Manuscript accepted on :20-02-2023
Published online on: 03-03-2023
Plagiarism Check: Yes
Reviewed by: Dr. Jagdish Joshi
Second Review by: Dr. Deepak Bansal
Final Approval by: Dr. Patorn Piromchai
Pharmacy faculty, Al-Isra University, Amman, Jordan.
Corresponding Author E-mail: tagreedaltaei@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2644
Abstract
One of the most common and deadly diseases is tuberculosis, which has been known to be originated in ancient times. The assessment of the effectiveness of treatment regimens involves the monitoring of adverse events and the estimation of biomarkers. Serum biomarkers: Chemokine, Hematology, Liver function tests, and Kidney function tests were studied in forty tuberculosis patients of pulmonary and extra-pulmonary with its correlation. The monitoring and follow-up were assessed for the presence of any adverse effects, and compliance to treatment by Isoniazid 300 mg/kg, and Rifampicin 600 mg/kg during the study period. A significant difference was recorded between pulmonary and extra-pulmonary patients of the serum chemokine CXCL8 after one and two months of the treatment. The serum CXCL8 was increased in pulmonary and decreased in extra-pulmonary TB patients. The conclusion of this study described that chemokines play a role in mediating an effective immune-modulatory role during the treatment of TB infection and the therapeutic drug monitoring for compliance with TB treatment. A significant difference was noticed in the levels of liver enzymes (AST and ALT) between pulmonary and extra-pulmonary tuberculosis. Kidney function parameters showed a difference in creatinine levels between the two studied groups.
Keywords
Chemokines; Isoniazid; Rifampicin; Tuberculosis (TB)
Download this article as:| Copy the following to cite this article: Odeh A, Altaei T. Impact of Isoniazid and Rifampicin on Chemokines with Clinical Parameters in Tuberculosis Patients. Biomed Pharmacol J 2023;16(1). |
| Copy the following to cite this URL: Odeh A, Altaei T. Impact of Isoniazid and Rifampicin on Chemokines with Clinical Parameters in Tuberculosis Patients. Biomed Pharmacol J 2023;16(1). Available from: https://bit.ly/3kFhftZ |
Introduction
Tuberculosis (TB) is a widespread and global disease, it is the most widespread and difficult to decline, through the air transmitted by a bacterium called Mycobacterium tuberculosis (Mtb) through sneezing, speaking, and coughing1-3. Many infected individuals develop asymptomatic latent TB, while approximately 5–10 percent of latently infected individuals will progress to active pulmonary TB4.
MTB is an obligate aerobe that can play like an infectious agent2 and can attack the lungs and the members of the body like the central nervous system, musculoskeletal system, and lymph nodes1, 5, 6. Patients of pulmonary TB can have a fever, low appetite, fatigue, weight loss, night sweating, and cough, these manifestations can stay for weeks or months2. The immune system fails to repel the attack and the symptoms will appear after the penetration of bacteria. The immune system will destroy the foreign body, and the bacteria will remain inhibited in the body, and this has no indications or clinical symptoms of disease unless the bacteria reacted again1, 6.
The long treatment regimens of drugs used, the relative inefficacy of the current TB vaccine, and the increase in drug-resistant TB cases, stress the importance of understanding host immune responses that mediate Mtb control4.
The various mechanisms that regulate the spread of tuberculosis are outlined in a way that explains how these cells can be recruited into the lung. The recruitment of these immune cells is controlled by the presence of certain chemoattractant molecules and their ligands. These molecules, which are known as chemokines, can guide leukocyte migration by binding to receptor G proteins7.
Through studies conducted on animal models of tuberculosis and human studies, researchers have gained a deeper understanding of the various mechanisms involved in the disease’s spread. Mycobacterium tuberculosis can enter the lungs through the inhalation of droplets8. Mtb then replicates and blocks the macrophage-killing capabilities of alveolar macrophages, which then take up mycobacteria. Upon infection, infected macrophages release chemokines and cytokines, which stimulate immune cells’ recruitment into the lung9.
Different chemokines are responsible for the recruitment of diverse immune cells into the lung. The levels of these chemicals are observed to increase as early as day 12 following infection10. This suggests that the presence of CXCL-3 and CXCL-5 triggers the recruitment of NK and neutrophils. In addition, certain lung epithelial cells can also sense Mtb and release chemokines, which leads to the potentiation of the recruitment of immune cells. These cells are known to produce CXCL-8 and CCL-211, 12.
Recent studies have shown that the presence of specific chemokines, such as those produced by neutrophils, can be used as a potential marker of treatment responsiveness in patients with acute tuberculosis13, 14.
Isoniazid, which was introduced during the 1950s, is one of the most commonly used drugs for treating tuberculosis15. Due to the emergence of tuberculosis caused by the human immunodeficiency virus, the use of INH has increased significantly16, 17. It is commonly used by critically ill patients who have multiple medications that they are taking to treat the disease. These patients may potentially interact with other drugs18, 19. Bacteriocins such as rifampicin are known to kill harmful gram-positive bacteria, such as Mycobacterium tuberculosis. They can also inhibit the synthesis of bacterial protein2, 3.
The goal of this study is to:
To identify a potential host marker for response to therapy, researchers have conducted studies on the plasma chemokines of patients with tuberculosis who are taking INH or Rifampicin.
Evaluating the effects of administered drugs on studied parameters: Hematology; Hb, Monocytes, Lymphocytes, Neutrophils, WBCs, MCV, MCHC, MCH, PCV. Liver function tests; Aspartate aminotransferase (AST), Alanine aminotransferase (ALT), Alkaline phosphatase (ALP), Bilirubin, Albumin. Kidney function tests; Creatinine, Urate, and Blood Urea Nitrogen (BUN).
Find the correlation of serum chemokine level to the studied parameters.
Patient monitoring during treatment, post-treatment follow-up, and compliance/adherence.
Patients and Methods
The materials used were Human IL-8/CXCL8 Quantikine ELISA Kit, Hb, Monocytes, Lymphocytes, Neutrophils, WBCs, MCV, MCHC, MCH, and PCV. AST, ALT, Bilirubin, Albumin, Creatinine, Urate, BUN.
Methods
Study design
The study was conducted among TB patients who visited Al Basheer Hospital Amman/Jordan, and Al Nadeem Hospital Madaba/Jordan, for treatment between September 2018 and March 2019. The study has scientific approval from the Faculty of Pharmacy /Isra University (IU/6/909) and approval from the ethical committee of the Ministry of Health Amman, Jordan (MOH REC 1800107).
Forty Patients were included in this study, were using treatment with antituberculous drugs Isoniazid and Rifampicin according to the scheduled regimen, and gave in-regime consent. Study subjects received 300 mg of isoniazid, and 600 mg of rifampin, according to their body weight (the body weight bands were 70-80 kg).
Basic demographic and clinical information was collected from all participants, including age, sex, body weight, comorbidities (including HIV infection and DM), and concomitant drug use.
Patients
Tuberculosis patients from the Center of chest diseases and the health of expatriates in Amman and Madaba cities were enrolled in this study. All patients presented with either positive sputum smear microscopy and/or a positive culture for Mtb at enrolment. Drug susceptibility testing was performed at the start of treatment. The study was performed with the cooperation of specialists in the same Centre and the patients.
Inclusion criteria
Tuberculosis patients presented with either positive sputum smear microscopy and/or a positive culture for Mtb. TB patients treated by INH and/ or Rifampicin. Patients cooperate and sign informed consent.
Exclusion criteria
Resistance to isoniazid or rifampicin will be a criterion for exclusion.
Any condition that would cause liver injury. TB patients are treated with other medications. Pregnant and lactating women
Blood sampling and Laboratory Testing
The blood samples collected from enrolled TB patients had been frozen at -20° C, serum was prepared, and studied data was collected from all patients.
The laboratory investigations were carried out on all enrolled patients: Hematology; Monocytes, Lymphocytes, Neutrophils, WBCs, MCV, MCHC, MCH, PCV, Hb. Liver function tests; AST, ALT, ALP, Bilirubin, Albumin. Kidney function tests; Creatinine, Urate, and BUN.
Human Chemokine IL-8/CXCL8 Immunoassay ELISA
According to the manufacturer’s instructions for the kit.
Patient Monitoring During Treatment
Therapeutic monitoring of enrolled TB patients looking for the presence of any adverse effects of the drugs used.
Adherence/Compliance & Treatment Follow-Up.
The adherence/compliance of enrolled TB patients to their medication regimen was followed up and assessed by stopping the administration of their medication due to the high levels of liver enzymes and the patients’ admission because of discontinuing the treatment.
Statistical analysis
The results are presented as a percentage or mean. The statistical significance of the study was computed by comparing the results of the linear regression analysis and the t-test. Logistical and univariate regression analyses were also utilized to identify the factors that affected the treatment response.
The receiver-operating characteristics analysis was performed on a sample of patients with different chemokine levels. The analyzed parameters were then used to perform correlation analyses and independent t-tests. The results of the study were then validated using the SPSS 19 software.
Results
Patients
Forty TB patients were enrolled in this study, 55% of patients had pulmonary TB with 80% of them being male and 20% being female, the average age was 42 years, and 45% of patients had extra pulmonary TB with 33% of them were male and 67% were female, the average age was 37 years.
The patients presented with a 2 to 3-week history of cough (93%), fever (40%), self-reported weight loss (60%), night sweats (73%), and/or chest pain (80%), and joint pain (80%) in pulmonary TB patients, compared to none of the mentioned symptoms were recorded in extra-pulmonary.
The highest incidence of pulmonary TB patients was found in 73%, 13.3 % of Jordanian, Asian & Syrian, respectively, while extra-pulmonary TB patients were found in 66% of Jordanians, and 8.3% were Asian and Egyptian. The highest percentage of smokers was found in pulmonary TB patients (53%), while in extra-pulmonary TB patients were found 33. The demographic and clinical characteristics of all enrolled TB patients were shown in table 1.
Table 1: Demographic and clinical characteristics of enrolled TB patients.
| PULMONARY N (%) 22 (55%) | EXTRA-PULMONARY N (%) 18 (45%) | ||
| AGE (mean) | 42 | 37 | |
| GENDER
Female Male |
8 (20%) 32 (80%) |
27 (67%) 13 (32.5%) |
|
| STATE
|
Married | 21 (52.5%) | 17 (42.5%) |
| Single | 11 (27.5%) | 17 (42.5%) | |
| Divorced | 8 (20%) | 7 (17.5%) | |
| NATIONALITY
|
Jordanian | 29 (72.5%) | 26 (65%) |
| Asian | 5 (12.5%) | 3 (7.5%) | |
| Syrian | 5 (12.5%) | 0 | |
| Egyptian | 0 | 3 (7.5%) | |
| Sudanese | 0 | 3 (7.5%) | |
| EDUCATION
|
Ungraduated from the secondary school | 35 (87.5%) | 27 (67.5%) |
| Bachelor’s degree | 5 (12.5%) | 13 (32.5%) | |
|
SMOKING |
21 (52.5%) |
13 (32.5%) |
|
Evaluation of serum chemokine in TB patients
The estimation of serum chemokine in TB patients treated by INH and/ or Rifampicin showed that the serum chemokine levels represent a significant difference between pulmonary and extra-pulmonary TB patients, P< 0.05, but there is no significant difference in the total mean of serum chemokine level between pre and post levels in each group, but 50% of the patients have a significant reduction, the other half represents nonsignificant elevation or no difference in the serum chemokine levels in both pulmonary and extra-pulmonary TB patients, as shown in figure 1.
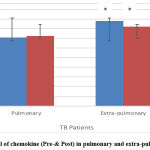 |
Figure 1: Serum level of chemokine (Pre-& Post) in pulmonary and extra-pulmonary TB patients. |
Two Way ANOVA test was applied to compare the serum level of chemokine between the two groups; pulmonary and extra-pulmonary TB patients, there was a significant difference after one- and two-months post-treatment in extra-pulmonary TB patients 82.1617±24.919 (P=0.039) and 55.7500±6.010 (P=0.0003), respectively showed in table 2 and figure 2.
Table 2. Serum chemokine levels of pulmonary & extra-pulmonary TB patients.
| Pulmonary (M±SE) | Extrapulmonary (M±SE) |
P Value |
|
| Stage 1: Pre-treatment | 71.1540±20.455 | 87.9958±37.593 | 0.17 |
| Stage 2: After one month | 72.2587±9.504 | 82.1617±24.919 | 0.039 |
| Stage 3:After two months | 74.1950±2.637 | 55.7500±6.010 | 0.0003 |
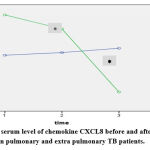 |
Figure 2: The serum level of chemokine CXCL8 before and after treatment in pulmonary and extra pulmonary TB patients. |
Receiver-operating characteristics (ROC) analysis of chemokine level
The data presented from the analysis of ROC indicated that there is a possible role of chemokine concentrations as a predictor of treatment response, AUC= 0.891, SD= 0.051 with a confidence interval of 95% of the difference, Figure 3.
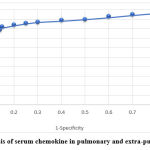 |
Figure 3: ROC analysis of serum chemokine in pulmonary and extra-pulmonary TB patients. |
Evaluating the effects of administered drugs on clinical parameters
The serum level of the studied parameters; Hematology; Hb, Monocytes, Lymphocytes, Neutrophils, WBCs. Liver function tests; Aspartate aminotransferase (AST), Alanine aminotransferase (ALT), Alkaline phosphatase (ALP), Bilirubin, Albumin. And Kidney function tests; Creatinine, Urate, Blood Urea nitrogen (BUN) in pulmonary and extra-pulmonary TB patients, figures 4-6.
There was a significant difference between pulmonary and extra-pulmonary TB patients in WBCs, neutrophils, and lymphocytes, while in monocytes there was no significant difference, also there was no significant difference in the serum levels of MCV, MCHC, MCH, PCV, and Hb between the two groups of patients, figure 4 (A & B).
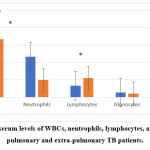 |
Figure 4 A: The serum levels of WBCs, neutrophils, lymphocytes, and monocytes in pulmonary and extra-pulmonary TB patients. |
Independent T-test was applied to compare the hematology parameters between the two groups of TB patients post-treatment, there was a significant difference in PCV level only in pulmonary 36.95±1.62 more than extra-pulmonary TB patients 34.15±5.44, P=0.0001. MCV, MCHC, MCH, WBCs, neutrophils, lymphocytes, and monocytes showed no significant difference as shown in table 3.
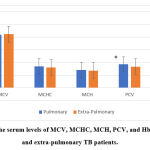 |
Figure 4 B: The serum levels of MCV, MCHC, MCH, PCV, and Hb in pulmonary and extra-pulmonary TB patients. |
Table 3: Hematology parameters of pulmonary and extra pulmonary TB patients.
| Pulmonary (M±SE) | Extrapulmonary (M±SE) | P value | |
| WBCs / µl | 7.76±2.28 | 6.67±4.32 | 0.347 |
| MCV fl | 83.43±4.71 | 84.44±7.02 | 0.646 |
| MCHC g/dl | 33.65±1.10 | 32.08±1.78 | 0.367 |
| MCH pg | 27.83±2.03 | 27.12±3.06 | 0.498 |
| PCV % | 36.95±1.62 | 34.15±5.44 | 0.0001 |
| Neutrophils x103 | 4.65±2.33 | 1.96±1.00 | 0.240 |
| Monocytes x103 | 0.54±0.24 | 0.39±0.12 | 0.308 |
| Lymphocytes x103 | 1.31±0.42 | 2.20±0.42 | 0.877 |
Comparison of the pre and post-hematology values of pulmonary and extra-pulmonary TB patients showed no significant differences as in figure 5.
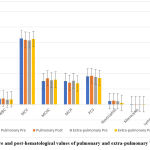 |
Figure 5: Pre and post-hematological values of pulmonary and extra-pulmonary TB patients. |
Studying the liver function tests in both groups of pulmonary and extra-pulmonary TB patients showed that there was a significantly elevated level of AST (90.22±122.2) and ALT (71.22±95.57) in pulmonary TB more than in extra-pulmonary TB patients; 23.36±9.99, 19.94±8.58, respectively, as shown in figure 6.
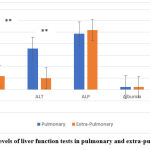 |
Figure 6: The serum levels of liver function tests in pulmonary and extra-pulmonary TB patients. |
Independent T-test was applied to compare the liver function parameters between the two studied groups after treatment, there was a highly significant difference in AST levels of 90.22±122.2, and 23.36±9.99 (P=0.003) of pulmonary and extra-pulmonary TB patients, respectively. The serum level of ALT showed a highly significant difference; 71.22±95.57, and 19.94±8.58 (P=0.002) of pulmonary and extra-pulmonary TB patients, respectively. Alkaline Phosphate, Albumin, and Bilirubin showed no significant difference as shown in table 4.
Table 4: Liver function parameters of pulmonary and extra pulmonary TB patients.
| Pulmonary (M±SE) | Extrapulmonary (M±SE) | P value | |
| AST µg/l | 90.22±122.2 | 23.36±9.99 | 0.003 |
| ALT µg/l | 71.22±95.57 | 19.94±8.58 | 0.002 |
| ALP µg/l | 97.15±38.08 | 103.5±16.19 | 0.415 |
| Albumin gm/dl | 4.35±0.70 | 4.34±0.78 | 0.224 |
| Bilirubin µmole/l | 5.45±2.73 | 5.54±2.24 | 0.298 |
A significant difference was shown in the values of AST (P=0.01), and ALP (P= 0.001) in the comparison of pre and post-values in both groups, while ALT values significantly declined in the extra-pulmonary group only (P=0.002) As in figure 7.
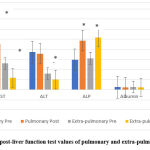 |
Figure 7: Pre and post-liver function test values of pulmonary and extra-pulmonary TB patients. |
Kidney function results described the difference in creatinine levels between the two studied groups pulmonary and extra-pulmonary TB patients; 66.87±18.25, and 52.62±16.91, respectively, figure 8.
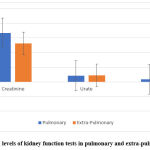 |
Figure 8: The serum levels of kidney function tests in pulmonary and extra-pulmonary TB patients. |
An Independent T-test was applied to compare the kidney function parameters between the two groups showed no significant differences in Creatinine, Urate, and BUN as shown in table 5.
Table 5: kidney function parameters of pulmonary and extra pulmonary TB patients.
| Pulmonary (M±SE) | Extra Pulmonary (M±SE) | P value | |
| Creatinine mmole/l | 66.87±18.25 | 52.62±16.91 | 0.062 |
| Urate mmole/l | 8.48±2.95 | 8.74±2.87 | 0.976 |
| BUN mmole/l | 3.60±1.19 | 3.45±1.31 | 0.823 |
Concerning the pre and post-values of the kidney function parameters; there was a significant difference in the creatinine results of pulmonary (P=0.001), and extra-pulmonary (P=0.002) as in figure 9.
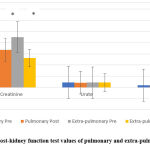 |
Figure 9: Pre and post-kidney function test values of pulmonary and extra-pulmonary TB patients. |
The Correlation of chemokine to the studied parameters
This section describes the correlation coefficients of serum chemokine level to the studied parameters; Hematology; Hb, Monocytes, Lymphocytes, Neutrophils, and WBCs. Liver function tests; Aspartate aminotransferase (AST), Alanine aminotransferase (ALT), Bilirubin, Albumin. Kidney function tests; Creatinine, Urate, Blood Urea Nitrogen (BUN) in pulmonary and extra-pulmonary TB patients.
The correlation of chemokine to hematology tests
Studying the correlation of serum chemokine level to the hematology tests showed that there is a non-significant negative correlation in pulmonary TB patients; r = -0.995, P=0.161, while in extra-pulmonary patients, there is a significant positive correlation, the correlation coefficients r= 0.588, P=0.02, as shown in figure 10 (A & B).
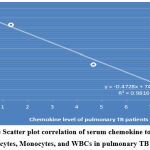 |
Figure 10 A: The Scatter plot correlation of serum chemokine to the Neutrophils, Lymphocytes, Monocytes, and WBCs in pulmonary TB patients. |
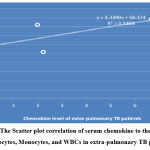 |
Figure 10 B: The Scatter plot correlation of serum chemokine to the Neutrophils, Lymphocytes, Monocytes, and WBCs in extra-pulmonary TB patients. |
The correlation of chemokine to liver function tests.
The correlation of serum chemokine level to the liver function tests showed that there is a significant weak positive correlation in pulmonary TB patients; r = 0.537, P=0.01, while in extra-pulmonary patients, there is a significant strong positive correlation, the correlation coefficients r= 0. 835, P=0.02, as shown in figure 11 (A & B).
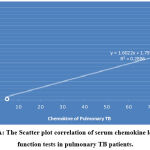 |
Figure 11 A: The Scatter plot correlation of serum chemokine level to liver function tests in pulmonary TB patients. |
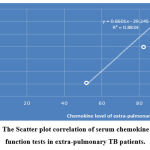 |
Figure 11 B: The Scatter plot correlation of serum chemokine level to liver function tests in extra-pulmonary TB patients. |
The correlation of chemokine to Kidney function tests.
Analysis of the correlation of serum chemokine level to the kidney function tests showed that there is a non-significant negative correlation in pulmonary TB patients; r = -0.819, P=0.14, while there is a significant strong positive correlation in extra-pulmonary patients, the correlation coefficients r= 0.768, P=0.03, as shown in figure 12 (A & B).
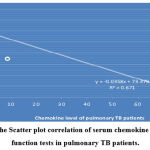 |
Figure 12 A: The Scatter plot correlation of serum chemokine level to Kidney function tests in pulmonary TB patients. |
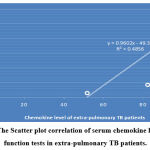 |
Figure 12 B: The Scatter plot correlation of serum chemokine level to Kidney function tests in extra-pulmonary TB patients. |
Patient monitoring during treatment, post-treatment follow-up, and Adherence.
The monitoring of enrolled pulmonary and extra-pulmonary TB patients during the treatment period and follow-up showed that there was a significant difference in the adherence to treatment in pulmonary TB patients (60%), and in the extra-pulmonary TB patients (83%), P<0.05.
The adverse effects were recorded in both groups of TB patients and showed that pulmonary have a severe symptom more than extra-pulmonary patients. At the beginning of treatment, many patients suffer from severe joint pain (80%). Red urine color (99%), depression (50%).
Discussion
Tuberculosis is an international public health problem. An improved understanding of the immunopathogenic of TB can facilitate the design of effective vaccines, new drug candidates, and the valuation of their efficacy20.
In this study, the female percentage was higher in extra-pulmonary TB patients compared to pulmonary, while the male percentage was higher in pulmonary TB patients compared to extra-pulmonary. The percentage of pulmonary was 55%, and 45% for extra-pulmonary TB patients, which agrees with the study of Toujani S, et al., (2015)21; mentioned that pulmonary tuberculosis is the most common form of TB presentation.
Smoking has a toxic effect on body organs including the liver, it causes necroinflammation by elevation of pro-inflammatory cytokines production such as IL-1, IL-6, and TNF-a, furthermore, smoking causes lipid peroxidation which activates stellate cells and gives rise to liver fibrosis, these factors lead to liver injury at the end. Smoking is a common cause of immunity imbalance by lymphocyte proliferation prevention which produce lymphocyte apoptosis, also by T cell suppression. Studies highlight the oxidative stress caused by cigarette consumption and by an accumulation of TB medication’s metabolites in different ways; cigarette smoking raises the serum and hepatic iron, likewise the accumulation of metabolites of INH and rifampicin in the liver cause cellular antioxidant defense mechanism, both will lead to hepatocytic death by oxidative stress22, 23. The high percentage of smokers in pulmonary tuberculosis is an important justification for the failure of treatment and the weak response to anti-tuberculosis drugs, because smoking raises the level of CXCL8 sharply, which leads to a reduction in the phagocytosis function24. The present study agrees with it.
High chemokine levels could promote Mycobacterium tuberculosis proliferation or alter anti-Mycobacterium tuberculosis immune responses, resulting in higher bacterial burdens25, this study agrees with it, which explains the decreased efficacy of drugs used for TB treatment and failure of therapy.
A study by Aryanpur M, et al. (2016)24 described that the measurement of TNF-a and CXCL-8 in the serum showed an opposing relationship between the phagocytic capability of macrophages and smoking which means that the manifestation of immune cells is less and far to be noticed in smoking patients than the non-smoking, while smoking did not affect CXCL-8 levels, these differences are biomarkers of the smoker patient’s response to TB infection. The present study showed that the number of smokers of pulmonary TB patients was higher than extra-pulmonary TB patients, which may explain the different responses to anti-tuberculosis treatment.
Mycobacterium tuberculosis by activation of macrophages could induce the sequences of cell activation and releases of inflammatory chemokines such as CXCL-8, which induces the immune system network. The present work studied the quantitative analysis of serum chemokines CXCL-8, which is agreeing with the studies of Garofalo R, et al. (1996), King C, et al. (1998), and Asokananthan N, et al. (2002)26-28 that explained the lung epithelial cells directly contribute to immune responses by releasing active substances, these cells have been shown to secrete IL-6 and IL-8/CXCL8 when stimulation happened by inflammatory mediators. Also, these study results are agreed with the studies of Kunkel SL, et al. (1991) and Richman-Eisenstat, et al. (1993)29, 30 that describe the active substance produced by lung epithelial cells as IL-8/CXCL8 which is one of the most important mediators of lung inflammation.
Pulmonary fibroblasts within human tuberculosis granulomas express CXCL831, and CXCL8 levels in sputum from TB patients closely counterpart and precede mycobacterial rescue in patients administering therapy for tuberculosis, suggesting that CXCL8 may be a useful early replacement biomarker in response to tuberculosis therapy32. The results of this study are agreeing with that.
The presented data from the analysis of ROC indicated that there is a role of chemokine CXCL-8 response as a predictor of a treatment success response. The clinical use of this test is to explore whether a patient should change the treatment regimen, assuming the patient will not respond to treatment or is not adherent to treatment.
Biomarkers of inflammation and infections are quantitative and provide a piece of information to improve the predictive chance for a successive treatment. Assessment of the serum levels of chemokines in pulmonary and extra-pulmonary TB patients treated by INH and/or Rifampicin as a means of determining the affected biomarker associated with response to therapy in TB patients; showed that the serum chemokine levels represent a significant difference between pulmonary and extra-pulmonary TB patients, P< 0.05, but there is no significant difference in the total mean of serum chemokine level between pre and post levels in each group, despite that 50% of the patients have a significant reduction, the other half represents non-significant elevation or no difference in the serum chemokine levels in both pulmonary and extra-pulmonary TB patients. This study agrees with the above-mentioned studies, and the cross-sectional study, which described that TB treatment changed the plasma level of cytokines and chemokines in TB patients33.
There was a significant difference between pulmonary and extrapulmonary TB patients in the PCV value, which agrees with the study by Al-Mohammadi MO, et al. (2011)34 who mentioned that the PCV is gradually improving when administering TB medications, but the smoking factor plays a role to evaluate the PCV level35.
Studies corroborate that INH increases the serum levels of ALT production >10 times higher than normal in an unclear mechanism, INH is metabolized by acetylation into acetylhydrazine (AcHz), hydrazine (Hz), and acetylisoniazid these metabolites are harmful on liver cells causing necrosis and lead to liver injury and hepatotoxicity36. A study of the liver function tests in the present work using an independent T-test showed that there was a significant difference in serum AST and ALT, while there was no significant difference in serum albumin and bilirubin between pulmonary and extra-pulmonary TB patients.
The isoniazid-induced liver injury is immune-mediated, but most cases are mild and resolve with immune tolerance37, there are two cases recorded in this research of isoniazid-induced toxicity, those two have a very high level of AST and ALT and correlated with the high level of serum chemokine CXCL8. We can notice hyperbilirubinemia in the pulmonary and the extrapulmonary, Rifampicin is a gold player to raise bilirubin to a high level, Rifampicin is not mainly responsible for damaging the liver, but this drug can support Isoniazid to liver injury38.
The pulmonary TB patients presented with symptoms like; cough, fever, weight loss, night sweats, and/or chest pain, and leg pain, two to three weeks earlier, but extra-pulmonary showed non-of the above-mentioned symptoms in this research. The present study agrees with a study conducted in Nepal, which mentioned the difficulty of detecting symptoms in extrapulmonary tuberculosis, because of the disappearance of such symptoms that are regularly associated with pulmonary tuberculosis 39.
A study by Xu W, et al. (2009)40 suggested prescribes supplements and vitamins to avoid the rise of liver enzymes, protect the liver from injury, and minimize the noncompliance of TB patients, the present work is agreeing with this suggestion.
Analysis of the correlation coefficient of serum chemokine level to the kidney function tests showed that there is a non-significant negative correlation in pulmonary TB patients, but there is a significant strong positive correlation in extra-pulmonary patients. From this, we conclude the effects of TB treatment in extra-pulmonary TB patients are more effective than treatment in pulmonary TB patients.
Conclusion
TB patients were closely monitored by testing the CXCL8 levels and clinical parameters, there was a significant difference between the two groups of serum CXCL8 levels, after one-month CXCL8 declined in the extrapulmonary TB patients, but the pulmonary TB patients had an opposite situation by increasing the level of CXCL8.
The correlation study showed a positive correlation coefficient between the serum chemokines with hematology, liver function, and kidney function parameters in extra-pulmonary TB patients, while there was a positive correlation coefficient of chemokine CXCL8 with liver function tests in pulmonary TB patients. The monitoring and following up of pulmonary and extra-pulmonary TB patients during the treatment period represent a significant difference in the adherence to treatment, which was higher in the extra-pulmonary TB patients, while the pulmonary TB patients suffered from adverse effects.
Conflict of Interest
The authors disclose that there is no conflict of interest.
References
- Desissa, F. Workineh, T. and Beyene, T. Risk factors for the occurrence of multidrug-resistant tuberculosis among patients undergoing multidrug-resistant tuberculosis treatment in East Shoa, Ethiopia. BMC public health, 2018; 18(1): 422.
CrossRef - Leung, A. N. Pulmonary tuberculosis: the essentials. The radiological society of north America, 1999; 210(2): 307-322.
CrossRef - Ilievska-Poposka, B. Metodieva, M. Zakoska, M. Vragoterova, C. and Trajkov, D. Latent Tuberculosis Infection-Diagnosis and Treatment. Open access Macedonian journal of medical sciences, 2018; 6(4): 651.
CrossRef - Dye, C. Glaziou, P. Floyd, K. and Raviglione, M. Prospects for tuberculosis elimination. Annual review of public health, 2013; 34: 271-286.
CrossRef - Gambhir, S. Ravina, M. Rangan, K. Dixit, M. Barai, S. and Bomanji, J. Imaging in extrapulmonary tuberculosis. International Journal of Infectious Diseases, 2017; 56: 237-247.
CrossRef - Pienaar E, Linderman JJ, Kirschner DE. Emergence and selection of isoniazid and rifampin resistance in tuberculosis granulomas. PLoS One. 2018;13(5): e0196322.
CrossRef - Zlotnik, A. and Yoshie, O. Chemokines: a new classification system and their role in immunity. Immunity, 2000; 12(2): 121-127.
CrossRef - Flynn, J.L. Lessons from experimental Mycobacterium tuberculosis infections. Microbes and Infection, 2006; 8(4): 1179-1188.
CrossRef - Guirado, E., Schlesinger, L. S. & Kaplan, G. Macrophages in tuberculosis: friend or foe. Seminars in immunopathology, 2013; 35: 563–583, https://doi.org/10.1007/s00281-013-0388-2.
CrossRef - Kang, D.D. Lin, Y. Moreno, J.R. Randall, T.D. and Khader, S.A. Profiling early lung immune responses in the mouse model of tuberculosis. PloS one, 2011; 6(1): e16161.
CrossRef - Lin, Y. Zhang, M. and Barnes, P.F. Chemokine production by a human alveolar epithelial cell line in response to Mycobacterium tuberculosis. Infection and immunity, 1998; 66(3): 1121-1126.
CrossRef - Wickremasinghe, M.I. Thomas, L.H. and Friedland, J.S. Pulmonary epithelial cells are a source of IL-8 in the response to Mycobacterium tuberculosis: essential role of IL-1 from infected monocytes in a NF-κB-dependent network. The Journal of Immunology, 1999; 163(7): 3936-3947.
CrossRef - Anbarasu, D. Raja, C.P. and Raja, A. Multiplex analysis of cytokines/chemokines as biomarkers that differentiate healthy contacts from tuberculosis patients in high endemic settings. Cytokine, 2013; 61(3): 747-754.
CrossRef - Mihret, A. Bekele, Y. Bobosha, K. Kidd, M. Aseffa, A. Howe, R. and Walzl, G. Plasma cytokines and chemokines differentiate between active disease and non-active tuberculosis infection. Journal of Infection, 2013; 66(4): 357-365.
CrossRef - Cohn, D.L. Treatment and prevention of tuberculosis in HIV-infected persons. Infectious disease clinics of North America, 1994; 8(2): 399-412.
CrossRef - Gourevitch, M.N. Hartel, D. Selwyn, P.A. Schoenbaum, E.E. and Klein, R.S. Effectiveness of isoniazid chemoprophylaxis for HIV-infected drug users at high risk for active tuberculosis. Aids, 1999; 13(15): 2069-2074.
CrossRef - Moore, M. McCray, E. & Onorato, I.M. Cross-matching TB and AIDS registries: TB patients with HIV co-infection, United States, 1993-1994. Public Health Reports, 1999; 114(3): 269.
CrossRef - Grange, J.M. Winstanley, P.A. and Davies, P.D. Clinically significant drug interactions with antituberculosis agents. Drug safety, 1994; 11(4): 242-251.
CrossRef - Schluger, N.W. Issues in the treatment of active tuberculosis in human immunodeficiency virus-infected patients. Clinical infectious diseases, 1999: 130-135.
CrossRef - Russell, D. G, Barry, C. E. rd, Flynn JL. Tuberculosis: What we do not know can, and does, hurt us. Science, 2010; 328: 852-856.
CrossRef - Toujani, S. Salah, N.B. Cherif, J. Mjid, M. Ouahchy, Y. Zakhama, H. Daghfous, J. Beji, M. Rhouma, N.M.B. and Louzir, B. La primo-infection et la tuberculosepulmonaire. Revue de pneumologie clinique, 2015; 71(2-3): 73-82.
CrossRef - Ramappa, V. and Aithal, G. P. (2013), Hepatotoxicity related to anti-tuberculosis drugs: mechanisms and management. Journal of clinical and experimental hepatology, 2013; 3(1): 37-49.
CrossRef - Wang, P. Pradhan, K. Zhong, X. B. and Ma, X. Isoniazid metabolism and hepatotoxicity. Acta pharmaceutica sinica B, 2016; 6(5): 384-392.
CrossRef - Aryanpur, M. Mortaz, E. Masjedi, M. R. Tabarsi, P. Garssen, J. Adcock, I. M. and Sharifi, H. Reduced phagocytic capacity of blood monocyte/macrophages in tuberculosis patients is further reduced by smoking. Iranian Journal of Allergy, Asthma, and Immunology, 2016; 15(3): 174-182.
CrossRef - Algood, H.M.S. Chan, J. and Flynn, J.L. Chemokines and tuberculosis. Cytokine &growth factor reviews, 2003; 14(6): 467-477.
CrossRef - Garofalo, R. Sabry, M. Jamaluddin, M. Yu, R.K. Casola, A. Ogra, P.L. and Brasier, A.R. Transcriptional activation of the interleukin-8 gene by respiratory syncytial virus infection in alveolar epithelial cells: nuclear translocation of the RelA transcription factor as a mechanism producing airway mucosal inflammation. Journal of Virology, 1996; 70(12): 8773-8781.
CrossRef - King, C. Brennan, S. Thompson, P.J. and Stewart, G.A. Dust mite proteolytic allergens induce cytokine release from cultured airway epithelium. The Journal of Immunology, 1998; 161(7): 3645-3651.
CrossRef - Asokananthan, N. Graham, P.T. Fink, J. Knight, D.A. Bakker, A.J. McWilliam, A.S. Thompson, P.J. and Stewart, G.A. Activation of protease-activated receptor (PAR)-1, PAR-2, and PAR-4 stimulates IL-6, IL-8, and prostaglandin E2 release from human respiratory epithelial cells. The Journal of Immunology, 2002; 168(7): 3577-3585.
CrossRef - Kunkel, S.L. Standiford, T. Kasahara, K. and Strieter, R.M. Interleukin-8 (IL-8): the major neutrophil chemotactic factor in the lung. Experimental lung research, 1991; 17(1): 17-23.
CrossRef - Richman-Eisenstat, J.B. Jorens, P.G. Hebert, C.A. Ueki, I. and Nadel, J.A. Interleukin-8: an important chemoattractant in sputum of patients with chronic inflammatory airway diseases. American Journal of Physiology-Lung Cellular and Molecular Physiology, 1993; 264(4): L413-L418.
CrossRef - O’Kane, C.M. Boyle, J.J. Horncastle, D.E. Elkington, P.T. and Friedland, J.S. Monocyte-dependent fibroblast CXCL8 secretion occurs in tuberculosis and limits survival of mycobacteria within macrophages. The Journal of Immunology, 2007; 178(6): 3767-3776.
CrossRef - Ribeiro-Rodrigues, R. Johnson, J.L. Ribeiro, F. Palaci, M. Sá, R.T. Maciel, E.L. Lima, F.E.P. Dettoni, V. Toossi, Z. Boom, W.H. and Dietze, R. Sputum cytokine levels in patients with pulmonary tuberculosis as early markers of mycobacterial clearance. Clin. Diagn. Lab. Immunol., 2002; 9(4): 818-823.
CrossRef - Idriss, H.T. and Naismith, J.H. TNFα and the TNF receptor superfamily: Structure‐function relationship (s). Microscopy research and technique, 2000; 50(3): 184-195.
CrossRef - Al-muhammadi, M. O. and Al-Shammery, H. G. Studying some hematological changes in patients with pulmonary tuberculosis in Babylon governorate. Medical Journal of Babylon, 2011; 8(4): 608-617.
- Bain, B. J. Rothwell, M. Feher, M. D. Robinson, R. Brown, J. and Sever, P. S. Acute changes in haematological parameters on cessation of smoking. Journal of the Royal Society of Medicine, 1992; 85(2): 80.
CrossRef - Wang, P. Pradhan, K. Zhong, X. B. and Ma, X. Isoniazid metabolism and hepatotoxicity. Acta pharmaceutica sinica B, 2016; 6(5): 384-392.
CrossRef - Metushi, I. Uetrecht, J., and Phillips, E. Mechanism of isoniazid‐induced hepatotoxicity: then and now. British journal of clinical pharmacology, 2016; 81(6): 1030-1036.
CrossRef - Thompson, N. P. Caplin, M. E. Hamilton, M. I. Gillespie, S. H. Clarke, S. W. Burroughs, A. K. and McIntyre, N. Anti-tuberculosis medication and the liver: dangers and recommendations in management. European Respiratory Journal, 1995; 8(8): 1384-1388.
CrossRef - Sreeramareddy, C. T. Panduru, K. V. Verma, S. C. Joshi, H. S. and Bates, M. N. Comparison of pulmonary and extrapulmonary tuberculosis in Nepal-a hospital-based retrospective study. BMC infectious diseases, 2008; 8(1): 8.
CrossRef - Xu, W. Lu, W. Zhou, Y. Zhu, L. Shen, H. and Wang, J. Adherence to anti-tuberculosis treatment among pulmonary tuberculosis patients: a qualitative and quantitative study. BMC health services research, 2009; 9(1): 169.
CrossRef








