |
| LA Conduit strains rate | -3.15 | ±1.34 | -2.74 | ±1.10 | 0.12 |
Readings of speckle tracking data showed significant less LA reservoir strain (P≤0001), LA Conduit strains (P=0.03) and LA contractile strain (P =0.003) in POAF (+ve). Remaining parameters were non-significant (Table 7).
 |
Figure 2a: ROC Curve shows sensitivity, specificity, and Receiver operating characteristic curve for LAVI and LA diameter. |
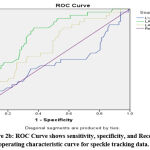 |
Figure 2b: ROC Curve shows sensitivity, specificity, and Receiver operating characteristic curve for speckle tracking data. |
 |
Figure 2c: ROC Curve shows sensitivity, specificity, and Receiver operating characteristic curve for speckle tracking data (Cont.) |
Table 7: ROC analysis data for different variables
| Variables | AUC | 95% CI | P value | Youden
index J |
Corresponding
cut-off point |
sensitivity | specificity | |
| Lower Bound | Upper Bound | |||||||
| LA diameter | 0.684 | 0.584 | 0.783 | 0.002 | 0.348 | 38.5 | 78.12% | 56.70% |
| LAVI | 0.692 | 0.583 | 0.801 | 0.001 | 0.337 | 27 | 75.00% | 58.80% |
| LV global strain | 0.452 | 0.328 | 0.575 | 0.412 | ||||
| LA reservoir strain | 0.738 | 0.646 | 0.831 | 0.000 | 0.441 | 25.6 | 81.2% | 62% |
| LA conduit strain | 0.627 | 0.519 | 0.735 | 0.022 | 0.246 | 15.3 | 81.25% | 43.3% |
| LA contractile strain | 0.678 | 0.577 | 0.779 | 0.000 | 0.339 | 12 | 87% | 46.3% |
| LA reservoir strain rate | 0.599 | 0.486 | 0.712 | 0.094 | 0.020 | 1.5 | 99.00% | 2.10% |
| LA conduit strain rate | 0.410 | 0.298 | 0.522 | 0.127 | 0.153 | -3.8 | 87.50% | 27.80% |
Age (P=0.03), LA diameter (P=0.04), and LAVI (P=0.03) were the only factors that were identified as potential predictors of POAF in multivariate logistic regression analysis (Table 8).
Table 8: Predictors of postoperative AF by multivariate logistic regression analysis
| Variables | Odds Ratio |
95% CI | P value | |
| Lower Bound | Upper Bound | |||
| Age | 0.94 | 0.88 | 0.99 | 0.03 |
| LA diameter | 0.86 | 0.75 | 0.99 | 0.04 |
| LAVI | 0.92 | 0.84 | 0.99 | 0.03 |
| LA reservoir strain | 1.75 | 0.65 | 4.69 | 0.26 |
| LA conduit strain | 0.60 | 0.22 | 1.62 | 0.31 |
| LA contractile strain | 0.65 | 0.24 | 1.77 | 0.40 |
| Post-operative MI | 1.55 | 0.50 | 4.80 | 0.45 |
Discussion
According to the most recent study, the prevalence of AF was 24.8%. (32 Patients from 129 patients). Clinical trials show that the incidence of POAF varies in proportion, as a result of many risk factors, but it also depends on the kind of cardiac surgery and the diagnostic standards for arrhythmias; the incidence of AF following CABG ranges from 15% to 40%.12-13 The incidence of POAF is 26%, according to a meta-analysis of 24 randomized clinical studies.14 Another study reported the incidence rate of POAF in the current study is like the incidence of arrhythmia in (23%) patients.15 Also, a larger number of patients (44%) who underwent CABG paired with valve surgery experienced POAF.16
In our study, patients with post-operative AF were older (61±7.9 years, p=0.045). This result may be related to the structural and functional changes that occur with ageing and the worsening of clinical problems. Numerous studies have shown advanced age as a risk factor for the emergence of POAF following heart surgery.16
A significant multicenter observational study conducted in 2018 involved 11239 consecutive patients without a history of atrial fibrillation who received isolated CABG between 1 January 2002 and 31 December 2010.17 They conclude that the incidence of AF significantly rises with age in both the general population and in post-CABG patients. Age was one of the predictors of incidence of AF post-operatively with mean age (67.59.5, p=0.0001). Additionally, a retrospective’s cohort study18, Age is a significant risk factor for AF, which affects 8% to 10% of people over the age of 80 in general. 314 One of the main substrates for AF, atrial fibrosis, is related with advanced age.18
Through this study, 25 patients who developed post-Operative myocardial infarction, were at high risk of developing AF (incidence of 31.3% of patients developed AF (p=0.05) more than patient without MI. These correlate with a study done by in 1999, the Trandolapril Cardiac Evaluation (TRACE) study included 6676 consecutive patients with acute myocardial infarction who were screened in 27 centers in Denmark for inclusion.19 The researchers looked at the incidence and prognostic significance of atrial fibrillation/-flutter following acute myocardial infarction. They found that 1395 patients (21%) experienced atrial fibrillation/-flutter at one or more of the specified times while hospitalized. They concluded that atrial fibrillation/flutter frequently follows acute myocardial infarction and showed that it was a distinct predictor of higher short- and long-term mortality.20
In our study, there were high association between AF and long hospital stay and post-operative mortality. POAF (+ve) was associated with long hospital stay (11.5 days ±4.3 vs 9.1± 3.6 in POAF (-ve), P=0.002), high mortality (12.5% vs 2.1% in POAF (-ve), P=0.03). Most of studies discussing the incidence of AF post cardiac surgery revealed high association with mortality and long hospital stay and high cost. Additionally, POAF lengthen hospital stay by 4.9 days, mostly because these patients attempted to restore sinus or control of heart rate, start and control of anticoagulants, aside from association between AF and other morbidities.21 Patients with POAF also had long hospital stays. According to a study in 2018, postoperative atrial fibrillation was linked to longer hospital stays and higher 1-year death rates (hazard ratio, 2.2; 95% confidence interval, 1.2-3.9). 22
Additionally, another cohort study suggested that older age, diabetes, and poor left ventricular function are independent risk factors for late mortality. They also suggested that a higher incidence of sudden death in postoperative AF patients may be related to the use of potentially dangerous anti-arrhythmic drugs or from acute myocardial infarction.23 Also, 25.1% (n=2290) of the 9,107 patients identified developed postoperative AF. POAF was linked to a greater risk of all-cause death compared to no AF, with an adjusted hazard ratio of 1.76 (1.33-2.33) and cardiovascular mortality at 2.43. (1.68–3.50). 24
In our study, 2D and Doppler study showing that patient with POAF had left trial diameter (40.09±3.47) higher than non POAF patient (p<0.001). With Youden index, the corresponding cut point 38.5 with sensitivity 78.12% and specificity 56.7%. Comparing our results with a retrospective analysis done in 2017, compared to the non-AF group, the atrium in the AF group had a substantially bigger mean anterior-posterior diameter (39.616.19 mm vs. 36.324.76 mm, p<0.001).25 Also, the POAF group had significant increased LA dimeter (40±5.2 vs 36.4±4.7, p=0.007), found that atrial fibrosis was associated with enlarged LA size that lead to incidence of POAF. However, LAVI enables a more accurate evaluation of LA. Left atrial size is typically utilized to assess structural changes in the LA. 26
In this study, the POAF (+ve) group, LAVI was higher than in the POAF (-ve) group (30.09 5.74 VS 26.5 5.4, p<0.001) with a cut point of 26 ml/m2, sensitivity 75%, and specificity 58.8. In a prospective study in 2013, 8 LAVI was significantly higher in patients with POAF (32.65.1 vs 27.37.2 in the NSR group), and another study reported that LAVI was a strong and independent predictor of POAF. LAVI > 32 ml/m2 was associated with a fivefold increase in risk of POAF, independent of age and other risk factors post cardiac surgery.27 Also, in a study conducted in 2015, discovered a strong correlation between increasing LAVI and incidence AF, with LAVI in AF being (24.0 6.4 vs. 30.3 9.0, p<0.001). 28
The abnormal LAVI cut-off that we found in our reference group (26 mL/m2) was a little lower than the value that recent recommendations suggest (34 mL/m2).29 The high mean body size of the participants in our cohort may have contributed to the slightly reduced LA volume once body size was considered.
Our research revealed no statistically significant differences between the two groups for various echocardiographic Doppler studies of the left atrium, including tissue Doppler studies for the mitral E, A, and S velocities, the E/A ratio, and the mitral deceleration time. The atrial strain curve is used in our work to estimate the likelihood of AF following coronary artery bypass surgery. The current study found that there is significant difference between POAF and non POAF regarding left atrial speckle tracking data. POAF (+ve) group had lower LA reservoir strain (27.9±7.42 vs 22.3±5.48; p<0.001), lower LA conduit strain (15.05±5.28 vs 12.8±4.35; P=0.03), LA contractile strain (12.86±5.49 vs 9.68 ±4.33; p=0.003). LA atrium reservoir strain had predicated value for detection of POAF with cut off <26, sensitivity 81.2% and specificity 62%, LA conduit with cut off <15.3, sensitivity 81.25% and specificity 43.3% and left atrial contractile with cut off<12, sensitivity 87% and specificity 46.3%. This finding is consistent with that conducted in 2013, found that patient with POAF (+ve) had lower LA global strain (reservoir strain) with cut off 27.7 with 81% sensitivity and 69% specificity (AUC 0.79, 95%CI, 0.65-0.93, p=0.003) also concluded that LA reservoir strain was independent predictor for POAF.8
Further study in 2016, revealed that patients with POAF had lower LA reservoir strain (20.86.9 vs 3012.8) and conduit strain (14.67 vs 11.13.8) than non POAF patients. Atrial fibrosis, LA size, and LA reservoir were found to be related to POAF and to be useful in predicting POAF, they concluded.26 another study reported that strain echocardiography is beneficial for detecting LA dysfunction and mechanical dispersion, also reported this observation. They speculated that this information is useful for predicting new-onset AF. In comparison to those without AF, individuals with new onset AF showed significantly higher reservoir strain (31.4%7.7% vs. 38.0%7.3%) and LA contractile strain (16.6%4.3% vs. 20.6%4.3%; P0.01).29 They found that the la reservoir strain was an independent predictor of new onset AF (AUC: 0.75; 95% CI: 0.63-0.87).29
Although the exact reasons for AF are not entirely known, structural remodeling and growing LA fibrosis play a critical role in the development of a substrate for AF. Several medical disorders, including ischemic heart disease, hypertension, diabetes, and heart failure, contribute to the remodeling of LA. Remodeling decreases the LA’s compliance, which impairs the atrial reservoir’s ability to function. As a result, atrial reservoir strain serves as a gauge of both the compliance and operation of the atrial reservoir. Therefore, decreased reservoir strain may be a marker of LA remodelling.30
As a result, atrial reservoir strain is a more accurate indicator of atrial dysfunction than LAV since the latter is an indication of more extensive remodeling while the former is believed to represent continuously increased filling pressure. The multivariate logistic regression analysis of our study revealed that age (odds ratios 0.94; 95% CI, 0.88-0.99; P=0.03), left atrium size (odds ratios 0.86; 95% CI, 0.75-0.99; P=0.04), and LAVI (odds ratios 0.92; 95% CI, 0.84-0.99; p=0.03) were the independent variables linked to the development of POAF. This is despite the variables associated with the development of POAF.
In our investigation, strain characteristics were not identified as independent POAF predictors. Our findings may imply that established criteria of LA dysfunction are not more accurate than innovative echocardiographic approaches in predicting POAF when assessing LA functions. Ischemia has an acute impact on atrial strain measures, while LAVI is less affected and reflects subacute or chronic diastolic function.31-32 This may help to explain why LAVI is more effective at predicting POAF than atrial strain because all the patients in our study had confirmed ischemia and coronary artery disease.33A specific study examining LAVI’s superiority over strain analysis in foretelling POAF or POAF-related cardiac events does not exist, though. Additionally, most findings came from investigations that involved relatively small patient populations. 34
Another study carried out in 2020 found clinical predictors of POAF were age and heart rate (P<0.001).10 While, echocardiographic measures associated with POAF were LA and LV global longitudinal strain (P<0.001). These results were consistent with other study in 2015 who reported that age (OR 1.09, 95% CI, 1.01–1.16) and LASs (OR1.63, 95% CI, 1.19–2.22) were both independent predictors of POAF, suggesting that atrial function assessed by echocardiographic deformation may enhance the clinical profile for identifying patients at high risk for developing POAF. 35
Our findings highlight the necessity for additional large-scale research examining straightforward echocardiographic characteristics that may predict POAF so that high-risk patients can take precautions even before the surgery.
Conclusion
Patients following CABG surgery typically experience postoperative atrial fibrillation, which is an arrhythmia. Age, LA size and LAVI are significantly associated with the occurrence of POAF in our patients. To lessen the mortality and morbidity linked to POAF, it may be beneficial to identify patients who are at an elevated risk of developing POAF before surgery by evaluating their LAVI or atrial STE results.
References
- El-Essawi A, Abdelhalim A, Groeger S, Breitenbach I, Brouwer R, Kück F, Harringer W. Predictors of postoperative atrial fibrillation persisting beyond hospital discharge after coronary artery bypass grafting. Perfusion; 37(1):62-8 (2022)
CrossRef - Haghjoo M. Pharmacological and nonpharmacological prevention of atrial fibrillation after coronary artery bypass surgery. J Tehran Heart Center; 7:2–9 (2012)
- Rezk M, Taha A, Nielsen SJ, Gudbjartsson T, Bergfeldt L, Ahlsson A, Jeppsson A. Clinical course of postoperative atrial fibrillation after cardiac surgery and long-term outcome. The Annals of Thoracic Surgery.114(6): 2209-2215 (2022)
CrossRef - Kashiwagi M, Ojima T, Hayata K, Kitadani J, Takeuchi A, Kuroi A, Terada K, Tanimoto T, Tanaka A, Yamaue H. Risk Factors for Chronic Atrial Fibrillation Development After Esophagectomy for Esophageal Cancer. Journal of Gastrointestinal Surger; 26(12):2451-9 (2022)
CrossRef - Armbruster AL, Campbell KB, Kahanda MG, Cuculich PS. The role of inflammation in the pathogenesis and treatment of arrhythmias. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy; 42(3):250-62 (2022)
CrossRef - Hirose K, Nakanishi K, Daimon M, Yoshida Y, Ishiwata J, Nakao T, Morita H, Di Tullio MR, Homma S, Komuro I. Prevalence, determinants, and prognostic value of left atrial dysfunction in patients with chronic coronary syndrome and normal left ventricular ejection fraction. The American Journal of Cardiology, 15;187:30-7 (2023)
CrossRef - Bhat A, Gan GC, Chen HH, Khanna S, Nawaz S, Nunes MC, Dobbins T, MacIntyre CR, Tan TC. Association of left atrial metrics with atrial fibrillation rehospitalization and adverse cardiovascular outcomes in patients with nonvalvular atrial fibrillation following index hospitalization. Journal of the American Society of Echocardiography; 34(10):1046-55 (2021)
CrossRef - Her AY, Kim JY, Kim YH, Choi EY, Min PK, Yoon YW, Lee BK, Hong BK, Rim SJ, Kwon HM. Left atrial strain assessed by speckle tracking imaging is related to new-onset atrial fibrillation after coronary artery bypass grafting. Canadian Journal of Cardiology; 29(3):377-83 (2013)
CrossRef - Cianciulli TF, Saccheri MC, Lax JA, Bermann AM, Ferreiro DE. Two-dimensional speckle tracking echocardiography for the assessment of atrial function. World journal of cardiology; 2(7):163 (2010)
CrossRef - Sabry AS, Mansour HA, El-Azm TH, Akef ME, Mostafa SA. Clinical and Echocardiographic Predictors of Atrial Fibrillation after Coronary Artery Bypass Grafting. Journal of Atrial Fibrillation;13(4):25-29 (2020)
CrossRef - Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Juni P. 2018 ESC/EACTS Guidelines on myocardial revascularization. Kardiologia Polska (Polish Heart Journal; 76(12):1585-664 (2018)
CrossRef - Villareal RP, Hariharan R, Liu BC, Kar B, Lee VV, Elayda M, Lopez JA, Rasekh A, Wilson JM, Massumi A. Postoperative atrial fibrillation, and mortality after coronary artery bypass surgery. Journal of the American College of Cardiology;43(5):742-8 (2004)
CrossRef - Steinberg BA, Zhao Y, He X, Hernandez AF, Fullerton DA, Thomas KL, Mills R, Klaskala W, Peterson ED, Piccini JP. Management of postoperative atrial fibrillation and subsequent outcomes in contemporary patients undergoing cardiac surgery: insights from the Society of Thoracic Surgeons CAPS‐Care Atrial Fibrillation Registry. Clinical cardiology; 37(1):7-13 (2014)
CrossRef - Andrews TC, Reimold SC, Berlin JA, Antman EM. Prevention of supraventricular arrhythmias after coronary artery bypass surgery. A meta-analysis of randomized control trials. Circulation; 84(5):236-44 (1991)
- Banach M, Rysz J, Okonski P, Misztal M, Barylski M, Irzmanski R, Zaslonka J. Risk factors of atrial fibrillation following coronary artery bypass grafting a preliminary report. Circulation Journal; 70(4):438-41 (2006)
CrossRef - Shen J, Lall S, Zheng V, Buckley P, Damiano Jr RJ, Schuessler RB. The persistent problem of new-onset postoperative atrial fibrillation: a single-institution experience over two decades. The Journal of thoracic and cardiovascular surgery;141(2):559-70 (2011)
CrossRef - Filardo G, Damiano RJ, Ailawadi G, Thourani VH, Pollock BD, Sass DM, Phan TK, Nguyen H, Da Graca B. Epidemiology of new-onset atrial fibrillation following coronary artery bypass graft surgery. Heart;104(12):985-92 (2018)
CrossRef - Folla CD and Melo CC. Predictive factors of atrial fibrillation after coronary artery bypass grafting. Einstein (São Paulo); 14(4):480-5 (2016)
CrossRef - Pedersen OD, Bagger H, Køber L, Torp-Pedersen C. The occurrence and prognostic significance of atrial fibrillation/-flutter following acute myocardial infarction. European heart journal;20(10):748-54 (1999)
CrossRef - El-Chami MF, Kilgo PD, Elfstrom KM, Halkos M, Thourani V, Lattouf OM, Delurgio DB, Guyton RA, Leon AR, Puskas JD. Prediction of new onset atrial fibrillation after cardiac revascularization surgery. The American journal of cardiology;110(5):649-54 (2012)
CrossRef - Echahidi N, Pibarot P, O’Hara G, Mathieu P. Mechanisms, prevention, and treatment of atrial fibrillation after cardiac surgery. Journal of the American College of Cardiology;51(8):793-801 (2008)
CrossRef - Akintoye E, Sellke F, Marchioli R, Tavazzi L, Mozaffarian D. Factors associated with postoperative atrial fibrillation and other adverse events after cardiac surgery. The Journal of thoracic and cardiovascular surgery;155(1):242-51 (2018)
CrossRef - Ahlsson A, Fengsrud E, Bodin L, Englund A. Postoperative atrial fibrillation in patients undergoing aortocoronary bypass surgery carries an eightfold risk of future atrial fibrillation and a doubled cardiovascular mortality. European journal of cardio-thoracic surgery; 37(6):1353-9 (2010)
CrossRef - Batra G, Ahlsson A, Lindahl B, et al. Atrial fibrillation in patients undergoing coronary artery surgery is associated with adverse outcome. Upsala journal of medical sciences;124(1):70-7 (2019)
CrossRef - Wenqi L, Wenjun Z, Xiaokang O, Zi W, Yujian M, Jie T, Huaibin W. Predictors of postoperative atrial fibrillation after isolated on-pump coronary artery bypass grafting in patients≥ 60 years old. In The Heart Surgery Forum; 20: 1583 (2017)
CrossRef - Ozben B, Akaslan D, Sunbul M, Filinte D, Ak K, Sari İ, Tigen K, Basaran Y. Postoperative atrial fibrillation after coronary artery bypass grafting surgery: a two-dimensional speckle tracking echocardiography study. Heart, Lung, and Circulation; 25(10):993-9 (2016)
CrossRef - Osranek M, Fatema K, Qaddoura F, Al-Saileek A, Barnes ME, Bailey KR, Gersh BJ, Tsang TS, Zehr KJ, Seward JB. Left atrial volume predicts the risk of atrial fibrillation after cardiac surgery: a prospective study. Journal of the American College of Cardiology;48(4):779-86 (2006)
CrossRef - Russo C, Jin Z, Sera F, Lee ES, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Left ventricular systolic dysfunction by longitudinal strain is an independent predictor of incident atrial fibrillation: a community-based cohort study. Circulation: Cardiovascular Imaging; 8(8): e003520 (2015)
CrossRef - Kawakami H, Ramkumar S, Nolan M, Wright L, Yang H, Negishi K, Marwick TH. Left atrial mechanical dispersion assessed by strain echocardiography as an independent predictor of new-onset atrial fibrillation: a case-control study. Journal of the American Society of Echocardiography;32(10):1268-76 (2019)
CrossRef - Rasmussen SM, Olsen FJ, Jørgensen PG, Fritz-Hansen T, Jespersen T, Gislason G, Biering-Sørensen T. Utility of left atrial strain for predicting atrial fibrillation following ischemic stroke. The International Journal of Cardiovascular Imaging; 35(9):1605-13 (2019)
CrossRef - Mu YM, Kasamaki Y, Ozawa Y, Ohta M, Chen XF, Tang Q, Wang CM, Hirayama A. Correlation of left ventricular pressure changes and left atrial function on strain rate imaging during acute left ventricular ischemia. International Heart Journal;51(6):421-5 (2010)
CrossRef - Møller JE, Hillis GS, Oh JK, Seward JB, Reeder GS, Wright RS, Park SW, Bailey KR, Pellikka PA. Left atrial volume: a powerful predictor of survival after acute myocardial infarction. Circulation;107(17):2207-12 (2003)
CrossRef - Guenancia C, Toucas C, Fauchier L, Stamboul K, Garnier F, Mouhat B, Sagnard A, Lorgis L, Zeller M, Cottin Y. High rate of recurrence at long-term follow-up after new-onset atrial fibrillation during acute myocardial infarction. EP Europace; 20(12):179-88 (2018)
CrossRef - Wenqi L, Wenjun Z, Xiaokang O, Zi W, Yujian M, Jie T, Huaibin W. Predictors of Postoperative Atrial Fibrillation after Isolated On-Pump Coronary Artery Bypass Grafting in Patients≥60 Years Old. The heart surgery forum; 20(1): 38-42.
- Verdejo HE, Becerra E, Zalaquet R, Del Campo A, Garcia L, Troncoso R, Chiong M, Marin A, Castro PF, Lavandero S, Gabrielli L. Atrial function assessed by speckle tracking echocardiography is a good predictor of postoperative atrial fibrillation in elderly patients. Echocardiography; 33(2):242-8 (2016).
CrossRef







