Pavan Udavant1* , Pragati Gurav1
, Pragati Gurav1 , Gayatri Kanade1
, Gayatri Kanade1 , Neelam Dashputre1
, Neelam Dashputre1 , Rahul sable1
, Rahul sable1 , Shubham Khairnar1
, Shubham Khairnar1 , Dinesh Rishipathak2
, Dinesh Rishipathak2 , Sapana Ahirrao1
, Sapana Ahirrao1 and Hemant Raut1
and Hemant Raut1
1Department of Pharmacology, MET’s Institute of Pharmacy, Bhujbal Knowledge City, Adgaon, Nashik, Maharashtra, India 422003.
2Department of Pharmaceutical Chemistry, MET’s Institute of Pharmacy, Bhujbal Knowledge City, Adgaon, Nashik, Maharashtra, India 422003.
Corresponding Author E-mail: pavanudavant@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2592
Abstract
Introduction: Hepato-renal toxicity is a devastating, non-communicable disease. Because of a lack of information on low-cost management to combat the disease, this study postulates the ameliorative effect of selected phytoconstituents against toxicity. Aim and Objective: The current study reveals an active phytoconstituent, α- Pinene, that has the ability to combat the degenerative effects of CCl4. Methodology: Carbon tetrachloride (CCl4) is an organic xenobiotic molecule as well as the most potent hepatotoxic agent used (1200 mg/kg body weight; i.p.) to induce hepato-renal toxicity in experimental rats. To determine in vivo hepato-renal toxicity, three different doses (0.05 ml/kg body weight, 0.1 ml/kg body weight, and 0.15 ml/kg body weight; intraperitoneally) were chosen. Vitamin C at the dose of 250 mg/kg/p.o. was used as a standard, due to its maximum ameliorative activity against oxidative damage in CCl4-induced hepato-renal toxicity in rats. For 7 days, the animals were pre-treated with α-pinene and Vitamin C. CCl4 was charged only on the 7th day. Result and Conclusion: The related biochemical tests were studied. CCl4 intoxication reduces mitochondrial membrane potential in liver and kidney cells, which accelerates excessive intracellular ROS production, but α-pinene pretreatment successfully restores it in both liver and kidney cells. Pretreatment with α-pinene and vitamin C for 7 days increased intracellular ameliorative capability in hepatic and renal cells significantly (p 0.01). In conclusion, α-pinene is capable of restoring antioxidant status by quenching intracellular ROS. As a result, α-pinene has the potential to provide hepatoprotective and nephroprotective effects against CCl4-induced toxicity in rats.
Keywords
α- Pinene; Biochemical tests; CCl4; Hepato-renal toxicity; Oxidative damage; Vitamin C
Download this article as:| Copy the following to cite this article: Udavant P, Gurav P, Kanade G, Dashputre N, Sable R, Khairnar S, Rishipathak D, Ahirrao S, Raut R. Evaluation of Hepatoprotective and Nephroprotective Effect of Α- Pinene on Wistar Albino Rat. Biomed Pharmacol J 2023;16(1). |
| Copy the following to cite this URL: Udavant P, Gurav P, Kanade G, Dashputre N, Sable R, Khairnar S, Rishipathak D, Ahirrao S, Raut R. Evaluation of Hepatoprotective and Nephroprotective Effect of Α- Pinene on Wistar Albino Rat. Biomed Pharmacol J 2023;16(1). Available from: https://bit.ly/3IkrVqQ |
Introduction
A number of ethno-medical plants in traditional medicines are claimed by Ayurveda and Unani practitioners to have hepato- and nephroprotective activities, and a few of them have been validated for their protective activity after showing promising results. α -Pinene (C10H16) is a terpenoid hydrocarbon, a more specific group of monoterpenes, with a bicyclic structure containing a double bond 1. α -Pinene is an isomeric form of the terpenoid pinene 2. α-pinene has been found in 40 different essential oils, including those of many coniferous trees and rosemary 3. It is most widely used in the making of camphor, borneol, terpineol, and terpene resin. Moreover, it is dominantly utilized in the synthesis of medicines, synthetic perfumes, and other chemicals. Because of its aromatic potential, α-pinene is becoming more popular in the fragrance and chemical industries for the production of aroma chemicals 4.
Organ toxicity is highly related to oxidative stress, as the renal cells are repeatedly exposed to drug pressure, xenobiotics, pesticides, fungicides, anesthetic agents, and organic solvents and consequently generate ROS during the metabolism process. The cell macromolecules, specifically DNA, proteins, and lipids, are the targets of oxidation. Numerous oxidative changes to DNA have been characterized that can cause base disincorporation, mutations, single or double DNA strand breaks, and finally cellular death 5. The Cytochrome 450 enzymes are a major family of enzymes associated with drug metabolism in the liver 6. The oxidative imbalance during renal disorder is characterized by “critical episodes,” which drastically reduce the renal functional capacity and structural integrity and ultimately cause toxicity 7. Carbon tetrachloride (CCl4) is an odorless and colorless solvent that is widely used as an industrial chemical. The primary target organs for human exposure to carbon tetrachloride are the liver and kidneys. It triggers oxidative stress through the by-product of CCl4 metabolism, i.e., (•CCl3). Damage to the cellular antioxidant defence and/or the generation of free radicals (FR) exceeded the defence system’s potential to remove those radicals, resulting in peroxidation. In the cellular membrane, lipid peroxidation arises, leading to the accumulation of membrane byproducts such as malondialdehyde (MDA). Cellular destruction arises from an elevation in oxidative stress in cell membranes 8.
Materials and Methods
Chemicals
α-Pinene, Ascorbic acid, Carbon tetrachloride (CCl4), Sodium nitroprusside, Dimethylsulphoxide (DMSO), Olive oil, DPPH reagent, Griess reagent.
Experimental Design
Wistar albino rats of both sexes (6–8 weeks old) were divided into 6 groups, with 6 rats in each group. The experimental protocol was approved by the institutional animal ethics committee at the MET’s BKC Institute of Pharmacy, Nashik. [Approval No. MET-IOP-IAEC/2020-021/01]
Group-I: Control, Rats were administered DW p.o. for 7 days.
Group-II: Positive control, rats were administered CCl4 (1200 mg/kg/i.p.) mixed with equal volumes of olive oil on the 7th day.
Group-III: Standard group, Rats administered with Vitamin C (250mg/kg/p.o.) + CCl4 (1200 mg/kg/i.p.) for 7 days+ equal volumes of Olive oil on 7th day.
Group-IV: Test Group A, Rats administered with 0.05ml/kg body weight of α- Pinene for 7 days+ CCl4 (1200 mg/kg/i.p.) + equal volumes of Olive oil on 7th day.
Group-V: Test Group B, Rats administered with0.1 ml/kg body weight of α-Pinene for 7 days+ CCl4 (1200 mg/kg/i.p.) + equal volumes of Olive oil on 7th day.
Group-VI: Test Group C, Rats administered with 0.15 ml/kg body weight of α-Pinene for 7 days+ CCl4 (1200 mg/kg/i.p.) + equal volumes of Olive oil on 7th day.
Sample collection
After the completion of the experimental period, the animals were anaesthetized by the administration of an inhalational anesthetic agent in a high dose, i.e., diethyl ether, and finally euthanized by the method of cervical dislocation. Blood was collected to separate the serum, which was used for biochemical estimations. The liver and kidney were removed and cleaned in ice-cold saline before being processed for histopathological studies.
Biochemical analysis
The activities and levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) and SGOT and SGPT, alkaline phosphatase (ALP), total bilirubin (TB), creatinine, urea, uric acid (UA), triglycerides, cholesterol, and total protein content were determined in the serum of all the studied animals using kits (Prism Diagnostics, Thane, India) and
Histopathological analysis
The isolated liver and kidney were excised into thin tissues that were processed using the paraffin wax embedding technique and stained using the haematoxylin and eosin [H and E] staining procedure. A light microscope with a magnification of 40 X was used to examine these sections.
Statistical analysis
GraphPad InStat was utilized to analyze the data. The results of the antioxidant and biochemical assays were reported as mean ± SD (standard deviation). One way analysis of variance (ANOVA), followed by Dunnett’s test, was used to test for the level of significance (values of **p < 0.01, *p < 0.05).
In vitro Antioxidant activity
The activity level of various antioxidants and changes in nitric oxide levels were also assessed using two different in vitro assays.
DPPH scavenging activity
Reaction setup:
Blank: Methanol
Control: Methanol + DPPH (Purple colored)
Standard: Ascorbic acid + DPPH
Sample: α-Pinene + DPPH
0.1 mM of DPPH solution was added to 3 ml of α-pinene sample solutions (using methanol as a solvent) at various concentrations (20 g/ml, 40 g/ml, 60 g/ml, 80 g/ml, and 100 g/ml). After shaking vigorously, the solution was directed to stand for 30 minutes in a dark environment at room temperature. Subsequently, the absorption maxima of all the samples were measured using a UV-visible spectrometer at 517 nm. The antioxidant activity of the sample was compared with a known standard ascorbic acid solution. All samples were analyzed in triplicate. The following formula was used to calculate the % DPPH radical scavenging activity:
% DPPH radical scavenging activity = (Control OD –Sample OD / Control OD) X 100[9]
Assay of nitric oxide scavenging activity:
The procedure is premised on the method, where sodium nitroprusside in an aqueous medium at physiological pH spontaneously emits nitric oxide, which combines with oxygen to yield nitrite ions that can be noted using Griess reagent. Scavengers of nitric oxide contend with oxygen, resulting in reduced nitrite ion formation.
Reaction setup:
Blank: Ethanol + 10 mM Sodium nitroprusside + PBS (pH 7.4)
Control: Ethanol + 10 mM Sodium Nitroprusside + PBS (pH 7.4) + Griess reagent
Standard: 10 mM Sodium nitroprusside + PBS (pH 7.4) + Ascorbic acid + Griess reagent
Sample: 10 mM Sodium nitroprusside + PBS (pH 7.4) + α-Pinene + Griess reagent
2 ml of sodium nitroprusside (10 mM) in phosphate buffered saline was mixed with various concentrations (5-200 g/ml) of α–pinene dissolved in ethanol for the experiment and incubated at 25 C for 2 hours. The same reaction mixture was used, but instead of α–pinene, an equal amount of ethanol was used as a control.
After the incubation period, 0.5 ml of the incubated solution was removed and 0.5 ml of Griess reagent (1% sulfanilamide, 2% H3P04, and 0.1% N-(1-naphthyl) ethylenediamine dihydrochloride) was added. After 30 minutes of incubation at 546 nm, the absorbance of the chromophore produced upon complexation of the nitrite with sulfanilamide and subsequently pairing with naphthyl ethylenediamine dihydrochloride was measured. The relative inhibition of nitrite formation by α–pinene and the standard antioxidant ascorbic acid was calculated. The inhibition data (percentage inhibition) were linearized against the α–pinene and standard antioxidant concentrations. The IC50 of α–pinene, which is the inhibitory concentration required to reduce 50% of nitric oxide formation, was determined [10].
Result
In vivo Effect of α- Pinene on Liver and Kidney
Body weight
In this study, we looked at the positive effects of α-Pinene on CCl4-induced rats and discovered that rats given only CCl4 (Group II) had significant (* p < 0.05; ** p < 0.01) increases in body weight, as well as significant toxicity symptoms (Table 01).
Weight of liver and kidney
Organ weight changes are accepted as a reliable sign of chemically-induced damage. The rats given CCl4 intraperitoneally, i.e., the toxicity-induced group had a considerable rise in the weight of their liver and kidney. Therefore, the relative body weight and the weight of the liver and kidney were significantly increased (value (**p< 0.01, *p< 0.05) in toxicity, i.e., CCl4 (1200 mg/kg/i.p.)-induced rats (Table 1).
Table 1: Body, liver and kidney weight changes in control and experimental groups.
| Groups |
Body weight (g) |
Change in body weight |
Liver weight (g) |
Kidney weight (g) |
|
|
Initial |
Final |
||||
|
Control |
233.16± 8.97 |
237.66 ± 8.33 |
4.5 |
8.06 ± 0.04 |
1.00 ±0.11 |
| Positive control
(CCl4) |
228.5± 2.66 |
240.0 ±2.82 |
11.5 |
10.27±0.17 |
1.32 ±0.04 |
| Standard
(Vit. C) |
216.33± 6.91** | 220 ± 5.89** | 3.6 | 8.24 ± 0.04 | 1.03 ±0.05** |
| α- Pinene I | 201.6 ± 3.55* | 215 ± 1.7** | 13.4 | 9.92 ±0.11** | 0.99 ±0.01** |
| α -Pinene II | 219.83 ± 2.71* | 227.66 ± 3.55** | 7.83 | 9.26 ±0.11** | 0.93 ±0.03** |
| α- Pinene III | 202.66± 4.08** | 210.16 ± 3.92** | 7.5 | 8.66 ±0.18** | 0.89 ±0.02** |
Each value represents the mean ± SD for 6 rats with p value (value (**p< 0.01, *p< 0.05), which is significant when compared with Positive control (CCl4 treated group)
Biochemical analysis
The functionality of the liver and the kidney was established by assessing the serum levels of aspartate aminotransferase (AST)/SGOT and alanine aminotransferase (ALT)/SGPT, alkaline phosphatase (ALP), total bilirubin (TB), creatinine, urea, uric acid (UA), triglycerides, cholesterol, and total protein.
Liver Functional Assessments
The important cellular enzymes of hepatocytes like ALT, AST, ALP, total bilirubin, and triglycerides are released into the blood after necrosis; therefore, it can be said that the hallmark of liver damage, which is elevation of the levels of marker enzymes like ALT and AST in the blood, are the consequences of the necrosis and inflammatory changes in the liver. In this study, administration of CCl4 resulted in a considerable increase (**p < 0.01, *p < 0.05) in the levels of ALT, AST, ALP, total bilirubin, and triglycerides when compared with the normal control group (Table 2 and Figure 1).
Table 2: Effect of administration of α- Pinene on basic liver assessments.
| Parameters | Control | Positive Control | Ascorbic acid | α- Pinene I | α- Pinene II | α- Pinene III |
| SGOT/AST | 190.09±
13.02** |
326.43±
15.18** |
198.93±
8.89** |
255.95±
13.82 |
204.05±
6.74** |
192.34±
9.52** |
| SGPT/ALT | 172.63±
7.01** |
367.26±
17.12** |
183.11±
17.61** |
299.14±
7.31 |
225.61± 8.17** | 176.78± 10.04** |
| ALP | 210.8±
29.78** |
518.26±
19.92** |
234.31±
28.12** |
423.57±
23.43 |
375.13± 12.0** | 227.66±
11.44** |
| Cholesterol | 84.18 ±
10.2** |
142.87±
13.50** |
89.22 ±
9.54** |
108.98±
8.51 |
97.4±7.28** | 88.33± 7.42** |
| Triglycerides | 105.27±
14.12** |
291.85±
11** |
108.75±
8.47** |
147.61±
8.58
|
132.54±
11.66**
|
111.77±
7.44**
|
| Total Bilirubin | 13.5±3.6** | 33.58±
5.3** |
16.00±
2.05** |
21.39±4.20
|
17.29±3.59**
|
13.45±3.18**
|
Each value represents the mean ± SD for 6 rats with p value (value (**p < 0.01, *p < 0.05), which is significant when compared with Positive control (CCl4 treated group).
 |
Figure 1: statistical comparison of serum biochemical parameters viz. AST, ALT, ALP and total bilirubin which are important biomarkers in the assessment of liver function. |
Kidney Functional Assessments
In this study, administration of CCl4 resulted in a significant increase (value: **p < 0.01, *p < 0.05) in the levels of creatinine, urea, uric acid, triglycerides, and cholesterol and a decrease in the level of total protein compared with the normal control group. At the treatment doses of α–pinene (0.05, 0.1, and 0.15 mg/kg/d), there was a dose-dependent and significant (**p < 0.01, *p < 0.05) reduction in kidney biomarkers (Table 3 and Figure 2).
Table 3: Effect of administration of α- Pinene on basic kidney assessments.
| Parameters | Control | Positive Control | Ascorbic acid | α- Pinene I | α- Pinene II | α- Pinene III |
| Creatinine | 0.93±
0.03** |
4.93±
0.4** |
1.12±
0.11** |
2.42±
0.04 |
1.97±
0.06** |
1.18±
0.04** |
| Urea | 19.13±
3.1** |
67.08 ±
7.82** |
19.76±
2.37** |
53.52±
5.56 |
31.32±
2.59** |
19.83±
1.61** |
| Uric acid | 93.45±
6.79** |
154.51±
7.53** |
96.21±
5.8** |
110.56±
7.52 |
100.25±
9.06** |
92.47 ±
7.30** |
| Cholesterol | 84.18±
10.2** |
142.87±
13.5** |
89.22 ±
9.54** |
108.31±
4.83 |
99.06±
4.04** |
88.16±
3.71** |
| Triglycerides | 105.27±
14.12** |
291.85 ±
11** |
108.75± 8.47** | 147.61±
8.58
|
132.54±
11.66**
|
111.77±
7.44**
|
| Total Protein | 6.07±
0.6** |
3.46±
0.7** |
7.73±
0.25** |
4.23±0.4 | 5.48±
0.34** |
7.15±
0.17** |
Each value represents the mean ± SD for 6 rats with a p value of (**p < 0.01, *p < 0.05), which is significant when compared with the positive control (CCl4 treated group).
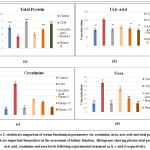 |
Figure 2: statistical comparison of serum biochemical parameters viz. creatinine, urea, uric acid and total protein which are important biomarkers in the assessment of kidney function. |
The values mentioned in the results are the average of six readings and the standard deviation for a group; statistically significant differences are indicated by asterisks (**p < 0.01 compared with the CCl4-toxic group). Group I: normal control; Group II: CCl4; Group III: vitamin C (250 mg/kg/p.o.); Group IV: α- Pinene (0.05 ml/kg i.p. ); Group V: α-Pinene (0.1 ml/kg i.p. ); Group VI: α-Pinene (0.15 ml/kg i.p.)
In vitro antioxidant activity
DPPH free radical scavenging activity
Table 4: % DPPH radical scavenging activity
| Concentration (μg/ml) | DPPH activity (%) (Mean±SD) | |
| Ascorbic acid | α- Pinene | |
| 20 | 54.92±0.49*** | 48.25±0.29*** |
| 40 | 59.04±0.30*** | 51.42±0.10*** |
| 60 | 64.12±0.51*** | 55.55±0.38*** |
| 80 | 70.79±0.66*** | 59.36±0.23*** |
| 100 | 76.82±0.51*** | 61.9±0.18*** |
| IC50 (μg/ml) | 5.4 | 29.94 |
Each value represents the mean ± SD with p value (value (***p ˂ 0.001), which is significant when compared with control.
The method relies on the generation of the non-radical form DPPH-H by the reaction of an alcoholic DPPH solution with a hydrogen-donating antioxidant. Table 4 shows an effective increase in the inhibition of DPPH radical concentration due to the scavenging ability of α–pinene, which has been reported to be concentration dependent. Ascorbic acid had a significantly greater scavenging effect (76.820.51%) than α- pinene (61.90.18%). Ascorbic acid (5.4) and α–pinene (29.94) were found to have IC50 values against DPPH scavenging activity (Table 4).
Nitric Oxide Scavenging Activity
Table 5 shows the nitric oxide scavenging effect of α–pinene and standard ascorbic acid. Standard ascorbic acid inhibited nitric oxide generation by 92.3 0.21% at 100 g/ml and α–pinene inhibited it by 46.19 0.18% at 100 g/ml. The IC50 values for ascorbic acid and α–pinene for nitric oxide scavenging were 33.04 g and 109.2 g, respectively (Table 5).
| Concentration (μg/ml) | Nitric Oxide Scavenging (%) (Mean±SD) | |
| Ascorbic acid | α- Pinene | |
| 5 | 30.9 ±0.34*** | 7.5 ±0.69*** |
| 25 | 49.03 ±0.40*** | 18.82 ±0.50*** |
| 50 | 59.62 ±0.31*** | 29.15 ±0.39*** |
| 75 | 74.49 ±0.36 | 35.4 ±0.53*** |
| 100 | 92.3 ±0.21*** | 46.19 ±0.18*** |
| IC50 (μg/ml) | 33.04 | 109.2 |
Each value represents the Mean ± SD with p value (***p< 0.001), which is significant when compared control.
Histopathological analysis
Histopathology of Liver
In Figure 3, [A] normal control animals from Group I indicate normal cellular architecture with proper structure of the central vein along with normal hepatocytes and no sign of degeneration or any histopathological changes. The liver sections of [B] Group II (Positive Control); [D] Group IV (Test 1) the central vein and hepatocytes of the CCl4-toxic rat were severely damaged and necrosed, with fatty and neutrophil infiltration and structural integrity loss. Indicated by an arrow in the figure. [E] Group V (Test 2) exhibits moderate damage with mild infiltration and vacuolations; however, [C] Group III (standard) and [F] Group VI (Test 3) exhibit mild infiltration of inflammatory cells, the absence of vacuoles, and normal architecture. α-Pinene reversed toxic changes in the liver in a dose-dependent manner, similar to the effect of standard Vitamin C.
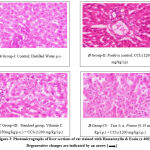 |
Figure 3: Photomicrographs of liver sections of rat stained with Hematoxylin & Eosin (x 400) Degenerative changes are indicated by an arrow [ → ] |
Histopathology of Kidney
Figure 4: Histopathological changes of nephrotic tissues (H&E); Magnification X400; [A] Group I (negative control) showing normal histological architecture and absence of histological alteration; [B] Group II (positive control); [D] Group IV (Test 1st); [E] Group V (Test 2) due to the nephrotoxic effect of CCl4 showing marked degeneration in the lining of the epithelium of all the tubules and also showing infiltration of inflammatory cells in between tubules; indicated by an arrow in the figure. However, [C] Group III (standard); [F] Group VI (test 3rd) showed mild infiltration and the absence of severe degeneration of nephrocytes or tubules.
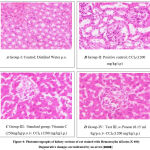 |
Figure 4: Photomicrographs of kidney sections of rat stained with Hematoxylin &Eosin (x 400) Degenerative changes are indicated by an arrow [→]. |
Discussion
The world of natural products, as opposed to that of synthetic pharmaceuticals, still aims to employ the potential of treatment today. They are working harder to alter food by substituting natural antioxidants for synthetic food additives. Functional foods are those that have preventive or therapeutic effects due to their unique constituents. A number of researchers have revealed that many medicinal and fragrant herbs, as well as the fruits and leaves of some berry plants, biosynthesize phytochemicals with antioxidant properties and can be used as a natural supply of molecules that scavenge free radicals. Thus, research is needed to develop effective defenses against harmful substances, and preliminary findings suggest that α–pinene, a functional plant, may play a role in this area [11, 12]. The most important task of biochemical and histopathological testing is determining the effectiveness and safety of α–pinene against CCl4 toxicity. In order to establish the toxicity of the numerous chemicals that are the primary indications of organ failure, biochemical and histopathological assays are required [13]. The protective effect of α–pinene against CCl4-induced degenerative changes in the liver, kidneys, and blood of rats was established in this study. CCl4 induces toxicity by increasing oxidative stress in rat serum, liver, and kidneys. The generation of CCl4 toxicity is associated with its alleviation by antioxidants.
Hypertrophy of organs is the first indication of the toxicity of chemical or biological substances. The rise in liver weight in the CCl4 group is owing to the aggregation of fat vacuoles observed through increased hepatic cholesterol and triglyceride levels. The important cellular enzymes of hepatocytes like ALT, AST, ALP, total bilirubin, and triglycerides are released into the blood after necrosis; therefore, it can be said that the hallmark of liver damage, which is elevation of the levels of marker enzymes like ALT and AST in the blood, is due to the necrosis and inflammatory changes in the liver.
In the current study, the serum of liver functional enzymes in CCl4-treated rats showed an increased level in contrast to vehicle-treated rats, which imparts structural alterations in the hepatic cell membrane, which is indicated by the loss of enzymes from the cell through the membrane, i.e., it’s possible that this is due to hepatocytes degradation and necrosis, which leads to an increase in the penetrability of the cell membrane, resulting in the leakage of marker enzymes into the blood system. Similarly, serum bilirubin is among the most important markers of liver injury since it shows the liver’s capability of converting bilirubin into bile. Total bilirubin levels in the serum of CCl4-treated rats were also elevated. These findings were also in agreement with the results of Nouran Md. Fahmy et al. However, in CCl4-treated rats, pre-treatment with α–pinene significantly reduced the levels of these liver functional enzymes and total bilirubin (*p < 0.05, **p < 0.01).
Because urea is the most notable nitrogen-containing metabolic product of protein metabolism, in cases of renal toxicity, the rate of urea production in the serum exceeds the rate of its clearance, resulting in uremic fluid [14]. It is known that CCl4 resists the inclusion of amino acids into proteins, which results in the elevation of serum urea levels. [15] In the present study, there was an elevated serum uric acid concentration corresponding to the body’s response to high level production of free radicals [16]. The alterations in levels of creatinine are dependent upon various factors, such as protein diet, catabolic state, and muscle mass [14]. The combination of α-pinene and CCl4 protects the kidneys and restores serum urea, uric acid, and creatinine levels to near normal levels.
The effects listed above demonstrate α–pinene’s nephroprotective effect. The probable mechanism involved in the hepatoprotective and nephroprotective activity can be either the scavenging of the free radicals generated in the liver and kidney or an increase in the protective enzymes. In-vitro testing of α–pinene revealed that it contains a higher percentage of antioxidants and is more effective in protecting against the toxic effects of carbon tetrachloride on the liver and kidney by lowering these effects on both biochemical and histopathological levels to levels closer to the negative control condition. Many phytoconstituents have antioxidant properties, which lower oxidative stress and, as a consequence, facilitate protection against organ toxicity.
Conclusion
In conclusion, the findings suggest that an active phytoconstituent with high antioxidant potential, such as α–pinene, could effectively improve the condition of patients suffering from hepatorenal toxicity. Throughout the study, it was discovered that a very small amount of α–pinene acted as an excellent scavenging agent and successfully alleviated certain degenerative changes in the liver and kidney caused by CCl4 intoxication. The study possesses great societal impact on community members as it is an alternative, cost-effective, and easily affordable therapeutic agent for the management of hepatorenal dysfunction.
Acknowledgement
The authors are sincerely thankful to our respected principal, Mr. Sanjay Kshirsagar sir (MET’s BKC Institute of Pharmacy, Nashik), for his keen support and belief.
References
- Winnacker, M., Pinenes: abundant and renewable building blocks for a variety of sustainable polymers. Angew. Chem. Int. Ed., 2018; 57(44): 14362-71.
CrossRef - Ochocka, J.R., Asztemborska, M., Sybilska, D., et al. Determination of enantiomers of terpenic hydrocarbons in essential oils obtained from species of Pinus and Abies. Pharm. Biol., 2002; 40(5): 395-99.
CrossRef - Vespermann, K.A., Paulino, B.N., Barcelos, M.C., et al. Biotransformation of α-and β-pinene into flavor compounds. Appl. Microbiol. Biotechnol., 2017; 101(5): 1805-17.
CrossRef - Alpha pinene market report, Company Analysis, History and Future Overview, Global Sales Trends by 2025, QYR Consulting, 2019-09-27.
- Poulsen, H.E. Oxidative DNA modifications. Exp. Toxicol. Pathol., 2005;57: 161-69.
CrossRef - Guengerich, F.P. Cytochrome p450 and chemical toxicology. Chem. Res. Toxicol., 2008; 21(1): 70-83.
CrossRef - Basile, D.P., Anderson, M.D. and Sutton, T.A.Pathophysiology of acute kidney injury. Compr. Phy., 2012;2(2): 1303.
CrossRef - Tomasi, A., Albano, E., Banni, S., et al. Free-radical metabolism of carbon tetrachloride in rat liver mitochondria. A study of the mechanism of activation. Biochem. J.,1987;246 (2): 313-17.
CrossRef - Tailor, Chandra Shekhar & Goyal, Anju. Antioxidant Activity by DPPH Radical Scavenging Method of Ageratum conyzoides Linn. Leaves. A. J. Ethno., 2014; 1: 244-49.
- Parul, R., Kundu, S.K. and Saha, P. In vitro nitric oxide scavenging activity of methanol extracts of three Bangladeshi medicinal plants. J. Pharm. Innov., 2013; 1(12, Part A): 83.
- Sacchetti G, Maietti S, Muzzoli M, et al. Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem. 2005; 9:621-32.
CrossRef - Yu LL, Zhou KK, Parry J. Antioxidant properties of cold pressed black caraway, carrot, cranberry, and hemp seed oils. Food Chemistry. 2005; 91:723-9.
CrossRef - Hamed Aramjoo, Pouria Mohammadparast-Tabas, et al. Protective effect of Sophora pachycarpaseed extract on carbon tetrachloride-induced toxicity in rats. BMC Complement Med Ther. 2022; 22: 76.
CrossRef - Mayne, P.D. The kidneys and renal calculi. Clinical chemistry in diagnosis and treatment. 6th ed. London: Edward Arnold Publications. 1994; 22-24.
- Davies, D.M., 1977. Textbook of adverse drug reactions. Oxford University Press.
- Hooper, D.C., Spitsin, S., Kean, R.B., et al. Uric acid, a natural scavenger of peroxynitrite, in experimental allergic encephalomyelitis and multiple sclerosis. Proceedings of the National Academy of Sciences. 1998; 95(2):675-80.
CrossRef








