Swastini IGAAP1* , Ni Nengah Sumerti2
, Ni Nengah Sumerti2 and Ni Ketut Nuratni2
and Ni Ketut Nuratni2
1Department of Medical Laboratory Technology, Poltekkes Kemenkes Denpasar, Denpasar, Indonesia.
2Department of Dental Health, Poltekkes Kemenkes Denpasar, Denpasar, Indonesia.
Corresponding Author E-mail: agungswastini18@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2619
Abstract
Snails are unpleasant yet beneficial. Rural people have used one to treat illnesses like toothache for years. We will test snail's mucus Achatina fulica's cytotoxic activity against Baby Hamster Kidney (BHK-21) fibroblast cells at 12.5%, 25%, 50%, 100% and its resistance to Phorpyromonas gingivalis, Fusobacterium nucleatum, E. Faecalis, and S. aureus using Microtetrazolium (MTT) assay. The test and comparison solution was incubated with 5x103/100 l cells in 96-well plates. 5 mg/mL MTT completed the solution's incubation. ELISA readers measured purple color intensity. The formula transformed absorbance data at 595 nm into percent alive cells. ELISA readers read data. ANOVA, parametric Kolmogorov-Smirnov data normality test were performed. The cytotoxicity statistical test shows the following results: 12.5 % (0.76875 ±0.01117), 25% (0.49350 ±0.004796), 50% (0.30250 ±0.006658) and 100% (0.171 ±0.10488). The lowest cytotoxicity of Achatina fulica snail mucus is 12.5% with an average of 0.768. Achatina fulica snail mucus resists Phorpyromonas gingivalis, Fusobacterium nucleatum, E. Faecalis, and S. aureus at 12.5%.
Keywords
Achatina fulica; BHK-21; cytotoxicity; fibroblast; periodontitis; Snail Mucus
Download this article as:| Copy the following to cite this article: IGAAP S, Sumerti N. N, Nuratni N. K. Cytotoxicity Test of Active Compounds Natural Ingredients of Snail Mucus (Achatina fulica) Against BHK-21 Fibroblast Cells. Biomed Pharmacol J 2023;16(1). |
| Copy the following to cite this URL: IGAAP S, Sumerti N. N, Nuratni N. K. Cytotoxicity Test of Active Compounds Natural Ingredients of Snail Mucus (Achatina fulica) Against BHK-21 Fibroblast Cells. Biomed Pharmacol J 2023;16(1). Available from: https://bit.ly/3yEnuBv |
Introduction
Periodontal diseases involve a wide variety of infections and chronic inflammatory conditions which affect the structures of the teeth (the gingiva, bone, and periodontal ligament), and affect eating, aesthetics, and speaking in particular. Periodontal disease is prevalent in adults, but aggressive periodontitis may occur in children. 1,2 People with periodontal diseases suffer from tooth loss and higher risk for systemic inflammation3. The loss of the tooth influences mastication, and subsequently ruin nutrition and diet4,5. Periodontal diseases are caused by bacteria in sub gingival dental plaque. 6,7 Among the gram-negative bacteria causing periodontal infections are Porphyromonas gingivalis8,9, Fusobacterium nucleatum as one of the most abundant gram-negative bacteria in periodontitis10,11, Enterococcus faecalis12–13and Staphylococcus aureus which is associated with the aggressive periodontitis.14,15 Localized periodontitis can be treated with mechanical debriment and good oral hygiene.16,17 Meanwhile, generalized periodontitis requires antibiotic therapy.18 This is because generalized periodontitis affects more than 30% of sites of the teeth.19 Therefore, new treatment modalities such as antimicrobial therapy for tissue repair and regeneration are indispensable.20
Snails’ mucus is a natural ingredient can be used as a traditional medicine for curing minor wounds and dental diseases. 21,22 In tropical country, various species of snails can be found, including land snail or Achatina fulica. However, the biological compound of the mucus is necessary to examine in order to determine whether a compound has the potential to be toxic to biological organisms, and if it so, to what extent.23 The examination to determine such necessity is through cytotoxicity test. 24,25 A cytotoxicity test is a biological evaluation of natural substances and is required for standard screening procedures. 26 The purpose of a cytotoxicity test is to determine the toxic effect of a substance directly on tissue culture.27
Wounds healing process includes homeostasis and inflammation, proliferation and maturation phases. 28,29 The proliferative phase increases the number of cells and wound healing factors, one of which is the proliferation of fibroblasts. Fibroblasts are the most common cells found in connective tissue. 30 It is used to synthesize several components of the extracellular matrix (collagen, elastin, reticular), several anionic macromolecules (glycosaminoglycans, proteoglycans) and multi-adhesive glycoproteins, laminins, and fibronectins that can promote cell attachment to substrates. 31,32 They also secrete cytokines and several growth factors, which can stimulate cell proliferation and inhibit the differentiation process. 33,34 The proliferation of fibroblasts determines the final outcome of wound healing. 35 Fibroblasts will produce collagen which will link the wound, and fibroblasts will also affect the re-epithelialization process which will make a wound closure.36,37 Snail mucus contains beta agglutinins (antibodies) in plasma (serum), achasin protein, glycoconjugates and acharan sulfate which play a role in the wound healing process by helping the blood clotting process and fibroblast cell proliferation. 38,39
In measuring the number of cells for proliferation and cytotoxicity assays, the MTT (Microtetrazolium) assay is one of the best methods to use. MTT assay is included to colorimetric procedure. Colorimetric procedures are considered economical, rapid, and able to measure multiple samples simultaneously.40 Besides, the colorimetric procedures can be automated, and preferred for evaluating the physiological state of microbes.41,42 The MTT assay method usually uses a 96-well microplate so that many samples can be studied simultaneously. A material can be said biocompatible when the material does not cause irritation to the tissue life, does not cause a toxic response, free of ingredients that can trigger an allergic reaction, and not has carcinogenic potential. Deciding biocompatible of a material can be carried out through a biocompatibility test or test toxicity.43,44 The first level of the biocompatibility test of a material can be done through in-vitro, namely the material to be tested contacted outside the body of microorganisms such as cell culture.45
The previous work related to snails’ mucus quantitative researches have been done to assess the composition of the snail.46–48 Land snails such as Achatina fulica, Lissachatina fulica, Hemiplecta distincta are concluded to have proteins from the mucus.46,47 Research has been done by Nugrahananto, et al (2014) to characterize proteins of snail mucus (Achatina fulica) in Yogyakarta that have antimicrobial activities to Streptococcus mutans and Actinobacillus actinomycetemcomitans.46 The results show that the proteins from Achatina Fullica is weighted 83.67 kDa (achasin), 50.81 kDa, 15 kDa, 11.45 kDa and 9.7 kDa (mytimacin-AF).46 The isolation and characterization of the protein was conducted using SDS-PAGE method, electro-elution, and dialysis.46 Another research by Noothuan, et al (2021) about a different snail mucus from Lissachatina fulica and Hemiplecta distincta was examined and proved that they have exhibits different pattern of protein. The protein concentration was determined using Bradford assay and the protein pattern of the two snails analyzed by 12.5% SDS-PAGE.47 The Lissachatina fulica mucus showed major bands at about 13, 37, 70, and > 200 kDa, whereas Hemiplecta Distincta showed major bands at approximately 11, 12, 14, 25, and 120 kDa.47 The snail mucus also exhibits various biological activities such as antimicrobial, antioxidant, anti-tyrosinase and antitumoral activities.48 Research by Trapella, et al (2018) about chemical composition and biological effect of the snail mucus has been conducted with snail species of Helix aspersa muller. 49 The method used in vitro experimental model. The results show that snail mucus exhibits glycolic acid and allantoin. It is also found that the mucus is lacked of cytotoxicity and induce cell proliferation.49 Most of the researches show the composition of the snail’s mucus.46,47 However, the qualitative-quantitative research about comprehensive chemical compounds from snail mucus, and their molecular formulas have not been widely studied, especially for periodontal disease.
Considering recent studies of developing medical substances from environment as an alternative to prevent further infections or wounds, it is worth to evaluate the comprehensive chemical compounds extracted fromsnail’s mucus, especially for peridontitis. Mammalian cell lines encompass 51% of approved biologics in industrial cell lines and the most used mammalian cell lines includes Baby Hamster Kidney (BHK) cells.50 BHK-21 has found applications in vaccines against mouth diseases and heterologous protein production and is the most important cell, the largest component of the dental pulp, periodontal ligament and gingiva.51,52 This study aims to analyze the cytotoxic activity of snail mucus Achatina fulica against BHK-21 fibroblast cells, carried out with various concentrations of the snail mucus at 12.5%, 25%, 50%, 100% to periodontitis bacteria of Porphyromonas gingivalis, Fusobacterium nucleatum, Enterococcus faecalis and Staphylococcus aureus. The percentage of concentrations used in this research is based on the research by Daud, et al (2018) which shows that at 11%, the snail mucus has the antibacterial activity.53 Meanwhile, at concentration 30% and 60%, snail mucus reduces the cell viability.54 The concentrations of 12.5%, 25%, 50% and 100% are also used in analyzing mucus antibacterial activity.47 Porphyromonas gingivalis contributes to chronic periodontitis. This bacterium creates virulence factors causing destruction to periodontal tissue.55 Research by Hendrawati, et al (2019) shows that 20% snail mucus gel can enhance the osteoblasts in rats suffering from periodontitis.56 Fusobacterium nucleatum causes lesions in periodontal disease, halitosis, dental pulp infection, oral cancer and systemic disease.57 Enterococcus faecalis appear more frequent in subgingival samples with periodontitis than from periodontally healthy one.58,59
Material and methods
The process of preparation to the generation of snail mucus is shown in Figure 1. The process is detailed according to the research design, preparation and generation of the snail mucus.
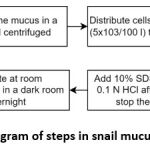 |
Figure 1: Diagram of steps in snail mucus generation. |
Research Design
The method used in this study is a qualitative-quantitative method with a “True Experiment Laboratory” research approach. The method was carried out by laboratory analysis of snail mucus (Achatina fulica) to obtain snail’s various chemical elements. This qualitative analysis is carried out using 16opica-standard testing techniques in the laboratory to identify the protein, antibacterial power or inhibition against Porphyromonas gingivalis, Fusobacterium nucleatum, Enterococcus faecalis and Staphylococcus aureus, and detect the anti-inflammation (Acharan sulfat). Quantitative research is carried out to determine the concentration of a compound in the sample, which can be in the form of moles, or percentages in grams. This technique requires high accuracy because errors in measurement will result in data errors in research. Quantitative analysis is generally carried out after qualitative analysis. The research was carried out at the Oral Biology Laboratory, Airlanggga University, Indonesia.
Preparation
The snails were obtained from community plantations in Nyalian village, Banjar Angkan District, Klungkung, Bali, Indonesia. The type of snail we used is Achatina fulica. The snail is weighted 200-250 grams each. The total snails we used were 25 snails. The object of the research is the chemical compound in the snail. Snail mucus was taken, then analyzed to obtain the active compound or chemical compounds contained in it. The tip of a syringe was used to stimulate the secretion of mucus.60,61
Generation of The Snail’s Mucus
A snail produces approximately 3-5 cc mucus. Snail mucus was collected in a bottle, then centrifuged. Then the snail mucus was tested for cytotoxicity using BHK-21 fibroblast cells, using the MTT Assay method. Cells in the number (5×103/100 l) were distributed to a 96-well plate and incubated with test and comparison solutions of various concentration series of 100 l for 24 hours in a CO2 incubator with 5% percentage at 37⁰C. After incubation, 100 l of solution with 5 mg/mL of MTT was added to each well. The reaction was stopped by adding a 10% SDS stopper into 0.1N HCl after 6 hours. After that, it was incubated at room temperature in a dark room overnight. The intensity of the purple color formed was measured with an Enzyme-linked immunosorbent (ELISA) reader or also known as microplate reader at a wavelength of 595 nm 60. The absorbance data obtained were converted into percent Live Cells with the formula 62:
Figure 2 shows the process where the first step is disinfected culture shock, then stock planting on RPMI media, centrifuge stock cell media RPMI. Next, is the picture of cell culture well plate 96 and then got the sample of 100%, 50%, 25% and 12.5%. At last, the data were read on ELISA reader.
 |
Figure 2: Steps in the process. A. Disinfected Culture stock. B. Stock planting on RPMI media. C. Centrifuge stock cell media RPMI. D. Cell culture well plate 96 well. |
Inhibition Test
The data collected is primary data in the form of zone diameter. The diameter of the inhibition zone of snail mucus against Porphyromonas gingivalis, Fusobacterium nucleatum, Enterococcus faecalis and Staphylococcus aureus is the diameter of the clear zone that appears on the disk diffusion as measured by a caliper (in millimeters) to determine the inhibition power. The qualitative analysis comprises the diameter of bacterial inhibition power that was categorized according to Davis and Stout (1971): very strong (clear zone >20mm), strong (10-20mm clear zone), moderate (5-10mm clear zone), weak (<5mm).63
Statistical Analysis
The analysis of normality test data used Kolmogorov-Smirnov. It was performed using SPSS version 25 and Program R. If the data are normally distributed, the test to determine significance is followed by a parametric statistical test using one-way ANOVA. If the data are not normally distributed, then the significance test is carried out with a non-parametric statistical test using Kruskal-Wallis.
Results and Discussion
Inhibition of Porphyromonas gingivalis
Porphyromonas gingivalis is known as major etiological agent in periodontal diseases which resulted in gingival inflammation9. Table 1 shows the results of the examination of the inhibition of Achatina fulica against Porphyromonas gingivalis bacteria (four repetitions). Table 2 shows the results of the examination of the inhibition diameter of the snail’s mucus against Porphyromonas gingivalis. The results of the inhibition of snail mucus against Phorpyromonas gingivalis is the largest in the treatment group with a concentration of 100%, with an area of 21.35 mm, and the smallest at a concentration of 12.5%. This indicates that the higher the concentration of snail mucus material, the wider the inhibition. So, in conclusion, the inhibition power of Achatina fulica to Porphyromonas gingivalis is very strong with 21.35 mm at 100% concentration and weak at 12.5% concentration. Analysis of differences in inhibition power between the treatment groups of Phorpyromonas gingivalis is given in Table 3. It presents that there is significant difference in the treatment group, where the difference between the 12.5% treatment group and the control group is the highest.
Table 1: Inhibition power of Phorpyromonas gingivalis
| Repetition | Phorpyromonas Gingivalis | |||||
| 100% | 50% | 25% | 12.5% | Control (+) | Control( -) | |
| 1. | 21.00 | 18.20 | 12.80 | – | 26.80 | – |
| 2. | 21.20 | 17.80 | 13.05 | – | 26.60 | – |
| 3. | 21.80 | 18.00 | 13.20 | – | 26.75 | – |
| 4. | 21.40 | 17.95 | 12.95 | – | 26.60 | – |
| Average | 21.35 | 17.98 | 13.00 | – | 26.68 | |
Table 2: Inhibition zone diameter in Phorpyromonas gingivalis bacteria
| Subject Group | N | Mean ± Inhibition Zone (millimeters)
|
Total Bacteria (CFU/m l) (No)
|
P |
| Control | 4 | 26±0.51
|
0.5 Mc Farland | 0.001* |
| Snail slime 12.5% | 4 | 0 ± 0.00
|
||
| Snail slime 25% | 4 | 13.00 ± 0.84
|
||
| Snail slime 50% | 4 | 17.98 ± 0.08
|
||
| Snail slime 100% | 4 | 21.35 ± 0,17 |
Table 3: Differences in inhibition of Phorpyromonas gingivalis bacteria.
| Variable | Group I | Group J | Mean difference (I-J) | P |
| Snail’s Mucus | Control | 12.5% | 26.68 | 1.00 |
| 25% | 21.35 | <0.001* | ||
| 50% | 17.98 | <0.001* | ||
| 100% | 13.00 | <0.001* | ||
| 12.5% | 25% | -13.00 | <0.001* | |
| 50% | -17.98 | <0.001* | ||
| 100% | -21.35 | <0.001* | ||
| Control | -26.68 | <0.001* | ||
| 25% | 50% | -4.98 | <0.001* | |
| 100% | -8.35 | <0.001* | ||
| Control | -13.68 | <0.001* | ||
| 12.5% | -13.00 | <0.001* | ||
| 50% | 100% | -3.37 | <0.001* | |
| Control | -9.00 | <0.001* | ||
| 12.5% | 17.98 | <0.001* | ||
| 25% | 4.98 | <0.001* | ||
| 100% | Control | 5.33 | <0.001* | |
| 12.5% | 21.35 | <0.001* | ||
| 25% | 8.35 | <0.001* | ||
| 50% | 5.33 | <0.001* |
Inhibition of Fusobacterium Nucleatum
The results of inhibition power of Fusobacterium Nucleatum in four repetitions are presented in Table 4. Meanwhile, table 5 shows the inhibition zone diameter of Fusobacterium Nucleatum. From table 5, it can be that the largest average diameter of the inhibition zone in the treatment group with various variations was at a concentration of 100%, with a diameter of 19.60 mm, while at the smallest 12.5% there is no inhibition zone. The table is revealing that the highest average at 100% concentration is categorized as strong diameter inhibition power. Whereas, the lowest concentration at 12.5% has weak diameter of bacterial inhibition power. From the category, it is noted that the inhibition power of Achatina fulica to Fusobacterium Nucleatum is categorized strong at 100% concentration and weak at 12.5% concentration. The statistical test on significance of differences in in inhibition is shown in table 6, where it notes that there is significant difference of inhibition power in each treatment group. Figure 3 shows the inhibition zone of the snail mucus against Fusobacterium nucleatum.
Table 4: Inhibition power of Fusobacterium nucleatum.
| Repetition | Fusobacterium Nucleatum | |||||
| 100% | 50% | 25% | 12.5% | Control (+) | Control( -) | |
| 1. | 19.20 | 16.20 | 12.40 | – | 25.20 | – |
| 2. | 19.60 | 16.60 | 12.20 | – | 25.60 | – |
| 3. | 20.20 | 16.95 | 12.80 | – | 25.80 | – |
| 4. | 19.80 | 16.80 | 12.60 | – | 26.00 | – |
| Average | 19.79 | 16.63 | 12.50 | – | 25.65 | – |
Table 5: Inhibition zone diameter of Fusobacterium nucleatum bacteria
| Subject Group | N | Mean ± Fusobacterium nucleatum Inhibition Zone (millimeters) | Total Bacteria (CFU/m l) (No) | P |
| Control | 4 | 25.65±,17 | 0.5 Mc Farland | 0.001* |
| Snail slime 12,5% | 4 | 0 ± 0.00 | ||
| Snail slime 25% | 4 | 12.50 ± 0.2 | ||
| Snail slime 50% | 4 | 16.63 ± 0.16 | ||
| Snail slime 100% | 4 | 19.70 ± 0.20 |
Table 6: Differences in inhibition of Fusobacterium nucleatum bacteria.
| Variable | Group I | Group J | Mean difference (I-J) | P |
| Snail’s Mucus | Control | 12.5% | 25.65 | 1.00 |
| 25% | 12.5 | <0.001* | ||
| 50% | 16.63 | <0.001* | ||
| 100% | 19.70 | <0.001* | ||
| 12.5% | 25% | -12.50 | <0.001* | |
| 50% | -16.63 | <0.001* | ||
| 100% | -19.70 | <0.001* | ||
| Control | -25.65 | <0.001* | ||
| 25% | 50% | -4.13 | <0.001* | |
| 100% | -7.20 | <0.001* | ||
| Control | -13.15 | <0.001* | ||
| 12,5% | -12.5 | <0.001* | ||
| 50% | 100% | -3.07 | <0.001* | |
| Control | -9.02 | <0.001* | ||
| 12.5% | 16.63 | <0.001* | ||
| 25% | 4.13 | <0.001* | ||
| 100% | Control | 5.95 | <0.001* | |
| 12.5% | 25.65 | <0.001* | ||
| 25% | 7.20 | <0.001* | ||
| 50% | 3.13 | <0.001* |
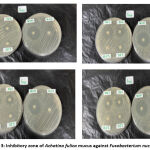 |
Figure 3: Inhibitory zone of Achatina fulica mucus against Fusobacterium nucleatum. |
Inhibition of E. Faecalis
Table 7 presents the inhibition zone results in four repetitions on E. Faecalis in the treatment group. Table 8 indicates that the largest inhibition zone in the treatment group is with a concentration of 100%, namely 21.93 mm, while the smallest was at a concentration of 12.5%, in which there is no inhibition zone. According to Davis and Stout (1971), the inhibition zone diameter of the concentration of 100% is categorized very strong. Meanwhile the lowest concentration at 12.5% is categorized weak. From the category, it is noted that the inhibition power of Achatina fulica to E. Faecalis is categorized very strong at 100% concentration and weak at 12.5% concentration. The analysis of differences in inhibition of E. Faecalis is presented in table 9. It shows that there is significant difference in inhibition between the treatment groups.
Table 7: Inhibition power of E. Faecalis bacteria.
| Repetition | E. Faecalis | |||||
| 100% | 50% | 25% | 12.5% | Control (+) | Control( -) | |
| 1. | 21.80 | 18.80 | 14.20 | – | 27.20 | – |
| 2. | 21.75 | 18.20 | 14.40 | – | 27.40 | – |
| 3. | 22.00 | 19.00 | 14.35 | – | 27.00 | – |
| 4. | 22.20 | 18.75 | 14.80 | – | 27.20 | – |
| Average | 21.93 | 18.68 | 14.43 | – | 27.20 | – |
Table 8: Inhibition zone diameter of E. Faecalis bacteria.
| Subject Group | N | Mean ± E. foecalis Zone (millimeters)
|
Total Bacteria (CFU/m l) | P |
| Control | 4 | 27.20±0,81 | 0.5 Mc Farland | 0.001* |
| Snail slime 12.5% | 4 | 0 ± 0.00 | ||
| Snail slime 25% | 4 | 14.43± 0.12 | ||
| Snail slime 50% | 4 | 18.68 ± 0.73 | ||
| Snail slime 100% | 4 | 21.93± 0,10 |
Table 9: Differences in inhibition of E. Faecalis bacteria.
| Variable | Group I | Group J | Mean difference (I-J) | P |
| Snail’s Mucus | Control | 12.5% | 27.20 | 1.00 |
| 25% | 12.77 | <0.001* | ||
| 50% | 8.52 | <0.001* | ||
| 100% | 5.27 | <0.001* | ||
| 12.5% | 25% | -14.43 | <0.001* | |
| 50% | -18.68 | <0.001* | ||
| 100% | -21.93 | <0.001* | ||
| Control | -27.20 | <0.001* | ||
| 25% | 50% | -4.25 | <0.001* | |
| 100% | -7.5 | <0.001* | ||
| Control | -12.77 | <0.001* | ||
| 12.5% | 14.43 | <0.001* | ||
| 50% | 100% | -3.25 | <0.001* | |
| Control | -8.52 | <0.001* | ||
| 12.5% | 18.68 | <0.001* | ||
| 25% | 4.25 | <0.001* | ||
| 100% | Control | -5.27 | <0.001* | |
| 12.5% | 19.20 | <0.001* | ||
| 25% | 6.80 | <0.001* | ||
| 50% | 3.80 | <0.001* |
Inhibition of S. aureus
Table 10 shows the inhibition power of the snail mucus of Achatina fulica against S. aureus bacteria in four repetitions. From table 10, it can be processed to analyze the inhibition zone diameter on S. aureus bacteria as presented in table 11. The results show that the average inhibition power in the treatment group with four repetitions with an inhibition zone area of 23.15 mm is at 100% concentration, while the lowest is in the treatment group with a concentration of 12.5%, with an inhibition power of 0 mm. Based on the inhibition zone diameter results, it can be concluded that the concentration of 100% as the highest average inhibition zone diameter is categorized very strong. Meanwhile, at concentration 12.5%, the number of mean S. aureus inhibition zone is categorized weak. So, it is concluded that the snail’s mucus from Achatina fulica to bacteria of S. aureus has very strong inhibition power at 100% concentration. From the tables 10 and 11, it can be seen that there are differences. As shown in table 12, it shows that there is significant difference in the treatment groups with various concentrations with p < 0.05.
Table 10: Inhibition power of S. aureus bacteria.
| Repetition | S. aureus | |||||
| 100% | 50% | 25% | 12.5% | Control (+) | Control( -) | |
| 1. | 22.80 | 19.60 | 16.20 | – | 27.20 | – |
| 2. | 23.00 | 19.80 | 15.40 | – | 27.40 | – |
| 3. | 23.20 | 19.40 | 15.80 | – | 27.00 | – |
| 4. | 23.60 | 19.80 | 16.40 | – | 27.20 | – |
| Average | 23.15 | 19.65 | 15.95 | – | 27.20 | |
Table 11: Inhibition zone diameter on S. Aureus bacteria
| Subject Group | N | Mean ± S. aureus Inhibition Zone (millimeters)
|
Total Bacteria (CFU/m l) | P |
| Control | 4 | 27.20±0.81 | 0.5 Mc Farland | 0.001* |
| Snail mucus 12,5% | 4 | 0 ± 0.00 | ||
| Snail mucus 25% | 4 | 15.90 ± 0.22 | ||
| Snail mucus 50% | 4 | 19.65 ± 0.95 | ||
| Snail mucus 100% | 4 | 23.15± 0.17 |
Table 12: Differences in inhibition of S. Aureus bacteria.
| Variable | Group I | Group J | Mean difference (I-J) | P |
| Snail’s Mucus | Control | 12.5% | 27.20 | 1.00 |
| 25% | 11.5 | <0.001* | ||
| 50% | 7.55 | <0.001* | ||
| 100% | 6.55 | <0.001* | ||
| 12.5% | 25% | -15.90 | <0.001* | |
| 50% | -19.65 | <0.001* | ||
| 100% | -23.15 | <0.001* | ||
| control | -27.20 | <0.001* | ||
| 25% | 50% | -3.75 | <0.001* | |
| 100% | -7.25 | <0.001* | ||
| control | -11.3 | <0.001* | ||
| 12.5% | -15.90 | <0.001* | ||
| 50% | 100% | -3.5 | <0.001* | |
| control | -7.55 | <0.001* | ||
| 12.5% | 19.65 | <0.001* | ||
| 25% | 3.75 | <0.001* | ||
| 100% | control | 3.25 | <0.001* | |
| 12.5% | 23.15 | <0.001* | ||
| 25% | 7.25 | <0.001* | ||
| 50% | 3.5 | <0.001* |
Antibacterial and Anti-Inflammatory Analysis with GCMS
Table 13 details the chemical compounds of snail’s mucus obtained from Achatina fulica. The content of active chemical compounds from snail slime was tested by GCMS (Gas Chromatography-Mass Spectrometry) which is to identify the active substance from snail’s mucus. It is noted that the most compound found is Achasin protein with an average of 102.20 mg/100g, and the least compound contained in the mucus is glycoconjugate in as much as 8.86 mg/100g. The average content of the compounds found using GCMS test is Heparan sulfate 16.45 mg/100g, Acharan sulfate 21.33 mg/100g, Achatin 86.12 mg/100g, Beta agglutinins 58.22 mg/100g, protein achasin 102.22 mg/100g, glycoconjugates 8.86mg/100g
Table 13: Chemical compounds obtained from Achatina fulica Mucus using GCMS for antibacterial and anti-inflammatory functions.
| Repetition | Heparan Sulfat
(mg/100 g) |
Acharan Sulfat (mg/100 g) | Achatin isolate (mg/100 g) | IonCa2+
(mg/100g) |
Beta Aglutinin mg/100g | Protein achasin (mg/100 g) | Glycoconjugate (mg/100 g |
| 1. | 16.60 | 21.35 | 36.10 | 86.15 | 58.21 | 102.20 | 8.90 |
| 2. | 16.50 | 21.30 | 36.00 | 86.10 | 58.19 | 102.15 | 8.87 |
| 3. | 16.30 | 21.37 | 36.08 | 86.13 | 58.25 | 102.25 | 8.82 |
| 4. | 16.40 | 21.33 | 36.06 | 86.11 | 58.23 | 102.30 | 8.85 |
|
Average |
16.45 | 21.33 | 36.06 | 86.12 | 58.22 | 102.22 |
8.86 |
Cytotoxicity Test
The results of the cytotoxicity test of BHK-21 fibroblast cells against snail mucus can be seen in table 14. Table 14 shows the highest toxicity to lowest toxicity respectively at the concentration of 12.5% (mean: 0.768), concentration of 25% (mean: 0.493), concentration of 50% (mean 0.302) and concentration of 100% (mean: 0.171). The concentration of the cell control at the average is 0.959 (mean: 0.88). Table 14 also shows clearly that there are differences in the control media, control cell and various concentration of Achatina fulica in which the least mean is at concentration of 12.5%, and the highest mean is at concentration of 100%.
Table 14: MTT Assay test results.
| Repetition | Control Media | Cell control | 100% | 50% | 25% | 12.5% |
| 1 | 0.082 | 0.974 | 0.162 | 0.297 | 0.493 | 0.784 |
| 2 | 0.097 | 0.952 | 0.184 | 0.299 | 0.496 | 0.764 |
| 3 | 0.081 | 0.951 | 0.163 | 0.302 | 0.487 | 0.758 |
| 4 | 0.092 | 0.959 | 0.175 | 0.312 | 0.498 | 0.769 |
| Average | 0.088 | 0.959 | 0.171 | 0.302 | 0.493 | 0.768 |
Table 15 shows the statistical test with Kolmogorov-Smirnov data that is normally distributed. Since all data were normally distributed, statistical tests to determine which group has the most significance were tested using the one-way ANOVA parametric statistical test. Subsequently, it is to determine which group has the most significance by using the one-way ANOVA parametric statistical test as shown in Table 16.
Table 15: Kolmogorov-Smirnov test results.
| Control Media | Cell control | Concen-
tration of 100% |
Concen-
tration of 50% |
Concen-
tration of 25% |
Concen-
tration of 12.5% |
||
| N | 4 | 4 | 4 | 4 | 4 | 4 | |
| Normal Parametersa,b | Mean | .08800 | .95900 | .17100 | .30250 | .49350 | .76875 |
| Std. Deviation | .007789 | .010614 | .010488 | .006658 | .004796 | .011117 | |
| Most Extreme Differences | Absolute | .279 | .250 | .277 | .280 | .208 | .241 |
| Positive | .279 | .250 | .277 | .280 | .174 | .241 | |
| Negative | -.196 | -.226 | -.195 | -.204 | -.208 | -.167 | |
| Test Statistic | .279 | .250 | .277 | .280 | .208 | .241 | |
| Asymp. Sig. (2-tailed) | .c,d | .c,d | .c,d | .c,d | .c,d | .c,d | |
Table 16: ANOVA test result.
| Sum of Squares | df | Mean Square | F | Sig. | |
| Between Groups | 2.368 | 5 | .474 | 5992.534 | .000 |
| Within Groups | .001 | 18 | .000 | ||
| Total | 2.370 | 23 |
Table 16 shows a significant difference between groups with various concentration and between treatment groups, where the significance is < 0.05. Table 17 indicates a significant difference the increase in snail mucus (%) to the number of living cells (cytotoxicity) with p < 0.05. From the tables above, it can be concluded that the minimum cytotoxicity value of snail mucus is at a concentration of 12.5%. This means that the increase in concentration above the concentration of 12.5% is cytotoxic to the number of cells.
Table 17: Cytotoxicity statistical test of BHK-21 fibroblast
| Snail’s Mucus (%) | x± SD | ||||||
| 100% | 50% | 25% | 12,5% | Control Media | Cell Control | ||
| 100% | 0.171 ± 0.10488 | 0 | 0.000a | 0.000b | 0.000c | 0.000d | 0.000e |
| 50% | 0.30250 ± 0.006658 | 0.000f | 0 | 0.000g | 0.000h | 0.000i | 0.000j |
| 25% | 0.49350 ± 0.004796 | 0.000k | 0.000l | 0 | 0.000m | 0.000n | 0.000o |
| 12.5% | 0.76875 ± 0.011117 | 0.000p | 0.000q | 0.000r | 0 | 0.000s | 0.000t |
Cytotoxicity to Fibroblast BHK 21
The use of traditional medicinal snail mucus must be carried out by knowledge of the safety level of preparations obtained through toxicity test so as not to cause harmful effects.64,65 Various ingredients or active chemical substances are found in the snails’ mucus, such as antibacterial and anti-inflammatory. The cytotoxicity test in this study was snail’s mucus of Achatina fulica against BHK-21 fibroblast cells. The use of cultured cells of BHK-21 is the most important cell and the largest component of the dental pulp, periodontal ligament and gingiva.51 This study used 12.5%, 25%, 50%, and 100% concentrations. This is to determine the potential toxicity of the active compound of snail’s mucus (Achatina fulica) against BHK-21 mice fibroblast cells in-vitro, using the MTT Assay method. Formazan can be generated even in cell-free conditions: MTT can be reduced by some particular compounds present in culture media such as polyphenols.66
The results of the snail’s mucus cytotoxicity test show that the average mean of four repetitions at a concentration of 12.5% is 0.768, while at a concentration of 50% is 0.493, the concentration of 25% was 0.302, while the concentration of 100% is 0.17. So at a concentration of 12.5 % is not toxic because it has more than 50% number of fibroblasts, while at a concentration of 25-100% it is toxic, this is due to the protein content of acharan sulfate.67
Fibroblast Proliferation
Snail mucus causes faster proliferation.39 These contents play an important role in cells and matrix cells interactions associated with normal and pathological conditions of cell recognition, adhesion, migration, and cell growth, and these active substances can also chemically stimulate the process of fibroplasia in the wound area. Increased proliferation of fibroblast cells can be used as a biological marker of the wound healing process, namely by the presence of a high percentage of increased fibroblast proliferation. The presence of a high percentage of live cells in BHK-21 fibroblast cells means that snail mucus has the effect of increasing fibroblast cell proliferation so that it can accelerate the wound healing process.68,69
The reduction of yellow MTT salt to purple formazan is performed by tetrazolium succinate reductase, which is included in the respiratory chain in the mitochondria of living cells.43,70 In this study, the average optical density of formazan in snail mucus with increasing concentrations of 12.5%, 25%, 50%, 100% showed a decrease (Table 14) due to the ability of living cells to reduce MTT salts. The principle of this assay is the breakdown of the yellow MTT tetrazolium ring (3-4-5-dimethylthiazol-2-yl)-2-5 diphenyl tetrazolium bromide) by the presence of dehydrogenase in the active mitochondria, resulting in an insoluble purplish-blue formazan product.71 The mechanism is that the yellow tetrazolium salt will be reduced in cells with metabolic activity, which has an important role, in this case, in the mitochondria of living cells that produce dehydrogenase. If dehydrogenase is inactivated due to cytotoxic effects, formazan will not be formed. In table 16, statistical calculations using One Way ANOVA followed by LSD with a significance level of 5% showed that the higher the snail mucus concentration, the lower the formazan density value was significant. Natural materials such as snails’ mucus, before being used as a medicine, must perform an enzymatic test process, not irritating, and have biocompatibility, or the material produced must not have a detrimental effect on the biological environment local and systemic.46 The basis of the MTT enzymatic test is to measure the ability of living cells based on mitochondrial activity from cell cultures.43 For this reason, natural ingredients have now been developed which can be used as alternative ingredients for healing inflammation.72 In the present study, the higher concentration, decreasing percentage rate of fibroblast cells. This is in accordance with the research conducted by (Apriasari, et al., 2014) on the toxicity of Mauli banana stem extract against BHK-21 fibroblast cells which proved that the higher the concentration of the extract, the lower the viability of fibroblast cells.73
Antimicrobial susceptibility testing (AST) performance of bacterial pathogens is an essential procedure to ensure and determine the susceptibility to antimicrobial agents and to analyse the resistance.74,75 Disk diffusion has been the pledge for antimicrobial susceptibility testing.76 On a larger scale, the AST helps in the evaluation of treatment services provided by hospital, clinics, and health programs to control and prevent infectious diseases.77,78 The determination of susceptibility and its resistance is by categorized the results of zone diameter of inhibition.74 The diameter of the inhibition zone around each antibiotic disk is measured in milimeters.79,80 The results of zone diameter of inhibition of Phorpyromonas gingivalis, E. Faecalis , and S. aureus show at concentration 100%, the zone diameter of inhibition are categorized very strong in 21.35, 21.93, 23.15 mm respectively. Meanwhile, the result of zone diameter of inhibition of Fusobacterium nucleatum is categorized strong with 19.7 mm diameter. The large zone diameter of inhibition indicates that the organism is susceptible, while the small or no zone inhibition shows resistance.76,81 So, it can be drawn in this study, that the snail’s mucus from Achatina fulica is resistant with concentration 12.5%.
Conclusion
This study conducted cytotoxicity test of snail mucus Achatina fulica with various concentration against BHK-21 fibroblast cells in mice. The concentration we used are 12.5%, 25%, 50% and 100%. Based on the results obtained, it can be concluded that the active compound of snails’ mucus in various concentrations has the highest cytotoxicity activity at a concentration of 12.5%, with an average of 0.768. After analyzing the active substances or chemical compounds of snail mucus, the antibacterial content is Achatin and acharan sulfate as antibacterial and painkillers, and for the anti-inflammation, it is obtained Heparan sulfate. The inhibition test of snail mucus against bacteria (Phorpyromonas gingivalis, Fusobacterium nucleatum, S. aureus, and E. foecalis, has a very strong category of inhibition. The results of the snail slime cytotoxicity test showed that more than 50% of the number of fibroblasts in BHK21 cells was at a concentration of 12.5%, meaning that the snail mucus at that concentration is not toxic. The future work of this research can be performed in the analysis of histological and clinical research to establish the snail mucus from Achatina fulica to be used for periodontitis therapy.
Acknowledgement
The author greatly appreciates the honorable Microbiology laboratory Faculty of Dentistry, Airlangga University and Director of Poltekkes Kemenkes Denpasar, Bali Indonesia. This research has been approved with Ethical Approval Number LB.02.03/EA/KEPK/0655/2021.
Conflict of Interest
The authors declare that they have no competing interest.
Reference
- Könönen E, Gursoy M, Gursoy UK. Periodontitis : A Multifaceted Disease of Tooth-Supporting Tissues. J Clin Med. 2019;8(1135):1-12.
CrossRef - Mazzoleni S, Ludovichetti FS, Bacci C, Zuccon A, Gracco A. Periodontitis in the Developmental Age : Pathogenesis , Epidemiology , Differential Diagnosis and Treatment . A Narrative Review. Inter Ped Dent Open Acc J. 2020;3(5):256-264. doi:10.32474/IPDOAJ.2020.03.000173
CrossRef - Martínez-garcía M, Hernández-lemus E. Periodontal Inflammation and Systemic Diseases : An Overview. Front Physiol. 2021;12(October):1-26. doi:10.3389/fphys.2021.709438
CrossRef - Boylan MR, Khalili H, Huang ES, et al. A prospective study of periodontal disease and risk of gastric and duodenal ulcer in male health professionals. Clin Transl Gastroenterol. 2014;5(2):e49-6. doi:10.1038/ctg.2013.14
CrossRef - Azzolino D, Passarelli PC, De Angelis P, Piccirillo GB, D’addona A, Cesari M. Poor oral health as a determinant of malnutrition and sarcopenia. Nutrients. 2019;11(12):1-17. doi:10.3390/nu11122898
CrossRef - Sedghi LM, Bacino M, Kapila YL. Periodontal Disease: The Good, The Bad, and The Unknown. Front Cell Infect Microbiol. 2021;11(December):1-26. doi:10.3389/fcimb.2021.766944
CrossRef - Corredor Z, Molina AS, Fong C, C LC, Olarte SG. Presence of periodontal pathogenic bacteria in blood of patients with coronary artery disease. Sci Rep. 2022;12(37):1-12. doi:10.1038/s41598-022-05337-1
CrossRef - Aleksijević LH, Aleksijević M, Škrlec I, Šram M, Šram M, Talapko J. Porphyromonas gingivalis Virulence Factors and Clinical Significance in Periodontal Disease and Coronary Artery Diseases. Pathogens. 2022;11(10). doi:10.3390/pathogens11101173
CrossRef - Liu Y, Zhang Y, Wang L, Guo Y, Xiao S. Prevalence of Porphyromonas gingivalis Four rag Locus Genotypes in Patients of Orthodontic Gingivitis and Periodontitis. PLoS One. 2013;8(4):4-9. doi:10.1371/journal.pone.0061028
CrossRef - Șurlin P, Nicolae FM, Șurlin VM, et al. Could periodontal disease through periopathogen fusobacterium nucleatum be an aggravating factor for gastric cancer? J Clin Med. 2020;9(12):1-15. doi:10.3390/jcm9123885
CrossRef - Stokowa-Sołtys K, Wojtkowiak K, Jagiełło K. Fusobacterium nucleatum – Friend or foe? J Inorg Biochem. 2021;224. doi:10.1016/j.jinorgbio.2021.111586
CrossRef - Chidambar CK, Shankar SM, Raghu P, Gururaj SB, Bushan KS. Detection of Enterococcus faecalis in subgingival biofilms of healthy, gingivitis, and chronic periodontitis subjects. J Indian Soc Periodontol. 2019;23(5):416-418. doi:10.4103/jisp.jisp_44_19
CrossRef - Sundaram G, Ramakrishnan T, Parthasarathy H, Raja M, Raj S. Detection of Enterococcus faecalis in subgingival biofilms of healthy, gingivitis, and chronic periodontitis subjects. Indian Society of Periodontology. 2018;(May):113-118. doi:10.4103/jisp.jisp
- Kim GY, Lee CH. Antimicrobial susceptibility and pathogenic genes of Staphylococcus aureus isolated from the oral cavity of patients with periodontitis. J Periodontal Implant Sci. 2015;45(6):223-228. doi:10.5051/jpis.2015.45.6.223
CrossRef - Vitkov L, Hartl D, Minnich B, Hannig M. Janus-faced neutrophil extracellular traps in periodontitis. Front Immunol. 2017;8(OCT). doi:10.3389/fimmu.2017.01404
CrossRef - Munasur SL, Turawa EB, Chikte UME, Musekiwa A. Mechanical debridement with antibiotics in the treatment of chronic periodontitis: Effect on systemic biomarkers—A systematic review. Int J Environ Res Public Health. 2020;17(15):1-19. doi:10.3390/ijerph17155601
CrossRef - Manresa C, Sanz-Miralles EC, Twigg J, Bravo M. Supportive periodontal therapy (SPT) for maintaining the dentition in adults treated for periodontitis. Cochrane Database of Systematic Reviews. 2018;2018(1):1-48. doi:10.1002/14651858.CD009376.pub2
CrossRef - Agossa K, Sy K, Mainville T, et al. Antibiotic use in periodontal therapy among french dentists and factors which influence prescribing practices. Antibiotics. 2021;10(3). doi:10.3390/antibiotics10030303
CrossRef - Llanos AH, Benítez Silva CG, Ichimura KT, et al. Impact of aggressive periodontitis and chronic periodontitis on oral health-related quality of life. Braz Oral Res. 2018;32. doi:10.1590/1807-3107bor-2018.vol32.0006
CrossRef - Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. 2017;3(June):1-14. doi:10.1038/nrdp.2017.38
CrossRef - Dinica RM, Sandu C, Botezatu AVD, et al. Allantoin from valuable romanian animal and plant sources with promising anti-inflammatory activity as a nutricosmetic ingredient. Sustainability (Switzerland). 2021;13(18). doi:10.3390/su131810170
CrossRef - Truchuelo MT, Vitale M. A cosmetic treatment based on the secretion of Cryptomphalus aspersa 40% improves the clinical results after the use of nonablative fractional laser in skin aging. J Cosmet Dermatol. 2020;19(3):622-628. doi:10.1111/jocd.13052
CrossRef - Leal J, Smyth HDC, Ghosh D. Physicochemical properties of mucus and their impact on transmucosal drug delivery. Int J Pharm. 2017;532(1):555-572. doi:10.1016/j.ijpharm.2017.09.018
CrossRef - Vidal MNP, Granjeiro JM. Cytotoxicity Tests for Evaluating Medical Devices: An Alert for the Development of Biotechnology Health Products. J Biomed Sci Eng. 2017;10(09):431-443. doi:10.4236/jbise.2017.109033
CrossRef - Srivastava GK, Alonso-Alonso ML, Fernandez-Bueno I, et al. Comparison between direct contact and extract exposure methods for PFO cytotoxicity evaluation. Sci Rep. 2018;8(1). doi:10.1038/s41598-018-19428-5
CrossRef - Jablonská E, Kubásek J, Vojtěch D, Ruml T, Lipov J. Test conditions can significantly affect the results of in vitro cytotoxicity testing of degradable metallic biomaterials. Sci Rep. 2021;11(1). doi:10.1038/s41598-021-85019-6
CrossRef - Arome D, Chinedu E. The importance of toxicity testing. Journal of Pharmaceutical and BioScience. 2014;4(2013):146-148.
- Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound Healing: A Cellular Perspective. Physiol Rev. 2019;99:665-706. doi:10.1152/physrev.00067.2017.-Wound
CrossRef - Gonzalez ACDO, Andrade ZDA, Costa TF, Medrado ARAP. Wound healing – A literature review. An Bras Dermatol. 2016;91(5):614-620. doi:10.1590/abd1806-4841.20164741
CrossRef - Khan S, Hashmi GS. Histology and functions of connective tissues: a review article. University Journal of Dental Science. 2015;4(2):1-2.
- Potekaev NN, Borzykh OB, Medvedev G v., et al. The role of extracellular matrix in skin wound healing. J Clin Med. 2021;10(24). doi:10.3390/jcm10245947
CrossRef - Tracy LE, Minasian RA, Caterson EJ. Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv Wound Care (New Rochelle). 2016;5(3):119-136. doi:10.1089/wound.2014.0561
CrossRef - de Araújo R, Lôbo M, Trindade K, Silva DF, Pereira N. Fibroblast Growth Factors: A Controlling Mechanism of Skin Aging. Skin Pharmacol Physiol. 2019;32(5):275-282. doi:10.1159/000501145
CrossRef - Xiao T, Yan Z, Xiao S, Xia Y. Proinflammatory cytokines regulate epidermal stem cells in wound epithelialization. Stem Cell Res Ther. 2020;11(1). doi:10.1186/s13287-020-01755-y
CrossRef - Addis R, Cruciani S, Santaniello S, et al. Fibroblast proliferation and migration in wound healing by phytochemicals: Evidence for a novel synergic outcome. Int J Med Sci. 2020;17(8):1030-1042. doi:10.7150/ijms.43986
CrossRef - Bainbridge P. Wound healing and the role of fibroblasts. J Wound Care. 2013;22(8):407-412. doi:10.12968/jowc.2013.22.8.407
CrossRef - Cañedo-Dorantes L, Cañedo-Ayala M. Skin acute wound healing: A comprehensive review. Int J Inflam. 2019;2019:1-15. doi:10.1155/2019/3706315
CrossRef - Igaap S, Putra Manuaba IB, Thahir H, et al. The effectiveness of giving snail slime (Acatina fulica) on the healing of pocket on the wistar rats with periodontitis. International Journal of Applied Pharmaceutics. 2019;11:19-21. doi:10.22159/ijap.2019.v11s4.35281
- Harti AS, Murharyati A, Dwi Sulisetyawati S, Oktariani M. The effectiveness of snail mucus (Achatina fulica) and chitosan toward limfosit proliferation in vitro. Asian Journal of Pharmaceutical and Clinical Research. 2018;11(Special Issue 3):85-88. doi:10.22159/ajpcr.2018.v11s3.30041
CrossRef - Benov L. Effect of growth media on the MTT colorimetric assay in bacteria. PLoS One. 2019;14(8). doi:10.1371/journal.pone.0219713
CrossRef - Benov L. Effect of growth media on the MTT colorimetric assay in bacteria. PLoS One. 2019;14(8):1-15. doi:10.1371/journal.pone.0219713
CrossRef - Benov L. Effect of growth media on the MTT colorimetric assay in bacteria. PLoS One. 2019;14(8). doi:10.1371/journal.pone.0219713
CrossRef - Ghasemi M, Turnbull T, Sebastian S, Kempson I. The mtt assay: Utility, limitations, pitfalls, and interpretation in bulk and single-cell analysis. Int J Mol Sci. 2021;22(23). doi:10.3390/ijms222312827
CrossRef - Chraniuk M, Panasiuk M, Hovhannisyan L, et al. Assessment of the Toxicity of Biocompatible Materials Supporting Bone Regeneration: Impact of the Type of Assay and Used Controls. Toxics. 2022;10(1). doi:10.3390/toxics10010020
CrossRef - Gawlikowski M, Fray M El, Janiczak K, Zawidlak-w B, Kustosz R. In-Vitro Biocompatibility and Hemocompatibility Study of New PET Copolyesters Intended for Heart Assist Devices. Polymers (Basel). 2020;12(2857):1-15. doi:10.3390/polym12122857
CrossRef - DN HM, Kriswandini IL, R EA. Antimicrobial proteins of Snail mucus (Achatina fulica) against Streptococcus mutans and Aggregatibacter actinomycetemcomitans. Dental Journal (Majalah Kedokteran Gigi). 2014;47(1):31-36. doi:10.20473/j.djmkg.v47.i1.p31-36
CrossRef - Noothuan N, Apitanyasai K, Panha S, Tassanakajon A. Snail mucus from the mantle and foot of two land snails, Lissachatina fulica and Hemiplecta distincta, exhibits different protein profile and biological activity. BMC Res Notes. 2021;14(1):1-7. doi:10.1186/s13104-021-05557-0
CrossRef - Ulagesan S, Kim HJ. Antibacterial and antifungal activities of proteins extracted from seven different snails. Applied Sciences (Switzerland). 2018;8(8):1-9. doi:10.3390/app8081362
CrossRef - Trapella C, Rizzo R, Gallo S, et al. HelixComplex snail mucus exhibits pro-survival, proliferative and pro-migration effects on mammalian fibroblasts. Sci Rep. 2018;8(1):1-11. doi:10.1038/s41598-018-35816-3
CrossRef - Kantardjieff A, Zhou W. Mammalian cell cultures for biologics manufacturing. Adv Biochem Eng Biotechnol. 2014;139(November 2013):1-9. doi:10.1007/10_2013_255
CrossRef - Meilena T, Fabiansyah JC, Djulaeha E, Hidayati HE. Toxicity Test on Taro Leaf Extract (Colocasia Esculenta L. Schoot) as Mouthwash to BHK-21 Fibroblast Cell Culture in Denture Users. Indonesian Journal of Dental Medicine. 2018;1(1):35-39. doi:10.20473/ijdm.v1i1.2018.35-39
CrossRef - Sartori R, Leme J, Caricati CP, Tonso A, Núñez EGF. Model comparison to describe BHK-21 cell growth and metabolism in stirred tank bioreactors operated in batch mode. Brazilian Journal of Chemical Engineering. 2018;35(2):441-458. doi:10.1590/0104-6632.20180352s20160592
CrossRef - Daud NS, Akbar AJ, Nurhikma E, Karmilah K. Formulation of Snail Slime (Achatina Fulica) Anti-Acne Emulgel using Tween 80-Span 80 as Emulsifying and HPMC as Gelling Agent. Borneo Journal of Pharmacy. 2018;1(2):64-67. doi:10.33084/bjop.v1i2.369
CrossRef - Messina L, Bruno F, Licata P, et al. Snail Mucus Filtrate Reduces Inflammation in Canine Progenitor Epidermal Keratinocytes (CPEK). Animals. 2022;12(14). doi:10.3390/ani12141848
CrossRef - Riana Septiwidyati T, Winiati Bachtiar E. The Role of Porphyromonas gingivalis Virulence Factors in Periodontitis Immunopathogenesis (Peran Faktor Virulensi Porphyromonas Gingivalis pada Imunopatogenesis Periodontitis). Dentika Dental Journal. 2020;23(1):7-12.
CrossRef - Hendrawati H, Agustha HN, Sari R. Topical application of snail mucin gel enhances the number of osteoblasts in periodontitis rat model. Dental Journal (Majalah Kedokteran Gigi). 2019;52(2):61-65. doi:10.20473/j.djmkg.v52.i2.p61-65
CrossRef - Brennan CA, Garrett WS. Fusobacterium nucleatum — symbiont, opportunist and oncobacterium. Nat Rev Microbiol. 2019;17(3):156-166. doi:10.1038/s41579-018-0129-6
CrossRef - Dai X, Ma R, Jiang W, et al. Enterococcus faecalis -Induced Macrophage Necroptosis Promotes Refractory Apical Periodontitis . Microbiol Spectr. 2022;10(4). doi:10.1128/spectrum.01045-22
CrossRef - Suárez L, Pereira A, Hidalgo W, Uribe N. Antibacterial, antibiofilm and anti-virulence activity of biactive fractions from mucus secretion of giant african snail achatina fulica against staphylococcus aureus strains. Antibiotics. 2021;10(12). doi:10.3390/antibiotics10121548
CrossRef - Harti AS, Murharyati A, Dwi Sulisetyawati S, Oktariani M. The effectiveness of snail mucus (Achatina fulica) and chitosan toward limfosit proliferation in vitro. Asian Journal of Pharmaceutical and Clinical Research. 2018;11(Special Issue 3):85-88. doi:10.22159/ajpcr.2018.v11s3.30041
CrossRef - Okeniyi FA, Oghenochuko OM, Olawoye SO, Animashahun RA, Adeyonu AG, Akpor OB. Antimicrobial potentials of mucus mucin from different species of giant African land snails on some typed culture pathogenic bacteria. Asian Journal of Agriculture and Biology. 2022;2022(4). doi:10.35495/ajab.2021.07.294
CrossRef - Kamiloglu S, Sari G, Ozdal T, Capanoglu E. Guidelines for cell viability assays. Food Front. 2020;1(3):332-349. doi:10.1002/fft2.44
CrossRef - S AAFG, Darwis W, Wibowo RH, Supriati R. Original article Antibacterial activity of the ethanolic extract of Sembung Rambat ( Mikania micrantha Kunth ) leaves against Bacillus subtilis. Journal of Science and Technology. 2021;10(1):6-11. doi:10.22487/25411969.2020.v10.i1.15476
CrossRef - Enomoto M, Iwata H, Iida M. Contribution of toxicologic pathologists for the safety of human health in biomedical research—past, present, and future of the jstp. J Toxicol Pathol. 2021;34(4):275-282. doi:10.1293/tox.2021-0028
CrossRef - Loko LEY, Medegan Fagla S, Orobiyi A, et al. Traditional knowledge of invertebrates used for medicine and magical-religious purposes by traditional healers and indigenous populations in the Plateau Department, Republic of Benin. J Ethnobiol Ethnomed. 2019;15(1):1-21. doi:10.1186/s13002-019-0344-x
CrossRef - Van Tonder A, Joubert AM, Cromarty AD. Limitations of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay when compared to three commonly used cell enumeration assays. BMC Res Notes. 2015;8(1):1-10. doi:10.1186/s13104-015-1000-8
CrossRef - Nirwana I, Munadziroh E, Yogiartono R, Thiyagu C, Ying C, DInaryanti A. Cytotoxicity and proliferation evaluation on fibroblast after combining calcium hydroxide and ellagic acid. J Adv Pharm Technol Res. 2021;12(1):27-31. doi:10.4103/japtr.JAPTR_154_20
CrossRef - Gugliandolo E, Macrì F, Fusco R, et al. The protective effect of snail secretion filtrate in an experimental model of excisional wounds in mice. Vet Sci. 2021;8(8). doi:10.3390/vetsci8080167
CrossRef - McDermott M, Cerullo AR, Parziale J, et al. Advancing Discovery of Snail Mucins Function and Application. Front Bioeng Biotechnol. 2021;9. doi:10.3389/fbioe.2021.734023
CrossRef - Śliwka L, Wiktorska K, Suchocki P, et al. The comparison of MTT and CVS assays for the assessment of anticancer agent interactions. PLoS One. 2016;11(5). doi:10.1371/journal.pone.0155772
CrossRef - Rai Y, Pathak R, Kumari N, et al. Mitochondrial biogenesis and metabolic hyperactivation limits the application of MTT assay in the estimation of radiation induced growth inhibition. Sci Rep. 2018;8(1):1-15. doi:10.1038/s41598-018-19930-w
CrossRef - Trinh XT, Long N van, van Anh LT, et al. A Comprehensive Review of Natural Compounds for Wound Healing: Targeting Bioactivity Perspective. Int J Mol Sci. 2022;23(17). doi:10.3390/ijms23179573
CrossRef - Maharani LA, Rosihan A, Diah S. Uji Sitotoksisitas Ekstrak Metanol Batang Pisang Mauli (Musa Sp) Terhadap Sel Fibroblas BHK (Baby Hamster Kidney) 21. Dentino Jurnal Kedokteran. 2014;2(2):210-214.
CrossRef - Khan ZA, Siddiqui MF, Park S. Current and emerging methods of antibiotic susceptibility testing. Diagnostics. 2019;9(2). doi:10.3390/diagnostics9020049
- Gajic I, Kabic J, Kekic D, et al. Antimicrobial Susceptibility Testing: A Comprehensive Review of Currently Used Methods. Antibiotics. 2022;11(4). doi:10.3390/antibiotics11040427
CrossRef - Hombach M, Zbinden R, Böttger EC. Standardisation of disk diffusion results for antibiotic susceptibility testing using the sirscan automated zone reader. BMC Microbiol. 2013;13(1):1-8. doi:10.1186/1471-2180-13-225
CrossRef - Benkova M, Soukup O, Marek J. Antimicrobial Susceptibility Testing: Currently Used Methods and Devices and the near Future in Clinical Practice. Vol 129.; 2020. doi:10.1111/jam.14704
CrossRef - Sawatzky P, Liu G, Dillon JAR, et al. Quality assurance for antimicrobial susceptibility testing of neisseria gonorrhoeae in Canada, 2003 to 2012. J Clin Microbiol. 2015;53(11):3646-3649. doi:10.1128/JCM.02303-15
CrossRef - Rahayu E, Lahay N. Antibacterial Inhibition Extract of Moringa Leaf and Basil Leaf Antibacterial Inhibition Test Against the Combination Extract of Moringa Leaf (Moringa oliefera) and Basil Leaf (Ocimum basilicum) as a Substitute for Feed Additive. Hasanuddin J Anim Sci. 2021;3(2):85-94. doi:10.20956/hajas.V3i2.20074
- Nassar MSM, Hazzah WA, Bakr WMK. Evaluation of antibiotic susceptibility test results: How guilty a laboratory could be? Journal of the Egyptian Public Health Association. 2019;94(1):1-5. doi:10.1186/s42506-018-0006-1
CrossRef - Yang K, Li HZ, Zhu X, et al. Rapid Antibiotic Susceptibility Testing of Pathogenic Bacteria Using Heavy-Water-Labeled Single-Cell Raman Spectroscopy in Clinical Samples. Anal Chem. 2019;91(9):6296-6303. doi:10.1021/acs.analchem.9b01064
CrossRef







