Partha Saha1 , Dipshikha Sharma2
, Dipshikha Sharma2 , Suvakanta Dash3
, Suvakanta Dash3 , Kumar Saurav Dey4
, Kumar Saurav Dey4 and Samir Kumar Sil1*
and Samir Kumar Sil1*
1Cancer Biology and Cell Physiology Lab., Department of Human Physiology, Tripura University, Suryamaninagar-799022, Tripura, India.
2Assam Science and Technology University, Guwahati-781013, Assam, India.
3Regional Institute of Pharmaceutical Science and Technology, Agartala-799005, Tripura, India.
4Guwahati Biotech Park, Guwahati-781039, Assam, India.
Corresponding Author E-mail: sks_ps_21@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2609
Abstract
Parkia javanica (Lamk.) Merr. is an ethnomedicinal leguminous plant species from northeastern India with a long history of medicinal use among various tribes of this region to treat cholera, dysentery, stomach aches, diarrhea and food poisoning, having antibacterial, wound-healing, anticancer and anti-inflammatory properties. Therefore, in this current study, the methanolic bark extract was carried out and fractionated by using flash chromatography, examined the cytotoxicity of the respective fractions on colon cancer cell lines, and evaluated the major phytochemical compounds present in the fractions using Gas Chromatography-Mass Spectrometry (GC-MS) chemical profiling. Chemical profiling of the fractions by GC-MS revealed in fraction-1 and -2, 2,4-Di-tert-butylphenol was the major compound (50.740% in fraction-1, 21.277% in fraction-2, and 7.859% in fraction-3) having reported anticancer activity. The gradation of the presence of this compound in the fractions was corroborated by the gradation of anti-colon cancer activity of the respective fractions on both the colon carcinoma cell lines. However, the presence of D-Allose in a substantial amount (20.870%) in only fraction-3 could not increase the anticancer activity of fraction-3 over the other two fractions. An in vitro cytotoxic assay guided evaluation of three flash chromatographic fractions (fraction-1, -2, and -3) of methanolic extract of Parkia javanica bark showed significant anticancer properties on two human colon carcinoma cell lines (HCT116 and SW480). The order of efficacy of the fractions was fraction-1> fraction-2 > fraction-3. In a time and dose-dependent experiment, fraction-1, being the most active one, showed an IC50 value of 16.25 µgml-1 (24 hrs), 9.94 µgml-1 (48 hrs), and 9.38 µgml-1 (72 hrs) on HCT116 and 35 µgml-1 (24 hrs), 20.14 µgml-1 (48 hrs), and 19.71 µgml-1 (72 hrs) on the SW480 cell line. Parkia javanica bark extract is bestowed with the potential of anti-colon cancer property and upon chemical profiling of different chromatographic fractions of the extract, 2,4-Di-tert-butylphenol has been identified as the primary anticancer component of the extract.
Keywords
Anti-Colon cancer; Bark extract; 2,4-Di-tert-butylphenol; Flash chromatography; GC-MS; Parkia javanica
Download this article as:| Copy the following to cite this article: Saha P, Sharma D, Dash S, Dey K. S, Sil S. K. Identification of 2,4-Di-tert-butylphenol (2,4-DTBP) as the Major Contributor of Anti-colon cancer Activity of Active Chromatographic Fraction of Parkia javanica (Lamk.) Merr. Bark Extract. Biomed Pharmacol J 2023;16(1). |
| Copy the following to cite this URL: Saha P, Sharma D, Dash S, Dey K. S, Sil S. K. Identification of 2,4-Di-tert-butylphenol (2,4-DTBP) as the Major Contributor of Anti-colon cancer Activity of Active Chromatographic Fraction of Parkia javanica (Lamk.) Merr. Bark Extract. Biomed Pharmacol J 2023;16(1). Available from: https://bit.ly/3j0CXaV |
Introduction
According to GLOBOCAN 2020 data, colon cancer ranks third among all newly diagnosed cancers and second among all cancer deaths globally, irrespective of gender or age1. It’s the second-most prevalent female cancer across the globe, and the third most common cancer reported in males2,3.
Numerous studies have revealed associations between colorectal cancer and eating choices, induced inflammation, and other factors, including diabetes mellitus, obesity, alcohol consumption, cigarette smoking, and genetic mutations4-10. 5-fluorouracil (5-FU) in conjunction with irinotecan, oxaliplatin, or levamisole is usually the conventional treatment11. Antibodies have also been created against the receptors of epidermal and vascular EGF (endothelial growth factor), which have improved the prognosis of colorectal cancer patients12.
Despite the fast advancements in the field of diagnosis and treatment of colon cancer in recent years, it remains a significant clinical concern globally due to its high prevalence13. Due to the toxicity and side effects of cytotoxic medications, as well as innate therapeutic resistance mechanisms in CRC tumor cells14, modern CRC therapy is still unsatisfactory. So, effective pure compounds must be introduced into the therapy of colon cancer that can help to reduce side effects and drug-related toxicity in humans.
Parkia javanica is one of the ethnomedicinal leguminous plants that have been traditionally used to cure a verity of ailments by several tribes in northeastern India15-17, among the other medicinal plants of this region18, 19. Previously, we discovered that the crude bark extract of this plant had anticancer potential on colon cancer cell lines20. The current study aimed to explore the key phytochemical compound(s) contributing to the anti-colon cancer property of the extract of Parkia javanica bark using different chromatographic subfractions of the extract.
Material and Methods
Identification of plant
P. javanica barks were obtained from Ambassa, Dhalai District of Tripura, India. The plant was identified by the renowned taxonomist of the Department of Botany, Tripura University, Prof. Badal Kumar Dutta. The corresponding specimen, identified as BD-01/06, was also sent to the Herbarium.
Preparation of the Plant extract
Around 200 gm of shaded dried P. javanica bark powder was mixed in 600 ml of solvent (methanol) for 48 hrs (with periodic shaking) at normal room temperature. After 48 hrs, the filtration process of the solvent was repeated until the solvent appeared clear. The solvent was concentrated at 60°C using a rotary evaporator before being lyophilized (at 4°C) to remove all traces of the solvent from the extract.
Conditions of Flash Chromatography
To fractionate the whole extract, a flash chromatographic technique (Teledyne ISCO Combi Flash Rf 150 model) was used. Column grade silica gel served as the stationary phase (mesh 60-120). Chloroform and methanol were the mobile phases. During the isolation procedure, the non-polar solvent was eluted initially, and then the polarity was gradually increased. The samples (12 ml) were automatically collected in test tubes using gradient elution. TLC was done again for each fraction using the same solvent system as in flash chromatography, and the fractions containing the equal Rf value pooled and evaporated to dryness, after which they crystallized to provide fine powdered crystals.
Gas Chromatography-Mass Spectrometry (GC-MS) analysis
To assess each fraction’s chemical profile, a liquid auto-sampler GC-MS (Clarus 680 GC & Clarus 600C MS, Perkin Elmer, made in USA) was used. TurboMass 5.4.2 was the software utilized in the system. The peaks were examined using the NIST-2008 data analysis software. An ‘Elite-5MS’ column was utilized in this instrument. 5% diphenyl and 95% dimethyl polysiloxane were used as the stationary phase. As the mobile phase, 99.99% helium gas (flow rate: 1 ml/minute) was employed. In splitless mode, the total injection volume was 2 µl. The injector (with injection volume of 2 µl) temperature was set at 280°C and the ion source at 180°C. The oven’s temperature: 60°C for 1 minute and after that increased the temperature at a speed of 7°C per minute up to 200°C (3 minutes holding time), then increased the temperature at a speed of 10°C per minute to 300°C (5 minutes holding time).
Reagents and cell lines used
Cancer as well as normal cell lines (HCT116, SW480: colon cancer; human fibroblast: normal) were used in this study. DMEM (HiMedia) was used as culture media for HCT116 and fibroblast cell lines. RPMI 1640 medium (HiMedia) was used for the SW480 cell line. 10% FBS, 2% penicillin-streptomycin (HiMedia) were supplemented with culture media.
MTT assay
HCT116, SW480 and fibroblast cells were dispersed at 104 cells per well in 96 well microplates and kept in a CO2 incubator. After attachment, the cells were treated with or without (vehicle-control) the flash chromatography-derived fractions and the positive control 5-fluorouracil (in depleted media) with a range of concentrations (the range was 200 µgml-1 to 1.56 µgml-1) and placed in a CO2 incubator for 24, 48, and 72 hrs. After incubation, the treated media was discarded and 100 µl of a working MTT solution (with PBS) of 0.5 mgml-1 was added to every single well of the 96-well plate, followed by 3 hrs of incubation. After the incubation, each well’s MTT medium was replaced by 100 µl of DMSO. Using a microplate reader, the absorbance was obtained by measuring it at 570 nm21.
Cell migration assay
Culture plates of 35 mm in diameter were used to seed colon cancer cells. After reaching optimal confluency, each culture plate was manually scratched with a microtip to generate a scratch of around 0.5 mm in width. The cells were then treated (at their respective IC50 concentrations) with or without (vehicle-control) the flash chromatography-derived active fraction. Microscopic images were acquired at various time periods (0 hr, 24 hrs, 48 hrs) after treatment. The chromatography-derived active fraction’s activity was determined by comparing the proportion of the closed area of the untreated (vehicle-controlled) scratch to that of the treated scratch22, 23.
Bright field microscopy imaging
In 35 mm culture plates, colon cancer cells were seeded and, after attachment, treated with or without (vehicle-control) the respective IC50 concentrations and incubated for 24, 48, and 72 hrs. Following incubation, the morphology of the treated cells was compared to the corresponding vehicle-controlled cells. An inverted microscope (Dewinter-Victory plus, Dewinter optical Inc., India) was used to capture each image.
Apoptotic study by acridine orange (AO) ethidium bromide (EB) staining
Apoptosis is linked to modifications in the shape of the nucleus were studied using an AO/EB fluorescent imaging method24, 25. 35 mm culture plates were used to seed the colon cancer cells. Following attachment, both the colon cancer cells were treated with or without (vehicle control) the flash chromatography-derived active fraction for 48 hrs at their respective IC50 concentrations. After being trypsinized, the cells were resuspended in cold PBS and stained with AO and EB (the concentration of both the stains is 100 µgml-1). Each time, 10 µl (9 µl of cell suspension and 1 µl of stain) of stained (AO:EB =1:1) cell suspension (treated and vehicle-controlled) was viewed under the fluorescence microscope (Zeiss, Germany).
Morphological evaluation of the nucleus using DAPI staining
The procedure described by Kntayya et al26 was followed to stain the cells with 4,6-diamidino-2-phenylindole (DAPI) with little modification. HCT116 and SW480 cells were grown on coverslips placed in culture plates and treated with or without the IC50 concentration of fraction-1 (the active fraction). The treated and untreated (vehicle- controlled) cells were then incubated for 48 hrs in the CO2 incubator. Following incubation, the cells were fixed with paraformaldehyde (4% in PBS), permeabilised with 0.1% Triton X-100 (in PBS) and stained with DAPI (2.5 µgml-1, in PBS). Changes in nuclear morphology were observed under the fluorescence microscope (Zeiss, Germany).
Statistical analysis
All tests were done three times, and the findings reported in this study as the mean SEM of three data sets. One-way ANOVA was used to calculate the p-value, which reflects the significance of differences between distinct sets of experimental data. P<0.05, P<0.01, and P<0.001 are denoted by *, **, and ***, respectively.
Results
Analysis of phytochemicals present in the fractions
The powdered crude methanolic extract of Parkia javanica bark (MEPJB) was fractionated using flash chromatography. This procedure produced three fractions from the crude methanolic extract, such as fraction-1, fraction-2, and fraction-3. The phytochemical composition of the three factions was evaluated using a gas chromatography-mass spectrometry (GC-MS) analysis. The major peak in the primary GC-MS chromatogram of fraction-1 had a retention time of 22.17 (Fig. 2A) with an area percentage of 50.740 (Table 1). The major peaks found in the primary GC-MS chromatogram of fraction-2 and fraction-3 had a retention time of 22.189 (Area% =21.277) and 26.215 (Area% =20.870) respectively, with some minor peaks (Fig. 2 B, C). The major phytochemical compound identified by mass spectrometry in fraction-1 and 2 was a phenolic compound named 2,4-Di-tert-butylphenol (2,4-DTBP) (Fig. 3) and in fraction-3 was a stereoisomer of D-glucose named D-Allose, according to area percentage. However, 2,4-DTBP was also found to be the second highest phytochemical present in the fraction-3. Aside from these two compounds, a further 13 compounds were also identified in small amounts among the three fractions of MEPJB (Table 1).
 |
Figure 1: Parkia javanica (Lamk.) Merr. plant (Ambassa, Tripura, India). |
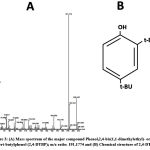 |
Figure 3: (A) Mass spectrum of the major compound Phenol,2,4-bis(1,1-dimethylethyl)- or 2,4-Di-tert-butylphenol (2,4-DTBP); m/z ratio: 191.1774 and (B) Chemical structure of 2,4-DTBP. |
Table 1: Identification of major phytochemical compounds present in the flash chromatography-derived fractions of methanolic extract of Parkia javanica bark (MEPJB) from the mass spectral data.
| Compound
No |
m/z ratio | Molecular
weight
|
Molecular formula
|
Area % in respective fractions | Identified Chemical Compounds in MEPJB |
| C: 1
|
191.1774
|
206
|
C14H22O
|
F1(50.740%)
F2(21.277%) F3 (7.859%) |
Phenol,2,4-bis(1,1-dimethylethyl)- OR 2,4-Di-tert-butylphenol (2,4-DTBP)
|
| C: 2 | 73.9704 | 340 | C22H44O2 | F1 (2.552%) | Methyl 14-methyl-eicosanoate |
| C: 3 | 149.0541; 72.8706 | 652 | C38H68O8 | F1 (4.023%)
F3 (3.533%) |
L-(+)-ascorbic acid 2,6-dihexadecanoate |
| C: 4 | 56.9816 | 276 | C17H24O3 | F1 (3.387%) | 7,9-di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione |
| C: 5 | 149.1226 | 278 | C16H22O4 | F1 (6.636%) | Dibutyl phthalate (DBP) |
| C: 6 | 73.9704 | 256 | C16H32O2 | F2 (3.300%) | Methyl 9-methyltetradecanoate |
| C: 7 | 72.9394 | 256 | C16H32O2 | F2 (5.716%) | n-Hexadecanoic acid |
| C: 8 | 149.0541 | 352 | C23H44O2 | F2 (5.281%) | Methyl 11-Docosenoate |
| C: 9 | 56.9128 | 436 | C31H64 | F2 (4.171%) | Hentriacontane |
| C: 10 | 56.9128 | 914 | C54H108Br2 | F2 (10.106%) | Tetrapentacontane, 1,54-dibromo- |
| C: 11 | 56.9128 | 696 | C41H77O2F5 | F2 (4.900%) | Octatriacontyl pentafluoropropionate |
| C: 12 | 59.8720 | 180 | C6H12O6 | F3 (20.870%) | D-Allose |
| C: 13 | 73.9017 | 228 | C14H28O2 | F3 (3.590%) | Methyl 11-Methyl-Dodecanoate |
| C: 14 | 54.9166 | 296 | C19H36O2 | F3 (4.066%) | Methyl 13-Octadecenoate |
| C: 15 | 56.9816 | 322 | C23H46 | F3 (2.352%) | 5-Methyl-Z-5-Docosene |
C: compound; F: fraction
Cytotoxicity of fractions on Colon cancer cell lines
The cytotoxic activities of three flash chromatographic fractions (fraction-1, -2, and -3) on the two cancer cell lines (colon adenocarcenoma: HCT116 and SW480) and the human fibroblast cell line (normal) were determined by treating all the cell lines with a range of concentrations (the range was 200 µgml-1 to 1.56 µgml-1) of each fraction for 24, 48, and 72 hrs. On the HCT116 cells, fraction-1 exhibited half-maximal inhibitory concentrations (IC50) of 16.25 µgml-1 (24 hrs), 9.94 µgml-1(48 hrs), and 9.38 µgml-1(72 hrs), whereas fraction-2 had IC50 values of 43.75 µgml-1(24 hrs), 35.71 µgml-1(48 hrs), and 34.38 µgml-1(72 hrs), but fraction-3 showed a substantially higher IC50 (>200 µgml-1 for 24 and 48 hrs; 166.67 µgml-1 for 72 hrs) than the other two fractions (Table 2). Similarly, on the SW480 cell line, fraction-1 showed an IC50 of 35 µgml-1 (24 hrs), 20.14 µgml-1 (48 hrs) and 19.71 µgml-1 (72 hrs), whereas, fraction-2 exhibited IC50 values of 67.86 µgml-1 (24 hrs), 36.02 µgml-1 (48 hrs) and 21.63 µgml-1 (72 hrs), but again, fraction-3 showed a much higher IC50 (>200 µgml-1 for 24 and 48 h; 155.26 µgml-1 for 72 hrs) than the other two fractions (Table 2). Here, fraction-1 emerged to be the most active fraction as it showed the lowest IC50 values for both colon cancer cell lines after 24, 48, and 72 hrs of incubation. However, the abovementioned IC50 concentrations (24, 48, and 72 hrs) of fraction-1 were almost nontoxic (more than 90% of the cells were alive) to normal human fibroblast cell line. The IC50 concentrations of the positive control (5-FU) for the colon cancer cell lines were found to be higher than the IC50 concentrations of fraction-1. Moreover, the IC50 concentrations of 5-FU for both the colon cancer cell lines showed toxicity to normal human fibroblast cell line (Fig. 4).
Table 2: IC50 concentrations (µg/ml) of the flash chromatography-derived fractions of methanolic extract of Parkia javanica bark (MEPJB) on colon cancer and normal cell lines.
|
Cell lines |
Incubation period | IC50 concentrations (µg/ml) | ||
| Fraction-1 | Fraction-2 | Fraction-3 | ||
|
HCT116 (Human colon cancer cell line) |
24 h | 16.25 | 43.75 | > 200 |
| 48 h | 9.94 | 35.71 | > 200 | |
| 72 h | 9.38 | 34.38 | 166.67 | |
|
SW480 (Human colon cancer cell line) |
24 h | 35 | 67.86 | > 200 |
| 48 h | 20.14 | 36.02 | > 200 | |
| 72 h | 19.71 | 21.63 | 155.26 | |
| FIBROBLAST
(Normal human connective tissue cell line) |
24 h | 68.18 | 82.5 | > 200 |
| 48 h | 50 | 71.87 | 177.78 | |
| 72 h | 45 | 65.91 | 67.5 | |
 |
Figure 4: MTT Assay: Effect of fraction-1, 2, 3, and 5-FU on HCT116 and SW480 colon cancer cell lines (A), and normal human fibroblast cell line (B). |
Fraction-1 alters the migration of colon cancer cell lines
As the cytotoxicity assay showed fraction-1 to be the most active one among the three-flash chromatography-derived fractions of MEPJB, its effect on cell migration was tested for both colon cancer cell lines (HCT116 and SW480) using a scratch assay. The cells were treated with or without the respective IC50 doses (treated with IC50 concentration of 24 hrs) of the active fraction-1 after creating a scratch on a uniform layer of cancer cells with the help of a microtip, and the scratched region was microscopically imaged between 0 and 48 hours. The overall cell density and motility of the vehicle-controlled and treated wounds were compared, revealing that fraction-1 inhibits cell migration, suggesting a potential anti-metastatic agent27. The gap size of the untreated (without fraction-1; vehicle-controlled) wounds of both the cancer cells was reduced after 24 and 48 hrs of incubation. Moreover, a substantial reduction in the overall cell density of the treated culture plates was also noticed after 24 and 48 hrs of incubation (Fig. 5).
 |
Figure 5: The cell migration assay of treated and untreated (vehicle-controlled) HCT116 (A), and SW480 (B) colon cancer cell lines. |
Morphological changes in colon cancer cells exposed to fraction-1
In the presence of respective IC50 doses (treated with IC50 concentration of 24 hrs) of fraction-1, colon cancer cells displayed considerable morphological changes as compared to the control (vehicle control). After 24 hrs of incubation, the colon cancer cells started changing their morphology and detaching from the surface. After 48 and 72 hrs, cells became totally rounded up, with a large number of cells that detached from the surface. Cell density also decreased after 48 and 72 hrs of incubation. An inverted microscope (Dewinter-Victory plus, Dewinter optical Inc., India) was used to capture images (Fig. 6).
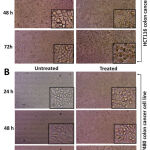 |
Figure 6: Treated (with fraction-1) and untreated (vehicle-controlled) colon cancer cells underwent morphological alterations (A. HCT116, B. SW480). |
Apoptosis induction by Fraction-1 on colon cancer cells (AO/EB dual staining)
It was discovered that fraction-1 induced time-dependent morphological alterations that might be linked to apoptosis27. To look into cell death and see if any apoptosis-related changes in the cell membrane took place in the presence of fraction-1, the AO/EB dual staining method (1:1) was used, and findings were obtained after 48 hrs of incubation. Ethidium bromide can penetrate nonviable cell membranes and attach to DNA. Acridine orange can easily penetrate through the viable cell membrane and attach to DNA. Nonviable cells glow red under fluorescence microscopy after AO/EB staining, whereas viable cells glow green. Most of the cells in the control group (vehicle-control) fluoresced light green after 48 hrs of incubation, whereas cells in the experimental group (treated with IC50 concentration of 48 hrs of fraction-1) fluoresced yellow-green or orange-green, representing the early and late apoptotic phases, respectively. Membrane blebbing as well as chromatin condensation were seen in the experimental group of cells, which are indicators of early apoptosis. Condensation of the nucleus (ring and necklace), granular nucleus, as well as apoptotic bodies in the experimental group of cells indicated late apoptosis (Fig. 7).
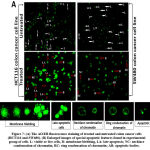 |
Figure 7: (A) The AO/EB fluorescence staining of treated and untreated colon cancer cells (HCT116 and SW480). |
Evaluation of nuclear morphology pre- and post-treatment, using DAPI staining
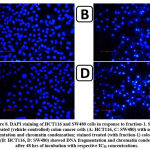
In response to fraction-1 (IC50 concentration of 48 hrs), DAPI staining was used to assess the nuclear alterations in HCT116 and SW480 cells, particularly DNA fragmentation and chromatin condensation. After 48 hrs of treatment, staining displayed DNA fragmentation and chromatin condensation in fraction-1-treated cells (Fig.8-B, D), but DNA fragmentation and chromatin condensation were not observed in untreated (vehicle controlled) cells (Fig.8-A, C).
Discussion
The Indo-Burma biodiversity hotspot, which includes northeast India, is one of the 25 global biodiversity hotspots, and as a result, this region of India is the richest reservoir of flora and fauna in the country28,29. The state of Tripura, being geographically located in this biodiversity hotspot, harbors a great diversity of medicinal plants, some of which have a long ethnomedicinal history15-19. Parkia javanica, a plant belonging to the fabaceae family, is widely distributed across the northeastern region of India, including the state of Tripura, and is used by various tribal populations of this region to cure a variety of ailments like stomach aches, cholera, diarrhea, dysentery, and food poisoning15-17. Many scientific studies have shown that this plant is antibacterial, anti-inflammatory, helps heal wounds, and fights leukaemia30-35. However, very few reports have been published regarding its anti-colon cancer property. Recently, anti-colon cancer activity of crude bark extract of Parkia javanica has been reported from our lab20. The current research work has been designed in the quest to identify the active compound(s) conferring the anti-colon cancer property of the methanolic extract. To achieve this, the whole extract was further fractionated through flash chromatography, and activity-guided chemical analysis of all the fractions was performed.
A time-dependent and dose-dependent in vitro cytotoxic assay of the three flash chromatography-derived fractions of the methanolic extract of Parkia javanica bark and 5-fluorouracil (positive control) on two colon cancer cell lines and a normal human fibroblast cell line revealed that all three fractions have cytotoxic effects on both the colon cancer cell lines. Out of three, fraction-1 was found to be the most active, followed by fraction-2 and fraction 3 (Fig. 4A). 5-FU showed a less cytotoxic effect on colon cancer cell lines compared to that of fraction-1 and 2 (Fig. 4A). Furthermore, the IC50 concentrations of 5-FU on cancer cells showed toxicity in normal human fibroblast cell line (Fig. 4B). The IC50 concentrations of fraction-1 and 2 (Table 2) on cancer cell lines were almost nontoxic to human fibroblast cell line (Fig. 4B). In a time and dose-dependent study, fraction-1, the most active fraction, demonstrated significant results in inhibiting cell motility, altering cell morphology, inducing apoptosis and altering nuclear morphology of the treated colon cancer cells HCT116 and SW480 (Fig. 5, 6, 7 and 8).
When the fractions of MEPJB were analysed by GC-MS, it revealed the presence of a total of 15 chemical compounds having an area percentage >2% (Table 1). The major compound found in fraction-1 (50.740%) and fraction-2 (21.277%) was 2,4-DTBP, which was also present in fraction 3 in a limited amount (7.859%) along with 20.870% of D-Allose. The gradation of the presence of 2,4-DTBP in different fractions was well corroborated by the gradation of anticancer activities of the fractions. In fraction-3, the effect of D-Allose was not very effective. The anticancer activity of 2,4-DTBP has been reported by many authors36, 37. Therefore, 2,4-DTBP may be the major contributor to the anti-colon cancer activity of the fractions. Further work in this direction is warranted.
Conclusion
Taking together all the results of the study indicated to anti-colon cancer potential of Parkia javanica crude bark extract and 2,4-DTBP may be the major contributing compound for this activity.
Acknowledgement
The authors acknowledge the technical support of Guwahati Biotech Park, Guwahati, Assam, India. The authors also acknowledge the technical help of Central Instrumentation Centre and Biotech Hub of Tripura University, Tripura, India.
Conflict of interest
The authors declare that they have no competing interests.
Funding Source
No funding sources.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71(3):209–49.
CrossRef - Huang WY, Ho CL, Lee CC, Hsiao CW, Wu CC, Jao SW, Yang JF, Lo CH, Chen JH. Oral tegafur-uracil as metronomic therapy following intravenous FOLFOX for stage III colon cancer. PLoS One. 2017 Mar 22;12 (3):e0174280. https://doi.org/10.1371/journal.pone.0174280
CrossRef - Dong Y, Wei MH, Lu JG, Bi CY. Long non-coding RNA HULC interacts with miR-613 to regulate colon cancer growth and metastasis through targeting RTKN. Biomedicine & Pharmacotherapy. 2019 Jan 1;109:2035-2042.https://doi.org/10.1016/j.biopha.2018.08.017
CrossRef - Bähr I, Goritz V, Doberstein H, Hiller GG, Rosenstock P, Jahn J, Pörtner O, Berreis T, Mueller T, Spielmann J, Kielstein H. Diet-induced obesity is associated with an impaired NK cell function and an increased colon cancer incidence. Journal of nutrition and metabolism. 2017 Oct:e4297025https://doi.org/10.1155/2017/4297025
CrossRef - Leon-Cabrera SA, Molina-Guzman E, Delgado-Ramirez YG, Vázquez-Sandoval A, Ledesma-Soto Y, Pérez-Plasencia CG, Chirino YI, Delgado-Buenrostro NL, Rodríguez-Sosa M, Vaca-Paniagua F, Ávila-Moreno F. Lack of STAT6 attenuates inflammation and drives protection against early steps of colitis-associated colon cancer. Cancer immunology research. 2017 May 1;5(5):385-396.
CrossRef - Giovannucci E. Insulin and colon cancer. Cancer Causes & Control. 1995 Mar;6(2):164-179.
CrossRef - Yuhara H, Steinmaus C, Cohen SE, Corley DA, Tei Y, Buffler PA. Is diabetes mellitus an independent risk factor for colon cancer and rectal cancer?. The American journal of gastroenterology. 2011 Nov;106(11):1911-1922. https://dx.doi.org/10.1038%2Fajg.2011.301
CrossRef - Cho E, Lee JE, Rimm EB, Fuchs CS, Giovannucci EL. Alcohol consumption and the risk of colon cancer by family history of colorectal cancer. The American Journal of Clinical Nutrition. 2012 Feb 1;95(2):413-419.https://doi.org/10.3945/ajcn.111.022145
CrossRef - Ji BT, Dai Q, Gao YT, Hsing AW, McLaughlin JK, FraumeniJr JF, Chow WH. Cigarette and alcohol consumption and the risk of colorectal cancer in Shanghai, China. European journal of cancer prevention. 2002 Jun 1;11(3):237-244.
CrossRef - Sarshekeh AM, Advani S, Overman MJ, Manyam G, Kee BK, Fogelman DR, Dasari A, Raghav K, Vilar E, Manuel S, Shureiqi I. Association of SMAD4 mutation with patient demographics, tumor characteristics, and clinical outcomes in colorectal cancer. PLoS One. 2017 Mar 7;12(3):e0173345.https://doi.org/10.1371/journal.pone.0173345
CrossRef - Ragnhammar P, Hafström L, Nygren P, Glimelius B. A systematic overview of chemotherapy effects in colorectal cancer. Actaoncologica. 2001 Jan 1;40(2-3):282-308. https://doi.org/10.1080/02841860121543
CrossRef - Eng C. The evolving role of monoclonal antibodies in colorectal cancer: early presumptions and impact on clinical trial development. The oncologist. 2010 Jan;15(1):73-84. https://dx.doi.org/10.1634%2Ftheoncologist.2009-0167
CrossRef - Li F, Li Q, Wu X. Construction and analysis for differentially expressed long non-coding RNAs and MicroRNAs mediated competing endogenous RNA network in colon cancer. PloS one. 2018 Feb 8;13(2):e0192494. https://doi.org/10.1371/journal.pone.0192494
CrossRef - Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer treatment reviews. 2007 Feb 1;33(1):9-23. https://doi.org/10.1016/j.ctrv.2006.09.006
CrossRef - Sinha SC. Medicinal plants of Manipur. Manipur Cultural Integration Conference, Imphal, 1996.
- Majumdar K, Datta BK, Roy D. Inventory and status of medicinal trees of Tripura. Indian Medicinal Plants. Aavishkar Publishers: Jaipur, India, 2009:93-123.
- Bhardwaj S., Gakhar SK. Ethnomedicinal plants used by the tribals of Mizoram to cure cuts & wounds. Indian J. Traditional knowledge. 2005;4(1):75- 80. http://nopr.niscair.res.in/handle/123456789/8497
- Das H B, Majumdar K, Datta BK, Roy D. Ethnobotanical uses of some plants by Tripuri and Reang tribes of Tripura. Natural Product Radiance. 2009;8(2):172- 180. http://nopr.niscair.res.in/handle/123456789/4040
- Roy M, Chakama B, Datta BK, Gupta AK. Traditional knowledge of medicinal plants used by Chakma tribal community of Tripura, India. Pleione. 2010;4(1):105-112.
- Saha P, Saha S, Sil SK. Anti-Colon Cancer Activity of Parkia javanicaBark Extract – An In Vitro IJPSDR. 2021;13(5). https://ijpsdr.com/index.php/ijpsdr/article/view/3329.
CrossRef - Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of immunological methods. 1983;65(1-2):55-63. https://doi.org/10.1016/0022-1759(83)90303-4
CrossRef - Ebeling S, Naumann K, Pollok S, Wardecki T, Vidal-y-Sy S, Nascimento JM, Boerries M, Schmidt G, Brandner JM, Merfort I. From a traditional medicinal plant to a rational drug: understanding the clinically proven wound healing efficacy of birch bark extract. Plos one. 2014;9(1):e86147. https://doi.org/10.1371/journal.pone.0086147
CrossRef - Krishnamoorthy JR, Sumitira S, Ranjith MS, Gokulshankar S, Ranganathan S, Mohanty BK, Prabhakaran G. An in vitro study of wound healing effect of a poly-herbal formulation as evidenced by enhanced cell proliferation and cell migration. Egypt. Dermatol. Online J. 2012;8(1):1-7.
- Toné S, Sugimoto K, Tanda K, Suda T, Uehira K, Kanouchi H, Samejima K, Minatogawa Y, Earnshaw WC. Three distinct stages of apoptotic nuclear condensation revealed by time-lapse imaging, biochemical and electron microscopy analysis of cell-free apoptosis. Experimental cell research. 2007;313(16):3635-3644. https://doi.org/10.1016/j.yexcr.2007.06.018
CrossRef - Liu K, Liu PC, Liu R, Wu X. Dual AO/EB staining to detect apoptosis in osteosarcoma cells compared with flow cytometry. Medical science monitor basic research. 2015;21:15-20. 12659/MSMBR.893327
CrossRef - Kntayya SB, Ibrahim MD, Mohd Ain N, Iori R, Ioannides C, Abdull Razis AF. Induction of apoptosis and cytotoxicity by isothiocyanate sulforaphene in human hepatocarcinoma HepG2 cells. Nutrients. 2018 Jun 4;10(6):718. https://doi.org/10.3390/nu10060718
CrossRef - Ramos-Silva A, Tavares-Carreón F, Figueroa M, De la Torre-Zavala S, Gastelum-Arellanez A, Rodríguez-García A, Galán-Wong LJ &Avilés-Arnaut H. Anticancer potential of Thevetiaperuviana fruit methanolic extract. BMC complementary and alternative medicine. 2017;17(1):1-11.
CrossRef - Mao A A, Hynniewta TM, &Sanjappa M. Plant wealth of Northeast India with reference to ethnobotany. Indian J. Traditional knowledge. 2009;8(1):96-103. http://nopr.niscair.res.in/handle/123456789/2979
CrossRef - Singh EJ, Singh NS, & Singh NR. Biodiversity conservation and natural resources in North East India-with special reference to Manipur. NeBIO. 2009;1(1):42-47.
- Saha S, Karmakar P, Sil SK. Chloroform fraction of Parkia javanica bark possesses antibacterial activity against multidrug resistant gram negative bacteria predominantly found in skin wound. Journal of Drug Delivery and Therapeutics. 2018 Sep 15;8(5):184-189. https://doi.org/10.22270/jddt.v8i5.1847
CrossRef - Sil SK, Saha S, Karmakar P. Reactive oxygen species as possible mediator of antibacterial activity of Parkia javanica, against bacterial species predominantly found in chronic wound. Journal of Drug Delivery and Therapeutics. 2018 Jan 14;8(1):43-47. https://doi.org/10.22270/jddt.v8i1.1657
CrossRef - Saha S, BasuMullick J, Ray Choudhury P, Saha P, Chakraborty D, Sil SK. Anti-Vibrio Activity of Parkia javanica: Studies on MIC, MBC, Growth Curve Analysis and ROS Generation on Four Vibrio cholarae Strains. Int. J. Curr. Microbiol. App. Sci. 2016;5(8):538-544. http://dx.doi.org/10.18782/2320-7051.2357
CrossRef - Mullick JB, Majumdar T, Reddy KV, Mukherjee S, Sil SK. Activity of the medicinal plant Parkia Javanica against multidrug-resistant Neisseria gonorrhoeae and other clinical isolates. Asian J. Pharm. Clin. Res. 2019;12:83-86. http://dx.doi.org/10.22159/ajpcr.2019.v12i9.33654
CrossRef - Saha S, Dinda M, Karmakar P, Sil SK. Immunomodulatory effect of Parkia javanica extract on intracellular expression of IL-6, IL-8, IL-12 and TNF- α. Journal of Drug Delivery and Therapeutics. 2018 May 14;8(3):58-63. http://dx.doi.org/10.22270/jddt.v8i3.1768
CrossRef - Saha R, Basu JM, Mookerjee A, Mohanta BC, Chaudhuri SK, Roy S, Dinda B, Sil SK. In vitro and in vivo studies on antiproliferative activity of Parkia javanica. Journal of Applied Bioscience. 2011;37(2):131-136.
- Sallam KM, Nasr ZS, El-Shershaby HM, Abed NN, Abd El-Ghany IY, Abd-Elkareim AS, Sidkey NM. Purification and radioiodination of 2, 4 di-tertiary-butyl phenol extracted from Lactococcuslactis subsp. lactis CAU: 3138-GM2 and its application on myeloma cells. Journal of Radioanalytical and Nuclear Chemistry. 2021 Jun 20:1-4. https://doi.org/10.1007/s10967-021-07838-1
CrossRef - Song YW, Lim Y, Cho SK. 2, 4‑Di‑tert‑butylphenol, a potential HDAC6 inhibitor, induces senescence and mitotic catastrophe in human gastric adenocarcinoma AGS cells. BiochimicaetBiophysicaActa (BBA)-Molecular Cell Research. 2018 May 1;1865(5):675-683. https://doi.org/10.1016/j.bbamcr.2018.02.003
CrossRef
List of abbreviations
MEPJB: Methanolic extract of Parkia javanica bark;
2,4-DTBP: 2,4-Di-tert-butylphenol;
MTT: 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide;
5FU: 5-fluorouracil;
GC-MS: Gas Chromatography-Mass Spectrometry;
NIST: National Institute of Standards and Technology;
M/Z: mass number/charge number.