Aliaa M. Radwan1* , Mustafa Karhib2, Shahenda A. Fatoh3, Ahmed Massoud3
, Mustafa Karhib2, Shahenda A. Fatoh3, Ahmed Massoud3 and Ehab Tousson3
and Ehab Tousson3
1Biochemistry Division, Chemistry Department, Faculty of Science, Tanta University, Egypt.
2Department of Medical Laboratory Techniques, Al-Mustaqbal University College, 51001 Hillah, Babylon, Iraq
3Zoology Department, Faculty of Science, Tanta University, Egypt.
Corresponding Author E-mail: alyaa_radwan@science.tanta.edu.eg
DOI : https://dx.doi.org/10.13005/bpj/2625
Abstract
Thioacetamide (TAA), well-known as a toxic agent and has been reported to be nephrotoxic due to oxidative stress induction and proinflammatory markers increase. Curcumin has been shown in various studies to have antioxidant and anti-inflammatory properties. This study aimed to investigate the ameliorative effect of curcumin against TAA-induced kidney toxicity in rats. In this study, 28 male albino rats were used, and kidney toxicity was induced by intraperitoneal injection of TAA at a dose of 200mg/kg, twice a week for 8 weeks then the rats were treated with curcumin at a dose of 200mg/kg, orally, daily for 2 weeks. Kidney functions in all the experimental groups as well as oxidative stress (MDA), catalase (CAT), and superoxide dismutase (SOD) were determined. In addition, DNA damage and the expression status of both proinflammatory cytokine (TNFα) and proliferating cell nuclear antigen (PCNA) were evaluated. Further, light microscopic studies were performed on kidney specimens. Our results demonstrated that TAA had a nephrotoxic effect, as evidenced by significantly increase in kidney functions as well as substantially increase in renal MDA associated with reduction in CAT and SOD antioxidant enzymes compared to control group. The administration of curcumin ameliorates the oxidative stress and upregulate the antioxidant parameters. Further, a signification increases in DNA damage, TNFα, and PCNA expression was seen in TAA-group which was then alleviated by curcumin treatment. In conclusion, the ameliorative effect of curcumin could be attributed to its ability to minimize oxidative stress, renal cell injury, and cytokine release.
Keywords
Antioxidant enzyme; Curcumin; Kidney toxicity; Oxidative stress; Thioacetamide; TNFα
Download this article as:| Copy the following to cite this article: Radwan A. M, Karhib M, Fatoh S. A, Massoud A, Tousson E. Curcumin Alleviates Thioacetamide-Induced Kidney Toxicity in Rats: Enhancing Antioxidant System, and Attenuating Oxidative Stress, DNA Damage, and Inflammation. Biomed Pharmacol J 2023;16(1). |
| Copy the following to cite this URL: Radwan A. M, Karhib M, Fatoh S. A, Massoud A, Tousson E. Curcumin Alleviates Thioacetamide-Induced Kidney Toxicity in Rats: Enhancing Antioxidant System, and Attenuating Oxidative Stress, DNA Damage, and Inflammation. Biomed Pharmacol J 2023;16(1). Available from: https://bit.ly/3I1JSKO |
Introduction
Thioacetamide (TAA) is a sulfur-containing compound commonly used in a variety of technical applications as pesticides and fungicides.1,2 TAA exposure to humans occurs through the generation of toxic fumes that are inhaled, ingested, or absorbed through the skin.3 TAA toxicity results from its rapid metabolism in the liver by monooxygenases to thioacetamide-S-oxide, resulting in the generation of peroxide radicals and further reactive oxygen species (ROS) production.4
ROS overwhelm the antioxidant defense mechanism, causing oxidation reactions to occur and causing damage to cellular biomolecules such as proteins, lipids, and DNA.5 As a result, oxidative stress occurs, leading to impairment of cellular structure and functions.6 TAA has been shown to be hepatotoxic and nephrotoxic and has been used for liver injury and renal toxicity in experimental models.7,8 Consequently, antioxidants, or free radical scavengers, have received a lot of attention in the prevention and treatment of TAA-induced liver and kidney damage.9
Several studies have been conducted to identify complementary medications to treat a variety of human ailments.10-13 The safety and possible therapeutic value of certain phytochemicals have been shown.14-17 Curcumin is a polyphenolic, yellow compound that was originally extracted from the Curcuma longa plant.18,19 For centuries, it has been used in traditional medicine as well as in food as a stabilizing agent and spice.20-22 It has been reported that curcumin has a variety of beneficial properties, including antimicrobial, anticancer, anti-inflammatory, and antioxidant.23 It can also act as a free radical scavenger, prevent ROS formation, and reduce oxidative stress.24
Many kidney disorders have an oxidative stress component to their pathophysiology, and oxidative stress, its mediators, and inflammation all play a role in the development of many of these diseases’ consequences.25-27 As a result, the current study sought to investigate curcumin’s ameliorative effects against TAA-induced renal toxicity in rats by measuring kidney functions, antioxidant status, oxidative stress marker, and the pro-inflammatory cytokine TNF-α.
Materials and Methods
Materials
Thioacetamide (99.0%) and curcumin were purchased from ADVENT CHEMBIO (Mumbai, India). All other chemicals involved in this study were of analytical grade.
Methods
Animals and Experimental protocol
A total of 28 male albino rats weighing 120-130 g were taken from the breeding unit of the Egyptian Organization for Biological Products and Vaccines (Abbassia, Cairo), kept in polypropylene cages at room temperature with a 12-hour light–dark cycle, and given a standard diet as well as unlimited access to tap water. The study was approved by the Research Ethical Committee (Faculty of Science, Tanta University, Egypt). The ethical committee’s number is IACUC-SCI-TU-0198.
The rats were equally divided into 4 different groups (n = 7) and categorized as follows: Group I: control group received saline as vehicle; Group II: curcumin group received curcumin daily at dose of (200 mg/kg body weight) by oral gavage for 2 weeks;28 Group III: TAA group injected intraperitoneally with 200 mg/kg body weight twice per week for 8 weeks;29 and Group IV: TAA+curcumin group injected intraperitoneally day after day with 200 mg/kg body weight thioacetamide for 8 weeks then treated with curcumin for 2 weeks.
At the end of the experiment, 40 mg/kg body weight of pentobarbital was used to anaesthetize the overnight-fasted rats. Then, blood and tissue samples were collected for further analysis.
Serum biochemical parameters
The blood samples were collected, clotted at room temperature, and the serum was extracted using a cooling centrifuge for 15 min at 3000 rpm. A commercial kit from Diamond, Egypt, was used to measure serum creatinine and urea after Patton and Crouch.30 The method planned by Abd Eldaim et al.31 was surveyed to determine potassium, calcium, sodium, and chloride ions levels using marketable kits of Indian Sensa-core electrolyte.
Tissue oxidative stress and antioxidant parameters
The kidney tissues from different experimental groups were excised, washed, and homogenized in saline, then centrifuged for 5 min at 4000 rpm. The resulting suspension was collected and stored at -80oC for further assessment. Biodiagnostic kits from Egypt were used in this study for the estimation of kidney malondialdehyde (MDA) and reduced glutathione (GSH) levels according to the methods of Ohkawa et al.32 and El-Aarag et al.33 respectively.
The activity of CAT was assessed spectrophotometrically at 240 nm by estimating the degradation rate of H2O2 as described by Aldubayan et al.34 Inhibiting adrenaline auto-oxidation to adrenochrome in an alkaline medium is the main strategy used to determine superoxide dismutase (SOD) activity as mentioned by Beauchamp and Fridovich.35
Comet Assay
The comet assay (single cell gel electrophoresis) method was used to analyze and quantify DNA damage in the kidney tissues from the various study groups according to Mutar et al.36
Histological processing
The kidney was promptly removed after necropsy and preserved for 24–48 hours by immersion in a 10% neutral buffered formalin solution. Following dehydration and paraffin embedding, serial slices (5 µm thick) were cut using a rotary microtome (Litz, Wetzlar, Germany). The sections were examined and photographed under a light microscope after being stained with haematoxylin and eosin.37
Immunohistochemical Studies
The avidin-biotin complex (ABC) kit (Elite–ABC, Vector Laboratories, CA, USA) with specific antibodies was used for detection of proliferating cell nuclear antigen (PCNA) (dilution 1:100, DAKO Japan Co, Ltd, Tokyo, Japan) and tumor necrosis factor alfa (TNFα) (dilution 1:200; DAKO Japan Co, Tokyo, Japan) in kidney sections as described by Tousson et al.38 and Elbandrawy et al.39 respectively. The brown hue of the stained cells was recovered using Image J’s color thresholding feature for quantitation.
Statistical analysis
Results were analysed using GraphPad Prism 8 software. Data are presented as mean ± standard error of mean (SEM). A one-way ANOVA followed by Tukey’s multiple comparison test was used to analyse the differences between groups. Differences were significant at p < 0.05.
Results
Impact of TAA and curcumin on kidney functions
Table 1 shows that the TAA group has a significant increase in urea, creatinine, potassium, and chloride ions with a considerable decline (p˂0.0001) in sodium and calcium levels when compared to the control group. TAA+curcumin treated group exhibited a remarkable reduction (p˂0.01, p˂0.05) in urea, creatinine, and chloride ions level relative to TAA group, however, the values were still markedly different from the control group.
Table 1: Effect of curcumin on serum kidney functions in TAA-intoxicated rats.
|
Groups |
Serum parameters | |||||
| Creatinine
(mg/dl) |
Urea
(mg/dl) |
Na+ ions
(mEq/L) |
K + ions
(mEq/L) |
Ca2+ions
(mEq/L) |
Cl– ions
(mEq/L) |
|
| Control | 0.47 ± 0.02799 | 31.58 ± 2.53 | 135 ± 0.65 | 4.11 ± 0.11 | 1.20 ± 0.007 | 100.5±3.22 |
| Curcumin | 0.45 ± 0.0228 | 32.65 ± 1.80 | 136.3 ± 0.4 | 3.91 ± 0.09 | 1.20 ± 0.009 | 98.5 ± 4.05 |
| TAA | 0.92* ± 0.0149 | 80.25* ± 3.03 | 82.5* ± 3.22 | 7.53* ± 0.54 | 0.33* ± 0.041 | 142.5* ± 3.2 |
| TAA + Curcumin | 0.81*# ± 0.0250 | 63.03*# ± 1.98 | 87.5* ± 3.22 | 6.46* ± 0.38 | 0.41* ± 0.057 | 127.5*# ± 2.3 |
Data are expressed as mean±SE. p˂0.05 is statistically significant, where *p and #p differences from control and TAA groups respectively.
Effect of TAA and curcumin on lipid peroxidation and antioxidant status
The renal MDA as indicator for tissue lipid peroxidation was observed to be significantly higher (P˂0.0001) in TAA-treated group compared to control. TAA-intoxicated rats treated with curcumin, on the other hand, showed a remarkable reduction (P˂0.0001) in MDA level compared to the TAA group. Furthermore, the level of GSH as well as the antioxidant activity of CAT and SOD enzymes were found to be markedly lower in TAA-intoxicated rats as compared with control. The treatment of TAA-intoxicated rats with curcumin (TAA + Curcumin) demonstrated a significant increase (P˂0.001) in non-enzymatic and enzymatic antioxidant parameters compared to TAA group (Table 2).
Table 2: Effect of curcumin on oxidative stress and antioxidant system in TAA-intoxicated rats
|
Groups |
Parameters | |||
| MDA
(µmol/g tissue) |
GSH
(µmol/g tissue) |
CAT
(U/g protein) |
SOD
(U/g protein) |
|
| Control | 0.373 ± 0.024 | 1.523 ± 0.101 | 3.133 ± 0.2603 | 3.473 ± 0.1467 |
| Curcumin | 0.366 ± 0.026 | 1.58 ± 0.0737 | 3.2 ± 0.2309 | 3.4 ± 0.127 |
| TAA | 1.458* ± 0.039 | 0.4643* ± 0.08 | 0.75* ± 0.0577 | 0.88* ± 0.05774 |
| TAA + Curcumin | 0.9083*# ± 0.07 | 1.312# ± 0.035 | 2.31# ± 0.2548 | 2.42*# ± 0.170 |
Data are expressed as mean±SE. p˂0.05 is statistically significant, where *p and #p differences from control and TAA groups respectively.
Effect of TAA and curcumin on DNA damage in kidney tissue
The results in Figure 1 demonstrated no significant change in DNA damage (tail length) in kidney tissues isolated from control and curcumin groups. Moreover, a remarkable increase (p˂0.0001) in DNA damage was found in rats treated with TAA alone compared to the control group. TAA+curcumin treated group, on the other hand, revealed a significant reduction (p˂0.0001) in DNA damage in comparison to TAA group.
Impact of TAA and curcumin in kidney structure
Normal histopathological structures of the glomeruli and tubules in the cortical and medullary portions were observed in the kidney sections of control and curcumin groups (Figures 2A&2B). In contrast, kidney section in treated rats with thioacetamide showed atrophy of the renal corpuscles with damage of Bowman’s capsules, severe tubular necrosis and atrophy and moderate inflammatory cellular infiltration (Figure 2C). On the other hand, kidney sections in post treated thioacetamide with curcumin (thioacetamide + curcumin group) exhibited moderate tubular necrosis and atrophy with mild inflammatory cellular infiltration (Figure 2D).
PCNA expression changes in kidney
Regarding PCNA expressions (nuclear reaction) in kidney sections in control and Cur groups showed faint to mild positive reactions in glomeruli and tubules in the cortical and medullary portions (Figs. 3A&3B). In contrast, kidney section in treated rats with thioacetamide showed strong positive reaction for PCNA (Fig. 3C). On the contrary, moderate positive reactions for PCNA was shown in thioacetamide+curcumin (Fig. 3D).
TNFα expression changes in kidney
Regarding TNFα expressions (cytoplasmic reaction) in kidney sections in control and Cur groups showed negative or faint positive reactions in glomeruli and tubules in the cortical and medullary portions (Figs. 4A&4B). In contrast, kidney section in treated rats with thioacetamide showed a moderately positive reaction for TNFα in glomeruli (Fig. 4C). On the contrary, mild positive reactions for TNFα was shown in thioacetamide+curcumin (Fig. 4D).
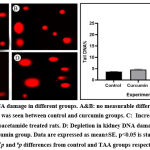 |
Figure 1: Kidney DNA damage in different groups. A and B: no measurable difference in kidney DNA damage (tail length) was seen between control and curcumin groups. |
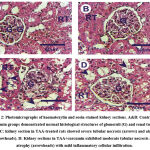 |
Figure 2: Photomicrographs of haematoxylin and eosin-stained kidney sections. A and B: Control and curcumin groups demonstrated normal histological structures of glomeruli (G) and renal tubules (RT). |
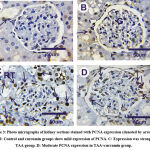 |
Figure 3: Photo micrographs of kidney sections stained with PCNA expression (denoted by arrows). A and B: Control and curcumin groups show mild expression of PCNA. |
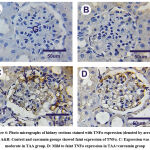 |
Figure 4: Photo micrographs of kidney sections stained with TNFα expression (denoted by arrows). A&B: Control and curcumin groups showed faint expression of TNFα. |
Discussion
The provoking of oxidative stress is the main reason of thioacetamide’s toxic effect on tissues such as brain, liver, and kidney which is used as a metal sulphide source. TAA toxicity is characterized by increased lipid peroxidation and reactive oxygen species liberation.40 In the current study, treatment with curcumin against kidney toxicity induced by TAA was studied in terms of antioxidant parameters, histological and immunohistochemical changes. TAA resulting in biochemical imbalances in kidney markers and functions. A marked increase in urea, creatinine, potassium and chloride ions levels were observed in TAA group compared to the normal healthy rats in the control group. Curcumin treatment caused noticeable recovery of these kidney function tests. Previously, similar findings were also reported.19,21 Curcumin has been shown to reduce tissue injury caused by certain toxins by hindering the cytochrome P450 (CYP) enzymes that convert these toxins into harmful compounds.41 Curcumin, on the other hand, has no effect on the activity of CYP2E1, the major enzyme responsible for TAA metabolism.42,43 From the data obtained in this study, we confirmed that curcumin reduce the pathological effects of TAA metabolites.
Furthermore, oxidative stress induction in the present TAA-treated group has been demonstrated by a decrease in kidney GSH content as well as an increase in MDA level.44 These findings are consistent with previous research, which found that TAA has an oxidative stress potency that surpasses the ability of the body’s antioxidative and protective mechanisms, resulting in damage and injury of various organs including liver and kidney.45-47 This was also confirmed by measuring the activity of antioxidant enzymes.48,49 The obtained data revealed that injection of TAA reduce the antioxidant capacity of rats represented in catalase and superoxide dismutase.
Curcumin treatment resulted in a prominent reduction of kidney MDA content, together with enhancing GSH level, CAT, and SOD antioxidant activities, confirming the antioxidant potential and anti-lipid peroxidative effect of curcumin.50 Our findings are in agreement with previous study which reported that ethanol extract of C. longa rhizomes ameliorated the TAA-suppressive impact on antioxidant enzymes SOD and CAT by keeping their activity at higher levels.21 By optimizing the level of renal antioxidant enzymes, oxidative stress caused by TAA toxicity was reduced. The antioxidant activity of curcumin may be recognized to the presence of different antioxidant functional groups such as phenyl rings, β-diketo group, and carbon-carbon double bonds in its structure.51
TAA-induced kidney toxicity has been described as multifaceted, involving several mechanisms, including covalent binding of TAA metabolites to renal cells macromolecules causing DNA damage.1,52 In the present study, higher DNA damage measured by comet assay was observed in rats injected with TAA. The administartion of curcumin to TAA-intoxicated rats significantly inhibited DNA damage. Furthermore, proinflammatory cytokine TNF-α was found to be elevated in TAA-intoxicated rats, indicating a high inflammatory state.53 Curcumin administration to the rats decreased the release of cytokines, supporting previous studies on curcumin’s inhibitory effects on TNF-α expression.54 Moreover, oral administration of curcumin to TAA-intoxicated rats for two weeks reduced renal proliferation and regeneration, as evidenced by a substantial decrease in PCNA positive-stained cells in kidney sections. These results supported previous findings that curcumin inhibited hepatocyte proliferation.20,55-57 These results were corroborated by histopathological findings that revealed the amelioration of kidney damage and inflammation in TAA-intoxicated rats treated with curcumin.
Conclusion
In conclusion, the current study indicated that curcumin reverse TAA-induced kidney toxicity by boosting the antioxidant system and alleviating oxidative stress, DNA damage, and inflammation. As a result, curcumin sheds light on potential therapies to prevent thioacetamide toxicity. However, due to curcumin’s low solubility and bioavailability, more research is needed to investigate the ameliorative and protective role of nanocurcumin.
Conflict of Interest
The authors declare no conflict of interest.
Funding Sources
There is no funding sources.
References
- Saggu S, Sakeran MI, Zidan N, Tousson E, Mohan A, Rehman H. Ameliorating effect of chicory (Chichorium intybus L.) fruit extract against 4-tert-octylphenol induced liver injury and oxidative stress in male rats. Food Chem. Toxicol., 2014; 72: 138-146.
CrossRef - Ghosh S, Sarkar A, Bhattacharyya S, Sil PC. Silymarin protects mouse liver and kidney from thioacetamide induced toxicity by scavenging reactive oxygen species and activating PI3K-Akt pathway. Front Pharmacol., 2016; 7: 481.
CrossRef - Apte UM, editor. Liver regeneration: basic mechanisms, relevant models and clinical applications. Academic Press; 2015.
- Stehbens WE. Oxidative stress, toxic hepatitis, and antioxidants with particular emphasis on zinc. Exp. Mol. Pathol., 2003; 75(3) :265-76.
CrossRef - Aljerf L, Williams M, Ajong AB, Onydinma UP, Dehmchi F, Pham VT, Bhatnagar S, Belboukhari N. Rev Chim. Comparative study of the biochemical response behavior of some highly toxic minerals on selenosis in rats. Chim., 2021; 72(2): 9-18.
CrossRef - Zaki NI, Abdelmoniem MM. Biochemical and molecular study on the beneficial effect of Solubilized coenzyme Q10 on thioacetamide-induced liver fibrosis in adult male rats. Egypt. J. Chem., 2021; 64(11): 6869-79.
CrossRef - Moustafa AH, Ali EM, Moselhey SS, Tousson E, El-Said KS. Effect of coriander on thioacetamide-induced hepatotoxicity in rats. Toxicol. Ind. Health., 2014; 30(7): 621-9.
CrossRef - Al-Attar AM, Alrobai AA, Almalki DA. Protective effect of olive and juniper leaves extracts on nephrotoxicity induced by thioacetamide in male mice. Saudi J. Biol. Sci., 2017; 24(1): 15-22.
CrossRef - Rice-Evans CA, Burdon RH. Free radical damage and its control. Elseiver. 1994: 113-130.
- Okada FK, Stumpp T, Miraglia SM. Carnitine diminishes etoposide toxic action on spermatogonial self-renewal and sperm production in adult rats treated in the prepubertal phase. J. Histochem. Cytochem., 2020; 68(5): 327-342.
CrossRef - Saad B, Azaizeh H, Said O. Tradition and perspectives of Arab herbal medicine: a review. Evid. Based Complement Alternat. Med., 2005; 2(4): 475-9.
CrossRef - Taylor RC. Alternative medicine and the medical encounter in Britain and the United States. Alternative Medicines. Routledge, 2022. 191-228.
CrossRef - Moura FA, de Andrade KQ, Dos Santos JC, Araújo OR, Goulart MO. Antioxidant therapy for treatment of inflammatory bowel disease: does it work?. Redox Biol., 2015; 6: 617-39.
CrossRef - Hussain Z, Thu HE, Shuid AN, Kesharwani P, Khan S, Hussain F. Phytotherapeutic potential of natural herbal medicines for the treatment of mild-to-severe atopic dermatitis: A review of human clinical studies. Biomed. Pharmacother., 2017; 93: 596-608.
CrossRef - Jaradat N, Zaid AN. Herbal remedies used for the treatment of infertility in males and females by traditional healers in the rural areas of the West Bank/Palestine. BMC Complement Altern. Med., 2019; 19(1): 1-2.
CrossRef - Abdel-Daim MM, Eissa IA, Abdeen A, Abdel-Latif HM, Ismail M, Dawood MA, Hassan AM. Lycopene and resveratrol ameliorate zinc oxide nanoparticles-induced oxidative stress in Nile tilapia, Oreochromis niloticus. Environ. Toxicol. Pharmacol., 2019; 69: 44-50.
CrossRef - Colson CR, De Broe ME. Kidney injury from alternative medicines. Adv. Chronic Kidney Dis., 2005; 12(3): 261-75.
CrossRef - El-Agamy DS. Comparative effects of curcumin and resveratrol on aflatoxin B1-induced liver injury in rats. Arch. Toxicol., 2010; 84(5): 389-96.
CrossRef - Salem NI, Noshy MM, Said AA. Modulatory effect of curcumin against genotoxicity and oxidative stress induced by cisplatin and methotrexate in male mice. Food Chem. Toxicol., 2017; 105: 370-376.
CrossRef - Wang ME, Chen YC, Chen IS, Hsieh SC, Chen SS, Chiu CH. Curcumin protects against thioacetamide-induced hepatic fibrosis by attenuating the inflammatory response and inducing apoptosis of damaged hepatocytes. J. Nutr. Biochem., 2012; 23(10): 1352-66.
CrossRef - Zhu Q, Zeng J, Li J, Chen X, Miao J, Jin Q, Chen H. Effects of compound Centella on oxidative stress and Keap1-Nrf2-ARE pathway expression in diabetic kidney disease rats. Evid. Based Complement Alternat. Med., 2020; 2020.
CrossRef - Kelly GS. Clinical applications of N-acetylcysteine. Altern. Med. Rev., 1998; 3(2): 114-27.
CrossRef - Prasad S, Tyagi AK, Aggarwal BB. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res. Treat., 2014; 46(1): 2-18.
CrossRef - Betancor-Fernández A, Pérez-Gálvez A, Sies H, Stahl W. Screening pharmaceutical preparations containing extracts of turmeric rhizome, artichoke leaf, devil’s claw root and garlic or salmon oil for antioxidant capacity. J. Pharm. Pharmacol., 2003; 55(7): 981-6.
CrossRef - Salama AF, Kasem SM, Tousson E, Elsisy MK. Protective role of L-carnitine and vitamin E on the kidney of atherosclerotic rats. Biomed. Aging Pathol., 2012; 2(4): 212-5.
CrossRef - El-Moghazy M, Zedan NS, El-Atrsh AM, El-Gogary M, Tousson E. The possible effect of diets containing fish oil (omega-3) on hematological, biochemical and histopathogical alterations of rabbit liver and kidney. Biomed. Prev. Nutr., 2014; 4(3): 371-7.
CrossRef - Izzularab BM, Tousson E, Abdo NI, Beltagy DM. Curative Consequences of Rocket Seeds (Eruca Sativa) Extract against Lead Nanoparticles Induced Renal Dysfunction in Rats. Biomed. Pharmacol. J., 2022; 15(1): 147-56.
CrossRef - Shapiro H, Ashkenazi M, Weizman N, Shahmurov M, Aeed H, Bruck R. Curcumin ameliorates acute thioacetamide‐induced hepatotoxicity. J. Gastroenterol. Hepatol., 2006; 21(2): 358-66.
CrossRef - Furtado KS, Prado MG, Aguiar e Silva MA, Dias MC, Rivelli DP, Rodrigues MA, Barbisan LF. Coffee and caffeine protect against liver injury induced by thioacetamide in male Wistar rats. Basic Clin. Pharmacol. Toxicol., 2012; 111(5): 339-47.
CrossRef - Patton CJ, Crouch SR. Spectrophotometric and kinetics investigation of the Berthelot reaction for the determination of ammonia. Anal. Chem., 1977; 49(3): 464-9.
CrossRef - Abd Eldaim MA, Tousson E, El Sayed IE, Awd WM. Ameliorative effects of Saussurea lappa root aqueous extract against Ethephon‐induced reproductive toxicity in male rats. Environ. Toxicol., 2019; 34(2): 150-9.
CrossRef - Ohkawa H, Ohishi W, Yagi K. Colorimetric method for determination of MDA activity. Biochemistry. 1979; 95: 351.
CrossRef - El-Aarag B, Attia A, Zahran M, Younes A, Tousson E. New phthalimide analog ameliorates CCl4 induced hepatic injury in mice via reducing ROS formation, inflammation, and apoptosis. Saudi J. Biol. Sci., 2021; 28(11): 6384-95.
CrossRef - Aldubayan MA, Elgharabawy RM, Ahmed AS, Tousson E. Antineoplastic Activity and Curative Role of Avenanthramides against the Growth of Ehrlich Solid Tumors in Mice. Oxid. Med. Cell Longev., 2019; 2019.
CrossRef - Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem., 1972., 247(10): 3170-5.
CrossRef - Mutar TF, Tousson E, Hafez E, Gazia MA, Salem SB. Ameliorative effects of vitamin B17 on the kidney against Ehrlich ascites carcinoma induced renal toxicity in mice. Environ. Toxicol., 2020; 35(4): 528-537.
CrossRef - Abosharaf HA, Salah M, Diab T, Tsubaki M, Mohamed TM. Biogenic silver nanoparticles induce apoptosis in Ehrlich ascites carcinoma. Biomed. Res. Ther., 2020; 7(11): 4100-4113.
CrossRef - Tousson E, Ali EM, Ibrahim W, Mansour MA. Proliferating cell nuclear antigen as a molecular biomarker for spermatogenesis in PTU-induced hypothyroidism of rats. Reprod. Sci., 2011; 18(7): 679-86.
CrossRef - Elbandrawy MM, Sweef O, Elgamal D, Mohamed TM, Elgharabawy RM. Ellagic acid regulates hyperglycemic state through modulation of pancreatic IL-6 and TNF-α immunoexpression. Saudi J. Biol. Sci., 2022; 29(5): 3871-3880.
CrossRef - Türkmen NB, Hande YÜ, TAŞLIDERE A, Şahin Y, Çiftçi O. The Ameliorate effects of nerolidol on thioacetamide-induced oxidative damage in heart and kidney tissue. Turk. J. Pharm. Sci., 2022; 19(1): 1-8.
CrossRef - Iqbal M, Sharma SD, Okazaki Y, Fujisawa M, Okada S. Dietary supplementation of curcumin enhances antioxidant and phase II metabolizing enzymes in ddY male mice: possible role in protection against chemical carcinogenesis and toxicity. Pharmacol. Toxicol., 2003; 92(1): 33-8.
CrossRef - de David C, Rodrigues G, Bona S, Meurer L, Gonzalez-Gallego J, Tunon MJ, Marroni NP. Role of quercetin in preventing thioacetamide-induced liver injury in rats. Toxicol. Pathol., 2011; 39(6): 949-57.
CrossRef - Fatima SN, Masood J. Fenugreek seeds attenuate thioacetamide induced liver damage. Pak. J. Pharm. Sci., 2021; 34(3): 933-942.
- El Awdan SA, Abdel Rahman RF, Ibrahim HM, Hegazy RR, El Marasy SA, Badawi M, Arbid MS. Regression of fibrosis by cilostazol in a rat model of thioacetamide-induced liver fibrosis: Up regulation of hepatic cAMP, and modulation of inflammatory, oxidative stress and apoptotic biomarkers. Plos one., 2019; 14(5): e0216301.
CrossRef - Pallottini V, Martini C, Bassi AM, Romano P, Nanni G, Trentalance A. Rat HMGCoA reductase activation in thioacetamide-induced liver injury is related to an increased reactive oxygen species content. J. Hepatol., 2006; 44(2): 368-74.
CrossRef - Said AM, Waheed RM, Khalifa OA. Protective role of rosemary ethanolic extract on thioacetamide induced hepatic encephalopathy: Biochemical and molecular studies. Aust. J. Basic Appl. Sci., 2019; 13(4) :1-6.
- l Awdan SA, Abdel Rahman RF, Ibrahim HM, Hegazy RR, El Marasy SA, Badawi M, Arbid MS. Regression of fibrosis by cilostazol in a rat model of thioacetamide-induced liver fibrosis: Up regulation of hepatic cAMP, and modulation of inflammatory, oxidative stress and apoptotic biomarkers. Plos one. 2019; 14(5): e0216301.
CrossRef - Narasimhaiah M, Arunachalam A, Sellappan S, Mayasula VK, Guvvala PR, Ghosh SK, Chandra V, Ghosh J, Kumar H. Organic zinc and copper supplementation on antioxidant protective mechanism and their correlation with sperm functional characteristics in goats. Reprod. Domest. Anim., 2018; 53(3): 644-54.
CrossRef - Bano K, Kumar B, Alyemeni MN, Ahmad P. Protective mechanisms of sulfur against arsenic phytotoxicity in Brassica napus by regulating thiol biosynthesis, sulfur-assimilation, photosynthesis, and antioxidant response. Plant Physiol. Biochem., 2022; 188: 1-11.
CrossRef - Gangarapu V, Gujjala S, Korivi R, Pala I. Combined effect of curcumin and vitamin E against CCl4 induced liver injury in rats. Am. J. Life Sci., 2013; 1(3): 117-124.
CrossRef - Farzaei MH, Zobeiri M, Parvizi F, El-Senduny FF, Marmouzi I, Coy-Barrera E, Naseri R, Nabavi SM, Rahimi R, Abdollahi M. Curcumin in liver diseases: a systematic review of the cellular mechanisms of oxidative stress and clinical perspective. Nutrients., 2018; 10(7): 855.
CrossRef - Chilakapati J, Korrapati MC, Hill RA, Warbritton A, Latendresse JR, Mehendale HM. Toxicokinetics and toxicity of thioacetamide sulfoxide: a metabolite of thioacetamide. Toxicology., 2007; 230(2-3): 105-16.
CrossRef - Khalil HM, Eliwa HA, El-Shiekh RA, Al-Mokaddem AK, Hassan M, Tawfek AM, El-Maadawy WH. Ashwagandha (Withania somnifera) root extract attenuates hepatic and cognitive deficits in thioacetamide-induced rat model of hepatic encephalopathy via induction of Nrf2/HO-1 and mitigation of NF-κB/MAPK signaling pathways. J. Ethnopharmacol., 2021; 277: 114141.
CrossRef - Bruck R, Ashkenazi M, Weiss S, Goldiner I, Shapiro H, Aeed H, Genina O, Helpern Z, Pines M. Prevention of liver cirrhosis in rats by curcumin. Liver Int., 2007; 27(3): 373-83.
CrossRef - Pan Z, Zhuang J, Ji C, Cai Z, Liao W, Huang Z. Curcumin inhibits hepatocellular carcinoma growth by targeting VEGF expression. Oncol Lett., 2018; 15(4): 4821-4826.
CrossRef - Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett., 2008; 269(2): 199-225.
CrossRef - Cheng CY, Lin YH, Su CC. Curcumin inhibits the proliferation of human hepatocellular carcinoma J5 cells by inducing endoplasmic reticulum stress and mitochondrial dysfunction. Int. J. Mol. Med., 2010; 26(5): 673-8.
CrossRef







