Manuscript accepted on :24-02-2022
Published online on: 19-01-2023
Plagiarism Check: Yes
Reviewed by: Dr. Francisco Solano , Dr. Ameer Ali Shakr Hadi
Second Review by: Dr. Loai Aljerf
Final Approval by: Dr. Jihan Seid Hussein
Antonio Ruggiero , Silvia Triarico
, Silvia Triarico , Alberto Romano
, Alberto Romano , Palma Maurizi
, Palma Maurizi , Giorgio Attina’
, Giorgio Attina’ and Stefano Mastrangelo
and Stefano Mastrangelo
Pediatric Oncology Unit, Fondazione Policlinico Universitario A. Gemelli IRCCS, Universita’ Cattolica Sacro Cuore, Rome, Italy.
Corresponding Author E-mail: antonio.ruggiero@unicatt.it
DOI : https://dx.doi.org/10.13005/bpj/2603
Abstract
Bisphosphonates are among the most widely used drugs in the world for their many clinical indications. Their mechanism of action is based on the increase in the level of bone mineralization through the inhibition of osteoclastic activity and the induction of osteoblastic activity. Recent studies also attribute to bisphosphonates an antineoplastic activity, due to the ability of these drugs to inhibit neo angiogenesis, inhibiting the proliferation of endothelial cells. Bisphosphonates have several common properties, including poorly absorbed orally, high affinity for bone mineral, inhibitory effects on osteoclastic bone resorption, prolonged bone retention, and elimination in the urine. Bisphosphonates are generally well tolerated but their use can be, however, burdened by serious side effects such as hypocalcaemia, renal impairment, and aseptic osteonecrosis of the jaw.
Keywords
Bisphosphonates; Bone Mineralization; Cancer; Pharmacology
Download this article as:| Copy the following to cite this article: Ruggiero A, Triarico S, Romano A, Maurizi P, Attina G, Mastrangelo S. Bisphosphonates: From Pharmacology to Treatment. Biomed Pharmacol J 2023;16(1). |
| Copy the following to cite this URL: Ruggiero A, Triarico S, Romano A, Maurizi P, Attina G, Mastrangelo S. Bisphosphonates: From Pharmacology to Treatment. Biomed Pharmacol J 2023;16(1). Available from: https://bit.ly/3XGY5Sf |
Introduction
Bisphosphonates are synthetic drugs characterized by a high tropism for bone tissue. The first uses of bisphosphonate drugs in the medical field date back to the early 1970s, when etidronate (a bisphosphonate lacking a nitrogen group) was used for the therapy of myositis ossificans, and for the prevention of hypertrophic bone formation after total hip replacement surgery 1-4. Subsequently, these drugs were used in imaging marked with Technetium 99, and again, they were added in some toothpastes because they were believed to be able to prevent stone formation in the dental pulp and decrease periodontal bone loss 5-6 To date, bisphosphonate drugs have many clinical indications and therefore are among the most widely used drugs in the world. It is estimated that there are 30 million prescriptions for bisphosphonate drugs each year in the United States alone, and they are used by at least 2.5 million people worldwide 6. Their mechanism of action lies precisely in increasing the level of bone mineralization by inhibiting osteoclastic activity and inducing osteoblastic activity 7-10. Recent studies have shown the existence of other possible mechanisms of action, including an antineoplastic activity, due to the ability of these drugs to inhibit the proliferation of endothelial cells, and consequently the neo angiogenesis, which is essential for the growth of a neoplasm 7,11-14. After therapy, several cases of gastrointestinal intolerance, symptomatic hypocalcaemia, some cases of fractures due to the stress that these drugs determine on bone remodelling (the reduction of bone remodelling hinders the removal of microfractures that are created in the bone) have been described, cases of influenza, myalgia, deterioration of renal function, cases of acute tubular necrosis, oesophageal erosions and ulcerations, antiangiogenetic effects, cases of anaemia, dyspnoea and oedema 15-16. In recent years, with the use of new types of bisphosphonate molecules and intravenous administration, a particular side effect has been found represented by avascular osteonecrosis of the jaws. This is a complication of their chronic use and was first described by Marx in a study examining 36 cases of osteonecrosis of the jaws in patients who had used bisphosphonates 17-18. It is therefore important to know the pharmacological characteristics of these drugs to allow their reasoned clinical use.
Pharmacokinetics
Bisphosphonate drugs are synthetic analogues of inorganic pyrophosphates in which the phosphoanhydrite bond has been replaced by a P-C-P bond that is not subject to hydrolysis, either in an acidic environment or by the action of pyrophosphatases (Figure 1) 7.
The ability of the bisphosphonates to bind to hydroxyapatite crystals and to prevent both crystal growth and dissolution was enhanced when the R1 side chain (attached to the geminal carbon atom of the P-C-P group) was a hydroxyl group (as in etidronate) rather than a halogen atom such as chlorine (as in clodronate). The presence of a hydroxyl group at the R1 position increases the affinity for calcium (and, thus, bone mineral) because of the ability of bisphosphonates to chelate calcium ions by tridentate rather than bidentate binding. 7,19.
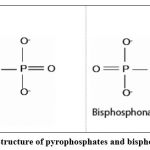 |
Figure 1: Structure of pyrophosphates and bisphosphonates. |
Based on recent discoveries concerning their molecular mechanism of action, bisphosphonates can be grouped in different classes: first-generation non-nitrogen-containing bisphosphonates (BPs), second-generation nitrogen-containing bisphosphonates 20.
The first-generation bisphosphonate drugs have a side chain in R2 that lacks a group containing a nitrogen atom, among them are identified: clodronate, etidronate and tiludronate 21.
The second generation of bisphosphonate drugs differently consists of those that present a nitrogen group in the R2 side chain of which: pamidronate, ibandronate, zoledronate, risedronate, alendronate, olpadronate and icadronate. The differences between the two generations of bisphosphonate drugs are substantial, not only chemically, but primarily in terms of efficacy and mechanism of action (Fig. 2).
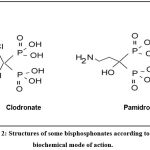 |
Figure 2: Structures of some bisphosphonates according to their biochemical mode of action. |
Within the same generation of bisphosphonate drugs, the potency of the molecules varies greatly when correlated in relation to the groups found on the R2 radical. The latest generation of bisphosphonate drugs, such as ibandronate and zoledronate, have, in fact, a potency of 5,000 and 10,000 times that of etidronate, respectively (Table I)22.
Table 1: Relative potency of the main bisphosphonate drugs
| Drug | Relative Potency |
| Etidronate | x 1 |
| Clodronate
Tiludronate |
x 10 |
| Pamidronate
Neridronate |
x 100 |
| Olpadronate
Alendronate |
x 1000 |
| Ibandronate
Resendronate |
x 5000 |
| Zoledronate | x 10000 |
Another way of classifying these drugs is by the different types of intake; those that are taken orally, such as aledronate, ibandronate and risedronate; those that are taken parenterally, usually intravenously, such as pamidronate and zoledronate. This differentiation is important not only from a therapeutic point of view, but also because of the complications that more often can occur in intravenous intake (e.g., the phenomenon of mandibular osteonecrosis). Bisphosphonate drugs taken orally are used for the prevention and treatment of osteoporosis; for the treatment of multiple myeloma, bone metastases caused by malignancies, and for Paget’s disease 15,23. Most bisphosphonate drugs taken intravenously are used, whose effectiveness is greater both for the characteristics of the molecules and for the plasma concentration that can be achieved. In fact, it must be considered that the bioavailability of oral bisphosphonate drugs is usually between 1% and 2%, but never exceeds 5% 23. Hence, the absorption in the intestine (first pass effect) is very low and 50% of what has been absorbed is deposited on the bone surface and then internalized by osteoclasts, while the other 50% is excreted unmetabolized in the urine 19,24. Therefore, it is difficult to achieve such a dosage to effectively treat malignant diseases. Moreover, their absorption is compromised by food, which creates a further reduction, and therefore administration on an empty stomach is necessary.
Therefore, bisphosphonates should be taken alone on an empty stomach first thing in the morning with at least 240 mL of water. After administration, the patient should not have food, drink, medications, or supplements for at least one half-hour (alendronate, risedronate) or one hour (ibandronate) 24-27. Alendronate, risedronate, and ibandronate are given orally, most commonly at weekly (alendronate, risedronate) or monthly (risedronate and ibandronate) intervals. The administration of zoledronic acid and pamidronate is intravenous, and there is also an intravenous preparation of ibandronate. Intravenous preparations are beneficial in patients who cannot tolerate oral bisphosphonates or where oral bisphosphonates are contraindicated, such as the presence or history of esophageal stricture.
It must also be considered that drugs taken intravenously may have a higher risk of side effects, especially osteonecrosis of the jaw. It has been noted, in fact, that the occurrence of osteonecrosis has an incidence of 1 case out of 100,000 per year for patients who have taken bisphosphonate drugs intravenously, while the annual incidence of osteonecrosis for those who take bisphosphonate drugs parenterally is between 0.8 and 12%, depending on the studies. Bisphosphonate drugs are able to chelate divalent ions, and thus also calcium ions, in three different ways, with the two phosphoric groups and with the substituent found in R 19,28,29.
This explains the ability of these drugs to penetrate into the bone and remain in the structure of this tissue for many years. In fact, it has been demonstrated that the drug remains in the bone tissue until complete remodelling of the bone occurs 22. Since bone turnover is rather slow, especially in elderly patients, and since bisphosphonates slow down this process even more, it has been estimated that the half-life of a bisphosphonate such as alendronate can be about 12 years 23.
In addition, the osteoblasts produce a substance called RANKL, or receptor activator of nuclear factor κβ ligand, which binds to RANK receptors on the surface of nearby monocytes. RANKL induces those monocytes to fuse together to form a multinucleated osteoclast cell and by activating these cells, they can start resorbing bones.
Pharmacodynamics
As described in much of the literature, the action of bisphosphonate drugs can be considered at three different levels: tissue, cellular, and molecular 5,19,30,31. At the tissue level, the main action of all these drugs is to decrease bone turnover. The first step of this process is the reduction of resorption, in fact it has been seen that in patients taking these drugs there is a conspicuous reduction of bone resorption markers in the urine, such as collagen polypeptides presenting cross-links. The decrease in bone formation secondary to resorption, a picture of reduced bone remodelling is therefore created 5. At the cellular level, the main target of the action of bisphosphonate drugs are osteoclasts, whose action is inhibited. These can inhibit the formation of new osteoclasts from monocytes, the activation of osteoclasts, reduce their maturation rate, and their activity, as well as their survival by promoting apoptosis5. Bisphosphonate drugs have been shown to be potent inhibitors of macrophage proliferation, which, in fact, are derived from the same cell line as osteoclasts 32,33.
At the molecular level, their action has not yet been clarified from all points of view, and not all bisphosphonate drugs act with the same mechanism of action. There are, in fact, substantial differences in the action of the two generations of bisphosphonate drugs. Those of the first generation, which lack the nitrogen group, are metabolized within the cells, especially in osteoclasts, in ATP analogues that are not subject to hydrolysis. These analogues accumulate in the cytosol and induce apoptosis 7. Second-generation bisphosphonate drugs, which contain the nitrogen group in the R2 chain have a mechanism of action that makes them much more potent than those of the previous generation. They act on the mevalonate pathway by inhibiting the key enzyme farnesyl diphosphate synthase (FPP synthase), and thus depriving cells of FPP and geranylgeranyl diphosphate (GGPP). These two molecules are essential for the posttranslational prenylation of some members of the G-protein superfamily, including some small GTPases such as Ras, Rac, and Rho. These proteins, once prenylated, are important in the regulation of several processes that are essential for cell activity and survival, and these proteins have also been shown to play a central role in the pathogenesis of certain types of malignancies. Therefore, bisphosphonate drugs, in addition to playing an important role in inhibiting decalcification induced by some diseases, also exhibit antineoplastic activity 7,32.
The anti-decalcifying action is carried out according to mechanisms of action that directly involve osteoclasts, but also through alterations in the communication that takes place between the different cells. In fact, some studies seem to show that this type of drugs stimulate the production of osteoclast inhibitory factor by osteoblasts 5,34,35. Many studies have highlighted that bisphosphonate drugs have a direct antineoplastic activity on many cell lines, both in vivo and in vitro. Their ability to induce apoptosis of neoplastic cells has been demonstrated in breast, prostate, ovarian, bladder, osteosarcoma, leukaemia and melanoma cancers, as well as in myeloma, a pathology in which bisphosphonate drugs are widely used 36.
Numerous in vitro studies have shown that bisphosphonates have direct cytostatic and proapoptotic effects on different human cancer cell lines in a concentration- and time-dependent manner.
The results show that several bisphosphonates (zoledronic acid, pamidronate, and incadronate) reduce myeloma cell proliferation and induce apoptosis, while the nonnitrogen-containing compound, clodronate, has little or no effect. It is also interesting to note that when bisphosphonates are combined with other chemotherapeutic drugs, marked synergy occurs. Thus, the antiproliferative and apoptotic effects of zoledronic acid on breast cancer cells in vitro are enhanced severalfold when zoledronic acid is combined with low concentrations of paclitaxel or tamoxifen 37.
The activity of these drugs is expressed at the level of G-protein, the decrease in prenylation of these molecules determines as the last step the activation of caspases, or those proteins responsible for cell apoptosis. However, it should be noted that the series of reactions leading to cell apoptosis vary depending on the type of bisphosphonate drug taken and the cell line on which it acts. For example, an induction of apoptosis independent of caspase activation has been described in osteosarcoma cells treated with zoledronate. In this case cell death was characterized by an increase in the expression of the oncogene Bax and a decrease in Bcl-2, there were then alterations at the nuclear level and activation of the mitochondrial pathway through translocation of the apoptosis-inducing factor and endonuclease. Finally, another mechanism by which bisphosphonate drugs containing a nitrogen group can induce apoptosis is similar to that of first-generation bisphosphonate drugs, i.e., there is the formation of an ATP analogue, which by accumulating in the cell is able to induce the blockade of ANT (adenine nucleotide translocase), which is believed to be involved in the mechanism of programmed cell death 7,38,39. Bisphosphonate drugs also exert their pharmacological action at other levels.
They are, in fact, able to inhibit, or at least delay the formation of metastases. This is because the G proteins that they inhibit are also responsible for the expression, by the neoplastic cells, of a series of molecules important for cell adhesion such as integrins. It has been seen that this action is expressed at quite low concentrations of these drugs, unlike the direct antineoplastic activity that is expressed for much higher concentrations. About this aspect, bisphosphonate drugs also act by reducing the degradation of the extracellular matrix, inhibiting the activity of metalloproteinases that for their proteolytic activity are essential for metastatic invasion [37-38]. Therefore, we can say that there is evidence that allows us to state that bisphosphonate drugs are capable of directly inhibiting the growth of neoplasms starting from hard tissues and soft tissues 40,41.
Of course, there will be greater efficacy for neoplasms originating from calcified tissue since these drugs accumulate more in this type of tissue. There are, on the other hand, neoplasms that produce calcified substances, even though they originate from soft tissues, and in this case too bisphosphonate drugs will have greater efficacy. However, it must be considered that bisphosphonates are molecules that, after administration, are immediately removed from the blood and accumulate in the bone tissue.42.
To avoid the administration of large doses of drug, ineffective for the therapeutic purpose that we have set (antineoplastic therapy) we can resort to an administration of small doses of drug at several times, in this way the soft tissues will be exposed to the drug for a longer period. Many bisphosphonates containing a nitrogen group, have been shown to inhibit the function of endothelial cells in vivo and in vitro. Among these we can mention: zoledronate, risedronate, alendronate, ibandronate and clodronate. These drugs, in vitro, not only inhibit the proliferation of endotheliocytes, but also their migration and organization in forming new capillary structures 43-47.
Due to the inhibition of Rho prenylation, there is a suppression of proliferation and adhesion by endothelial cells 48-50. Another hypothesis considered is that of an ability on the part of bisphosphonate drugs to inhibit the production of VEGF (vascular-endothelial growth factor), which is essential for the formation of new blood vessels 48,51-54.
In their study, Aksoy et al. propose an interesting hypothesis on the ability to inhibit neo angiogenesis: the hypercalcaemic effects of these drugs contribute substantially to their antiangiogenic activity. The direct consequence in clinical practice would be the lack of need for support with vitamin D and calcium during therapy with bisphosphonate drugs, support that is given in almost all cases, and that these authors believe is necessary only in those cases in which hypocalcaemia is symptomatic (about 5-17% of patients taking bisphosphonate drugs)51,55-57. Bisphosphonate drugs also appear to have the ability to target the tumor by modulating immune system responses. The fact that this system is conditioned, in some way, by the administration of bisphosphonate drugs was already evident in the first uses of these drugs, since they cause, following the first intravenous administration and in some patients, a flu-like reaction, with cold and not high fever 32,58-65.
Recent studies have shown that pamidronate, ibandronate, alendronate, risedronate and zoledronate cause a significant increase in T lymphocytes both in vitro and in vivo. These cells, once activated by unidentified mechanisms, appear to be able to selectively kill tumor cells 7,66-72.
Conclusion
Bisphosphonates constitute a class of drugs widely used to counteract loss of bone mineral density.
Although the exact molecular mechanisms, through which bisphosphonates are able to counteract the loss of bone mineral density, have not yet been identified exactly. Following their administration,
they are absorbed and deposited on hydroxyapatite crystals present in the sites of resorption of bone matrix. Once deposited at this level, bisphosphonates interact with osteoclasts, inhibiting their proliferation, shortening their average life, and decreasing their activity.
Their absorption is impaired by food, especially foods containing calcium, so bisphosphonates should be given when fasting and then only with water or intravenously. In addition, anticancer properties of these molecules have been reported and mainly attributed to an antiangiogenic effect. The side effects induced by bisphosphonates, and the intensity with which they occur, may vary from patient to patient, depending both on the active ingredient and on the sensitivity of everyone towards the same drug. Among the main side effects common to most of the active ingredients belonging to the class of bisphosphonates, we have nausea, abdominal pain, diarrhoea or osteonecrosis of the jaw. It is important, therefore, that these drugs are used according to their clinical indications thus reducing the risk of side effects for patients receiving them.
Conflict of Interest
The authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Funding Sources
The authors received no specific funding for this work.
References
- Allgrove J. Biphosphonates. Arch Dis Child. 1997;76(1):73-75. doi:10.1136/adc.76.1.73
CrossRef - Ezra A, Golomb G. Administration routes and delivery systems of bisphosphonates for the treatment of bone resorption. Adv Drug Deliv Rev. 2000;42(3):175-195. doi:10.1016/s0169-409x(00)00061-2
CrossRef - Russell RG, Croucher PI, Rogers MJ. Bisphosphonates: pharmacology, mechanisms of action and clinical uses. Osteoporos Int. 1999;9 Suppl 2:S66-S80. doi:10.1007/pl00004164
CrossRef - Sato M, Grasser W, Endo N, et al. Bisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure. J Clin Invest. 1991;88(6):2095-2105. doi:10.1172/JCI115539
CrossRef - Oades GM, Coxon J, Colston KW. The potential role of bisphosphonates in prostate cancer. Prostate Cancer Prostatic Dis. 2002;5(4):264-272. doi:10.1038/sj.pcan.4500607
CrossRef - Gutta R, Louis PJ. Bisphosphonates and osteonecrosis of the jaws: science and rationale. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104(2):186-193. doi:10.1016/j.tripleo.2006.12.004
CrossRef - Stresing V, Daubiné F, Benzaid I, Mönkkönen H, Clézardin P. Bisphosphonates in cancer therapy. Cancer Lett. 2007;257(1):16-35. doi:10.1016/j.canlet.2007.07.007
CrossRef - Triarico S, Maurizi P, Mastrangelo S, Attinà G, Capozza MA, Ruggiero A. Improving the Brain Delivery of Chemotherapeutic Drugs in Childhood Brain Tumors. Cancers (Basel). 2019;11(6):824. doi:10.3390/cancers11060824
CrossRef - Rinninella E, Ruggiero A, Maurizi P, Triarico S, Cintoni M, Mele MC. Clinical tools to assess nutritional risk and malnutrition in hospitalized children and adolescents. Eur Rev Med Pharmacol Sci. 2017;21(11):2690-2701.
- Triarico S, Rinninella E, Cintoni M, et al. Impact of malnutrition on survival and infections among pediatric patients with cancer: a retrospective study. Eur Rev Med Pharmacol Sci. 2019;23(3):1165-1175. doi:10.26355/eurrev_201901_17009
- Shaw NJ, Bishop NJ. Bisphosphonate treatment of bone disease. Arch Dis Child. 2005;90(5):494-499. doi:10.1136/adc.2003.036590
CrossRef - Srivastava T, Alon US. The role of bisphosphonates in diseases of childhood. Eur J Pediatr. 2003;162(11):735-751. doi:10.1007/s00431-003-1298-4
CrossRef - Ferrara P, Marrone G, Emmanuele V, et al. Homotoxicological remedies versus desmopressin versus placebo in the treatment of enuresis: a randomised, double-blind, controlled trial. Pediatr Nephrol. 2008;23(2):269-274. doi:10.1007/s00467-007-0440-3
CrossRef - Falsini B, Iarossi G, Chiaretti A, et al. NGF eye-drops topical administration in patients with retinitis pigmentosa, a pilot study. J Transl Med. 2016;14:8. doi:10.1186/s12967-015-0750-3
CrossRef - Ajong AB, Kenfack B, Ali IM, et al. Hypocalcaemia and calcium intake in pregnancy: A research protocol for critical analysis of risk factors, maternofoetal outcomes and evaluation of diagnostic methods in a third-category health facility, Cameroon. PLoS One. 2020;15(11):e0241812. doi: 10.1371/journal.pone.0241812.
CrossRef - Ajong AB, Kenfack B, Ali IM, et al. Ionised and total hypocalcaemia in pregnancy: An analysis of prevalence and risk factors in a resource-limited setting, Cameroon. PLoS One. 2022;17(5):e0268643. doi: 10.1371/journal.pone.0268643.
CrossRef - Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61(9):1115-1117. doi:10.1016/s0278-2391(03)00720-1
CrossRef - Rodan GA. Mechanisms of action of bisphosphonates. Annu Rev Pharmacol Toxicol. 1998;38:375-388. doi:10.1146/annurev.pharmtox.38.1.375
CrossRef - Ruggiero A, Rizzo D, Catalano M, Coccia P, Triarico S, Attiná G. Acute chemotherapy-induced nausea and vomiting in children with cancer: Still waiting for a common consensus on treatment. J Int Med Res. 2018;46(6):2149-2156. doi:10.1177/0300060518765324
CrossRef - Ebrahimpour A, Francis MD. Bisphosphonate therapy in acute and chronic bone loss: physical chemical considerations in bisphosphonate-related therapies. In: Bijvoet O, Fleisch HA, Canfield RE, Russell RGG eds. Bisphosphonates on Bones. Amsterdam, Holland: Elsevier Science; 1995:125–136
- Roelofs AJ, Thompson K, Ebetino FH, Rogers MJ, Coxon FP. Bisphosphonates: molecular mechanisms of action and effects on bone cells, monocytes and macrophages. Curr Pharm Des. 2010;16(27):2950-60. doi: 10.2174/138161210793563635.
CrossRef - Giordano P, Lassandro G, Barone A, et al. Use of Eltrombopag in Children With Chronic Immune Thrombocytopenia (ITP): A Real Life Retrospective Multicenter Experience of the Italian Association of Pediatric Hematology and Oncology (AIEOP). Front Med (Lausanne). 2020;7:66. doi:10.3389/fmed.2020.00066
CrossRef - Pazianas M, Abrahamsen B, Ferrari S, Russell RG. Eliminating the need for fasting with oral administration of bisphosphonates. Ther Clin Risk Manag. 2013;9:395-402. doi: 10.2147/TCRM.S52291.
CrossRef - Sarin J, DeRossi SS, Akintoye SO. Updates on bisphosphonates and potential pathobiology of bisphosphonate-induced jaw osteonecrosis. Oral Dis. 2008;14(3):277-285. doi:10.1111/j.1601-0825.2007.01381.x
CrossRef - Mashiba T, Hirano T, Turner CH, Forwood MR, Johnston CC, Burr DB. Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J Bone Miner Res. 2000;15(4):613-620. doi:10.1359/jbmr.2000.15.4.613
CrossRef - Timeus F, Crescenzio N, Longoni D, et al. Paroxysmal nocturnal hemoglobinuria clones in children with acquired aplastic anemia: a multicentre study. PLoS One. 2014;9(7):e101948. doi:10.1371/journal.pone.0101948
CrossRef - Falsini B, Ziccardi L, Lazzareschi I, et al. Longitudinal assessment of childhood optic gliomas: relationship between flicker visual evoked potentials and magnetic resonance imaging findings. J Neurooncol. 2008;88(1):87-96. doi:10.1007/s11060-008-9537-1
CrossRef - Kenny PA, Lee GY, Bissell MJ. Targeting the tumor microenvironment. Front Biosci. 2007;12:3468-3474. doi:10.2741/2327
CrossRef - Yoneda T, Hagino H, Sugimoto T, et al. Bisphosphonate-related osteonecrosis of the jaw: position paper from the Allied Task Force Committee of Japanese Society for Bone and Mineral Research, Japan Osteoporosis Society, Japanese Society of Periodontology, Japanese Society for Oral and Maxillofacial Radiology, and Japanese Society of Oral and Maxillofacial Surgeons. J Bone Miner Metab. 2010;28(4):365-83. doi: 10.1007/s00774-010-0162-7.
CrossRef - Ruggiero A, Rizzo D, Mastrangelo S, Battaglia D, Attinà G, Riccardi R. Interactions between antiepileptic and chemotherapeutic drugs in children with brain tumors: is it time to change treatment?. Pediatr Blood Cancer. 2010;54(2):193-198. doi:10.1002/pbc.22276
CrossRef - Ruggiero A, Maurizi P, Larocca LM, Arlotta A, Riccardi R. Childhood CD4+/CD56+ hematodermic neoplasm: case report and review of the literature. Haematologica. 2006;91(12 Suppl):ECR48.
CrossRef - Tanvetyanon T, Stiff PJ. Management of the adverse effects associated with intravenous bisphosphonates. Ann Oncol. 2006;17(6):897-907. doi:10.1093/annonc/mdj105
CrossRef - Yamamoto K, Yoshino S, Shue G, Nagashima M. Inhibitory effect of bone resorption and inflammation with etidronate therapy in patients with rheumatoid arthritis for 3 years and in vitro assay in arthritis models. Rheumatol Int. 2006;26(7):627-632. doi:10.1007/s00296-005-0042-y
CrossRef - Ruggiero A, Rizzo D, Trombatore G, Maurizi P, Riccardi R. The ability of mannitol to decrease cisplatin-induced nephrotoxicity in children: real or not?. Cancer Chemother Pharmacol. 2016;77(1):19-26. doi:10.1007/s00280-015-2913-6
CrossRef - Flanagan AM, Chambers TJ. Inhibition of bone resorption by bisphosphonates: interactions between bisphosphonates, osteoclasts, and bone. Calcif Tissue Int. 1991;49(6):407-415. doi:10.1007/BF02555852
CrossRef - Olson KB, Hellie CM, Pienta KJ. Osteonecrosis of jaw in patient with hormone-refractory prostate cancer treated with zoledronic acid. Urology. 2005;66(3):658. doi:10.1016/j.urology.2005.03.028
CrossRef - Jagdev SP, Croucher PI, Coleman RE. Zoledronate acid induces apoptosis of breast cancer cells in vitro—evidence for additive and synergistic effects with taxol and tamoxifen. Proc Am Soc Clin Oncol 2000; 19: 664a.
- Bezzi M, Hasmim M, Bieler G, Dormond O, Rüegg C. Zoledronate sensitizes endothelial cells to tumor necrosis factor-induced programmed cell death: evidence for the suppression of sustained activation of focal adhesion kinase and protein kinase B/Akt. J Biol Chem. 2003;278(44):43603-43614. doi:10.1074/jbc.M308114200
CrossRef - Celin MR, Simon JC, Krzak JJ, et al. Do Bisphosphonates Alleviate Pain in Children? A Systematic Review. Curr Osteoporos Rep. 2020;18(5):486-504. doi:10.1007/s11914-020-00621-3
CrossRef - Tuomela JM, Valta MP, Väänänen K, Härkönen PL. Alendronate decreases orthotopic PC-3 prostate tumor growth and metastasis to prostate-draining lymph nodes in nude mice. BMC Cancer. 2008;8:81. doi:10.1186/1471-2407-8-81
CrossRef - Fetoni AR, Ruggiero A, Lucidi D, et al. Audiological Monitoring in Children Treated with Platinum Chemotherapy. Audiol Neurootol. 2016;21(4):203-211. doi:10.1159/000442435
CrossRef - Toussaint ND, Elder GJ, Kerr PG. Bisphosphonates in chronic kidney disease; balancing potential benefits and adverse effects on bone and soft tissue. Clin J Am Soc Nephrol. 2009;4(1):221-33. doi: 10.2215/CJN.02550508.
CrossRef - Romano A, Capozza MA, Mastrangelo S, et al. Assessment and Management of Platinum-Related Ototoxicity in Children Treated for Cancer. Cancers (Basel). 2020;12(5):1266. doi:10.3390/cancers12051266
CrossRef - Chiaretti A, Conti G, Falsini B, et al. Intranasal Nerve Growth Factor administration improves cerebral functions in a child with severe traumatic brain injury: A case report. Brain Inj. 2017;31(11):1538-1547. doi:10.1080/02699052.2017.1376760
CrossRef - Bishop NJ, Williams DM, Compston JC, Stirling DM, Prentice A. Osteoporosis in severe congenital neutropenia treated with granulocyte colony-stimulating factor [published correction appears in Br J Haematol 1995 Jun;90(2):492]. Br J Haematol. 1995;89(4):927-928. doi:10.1111/j.1365-2141.1995.tb08441.x
CrossRef - Ruggiero A, Rizzo D, Attinà G, et al. Phase I study of temozolomide combined with oral etoposide in children with recurrent or progressive medulloblastoma. Eur J Cancer. 2010;46(16):2943-2949. doi:10.1016/j.ejca.2010.05.016
CrossRef - Chiaretti A, Aloe L, Antonelli A, et al. Neurotrophic factor expression in childhood low-grade astrocytomas and ependymomas. Childs Nerv Syst. 2004;20(6):412-419. doi:10.1007/s00381-004-0959-6
CrossRef - Hashimoto K, Morishige K, Sawada K, et al. Alendronate suppresses tumor angiogenesis by inhibiting Rho activation of endothelial cells. Biochem Biophys Res Commun. 2007;354(2):478-484. doi:10.1016/j.bbrc.2007.01.014
CrossRef - Riccardi A, Mazzarella G, Cefalo G, et al. Pharmacokinetics of temozolomide given three times a day in pediatric and adult patients. Cancer Chemother Pharmacol. 2003;52(6):459-464. doi:10.1007/s00280-003-0677-x
CrossRef - Ruggiero A, Triarico S, Trombatore G, et al. Incidence, clinical features and management of hypersensitivity reactions to chemotherapeutic drugs in children with cancer. Eur J Clin Pharmacol. 2013;69(10):1739-1746. doi:10.1007/s00228-013-1546-0
CrossRef - Aksoy S, Abali H, Dinçer M, Kilickap S, Güllü I, Tekuzman G. Hypocalcemic effect of zoledronic acid or other bisphosphonates may contribute to their antiangiogenic properties. Med Hypotheses. 2004;62(6):942-944. doi:10.1016/j.mehy.2004.02.001
CrossRef - Yamagishi S, Abe R, Inagaki Y, et al. Minodronate, a newly developed nitrogen-containing bisphosphonate, suppresses melanoma growth and improves survival in nude mice by blocking vascular endothelial growth factor signaling. Am J Pathol. 2004;165(6):1865-1874. doi:10.1016/s0002-9440(10)63239-7
CrossRef - Morabito N, Lasco A, Gaudio A, et al. Bisphosphonates in the treatment of thalassemia-induced osteoporosis. Osteoporos Int. 2002;13(8):644-649. doi:10.1007/s001980200087
CrossRef - Homik JE, Cranney A, Shea B, et al. A metaanalysis on the use of bisphosphonates in corticosteroid induced osteoporosis. J Rheumatol. 1999;26(5):1148-1157.
CrossRef - Tanvetyanon T. Is hypocalcemia during therapy with zoledronic acid or other bisphosphonates beneficial to cancer patients?. Med Hypotheses. 2004;63(4):764-765. doi:10.1016/j.mehy.2004.06.003
CrossRef - Chiaretti A, Ruggiero A, Barone G, et al. Propofol/alfentanil and propofol/ketamine procedural sedation in children with acute lymphoblastic leukaemia: safety, efficacy and their correlation with pain neuromediator expression. Eur J Cancer Care (Engl). 2010;19(2):212-220. doi:10.1111/j.1365-2354.2008.01006.x
CrossRef - Haworth CS, Selby PL, Webb AK, Mawer EB, Adams JE, Freemont TJ. Severe bone pain after intravenous pamidronate in adult patients with cystic fibrosis. Lancet. 1998;352(9142):1753-1754. doi:10.1016/S0140-6736(05)79826-3
CrossRef - Criscitiello C, Viale G, Gelao L, et al. Crosstalk between bone niche and immune system: osteoimmunology signaling as a potential target for cancer treatment. Cancer Treat Rev. 2015;41(2):61-8. doi: 10.1016/j.ctrv.2014.12.001.
CrossRef - Pazianas M, Miller P, Blumentals WA, Bernal M, Kothawala P. A review of the literature on osteonecrosis of the jaw in patients with osteoporosis treated with oral bisphosphonates: prevalence, risk factors, and clinical characteristics. Clin Ther. 2007;29(8):1548-1558. doi:10.1016/j.clinthera.2007.08.008
CrossRef - O’Sullivan M, Zacharin M. Intramedullary rodding and bisphosphonate treatment of polyostotic fibrous dysplasia associated with the McCune-Albright syndrome. J Pediatr Orthop. 2002;22(2):255-260.
CrossRef - Dwan K, Phillipi CA, Steiner RD, Basel D. Bisphosphonate therapy for osteogenesis imperfecta. Cochrane Database Syst Rev. 2016;10(10):CD005088. doi:10.1002/14651858.CD005088.pub4
CrossRef - Constantino CS, Krzak JJ, Fial AV, et al. Effect of Bisphosphonates on Function and Mobility Among Children With Osteogenesis Imperfecta: A Systematic Review. JBMR Plus. 2019;3(10):e10216. doi:10.1002/jbm4.10216
CrossRef - Falk MJ, Heeger S, Lynch KA, et al. Intravenous bisphosphonate therapy in children with osteogenesis imperfecta. Pediatrics. 2003;111(3):573-578. doi:10.1542/peds.111.3.573
CrossRef - Glorieux FH, Bishop NJ, Plotkin H, Chabot G, Lanoue G, Travers R. Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N Engl J Med. 1998;339(14):947-952. doi:10.1056/NEJM199810013391402
CrossRef - Roux S, Massicotte MH, Huot Daneault A, Brazeau-Lamontagne L, Dufresne J. Acute hypercalcemia and excessive bone resorption following anti-RANKL withdrawal: Case report and brief literature review. Bone. 2019;120:482-486. doi:10.1016/j.bone.2018.12.012
CrossRef - Kutluk MT, Hazar V, Akyüz C, Varan A, Büyükpamukçu M. Childhood cancer and hypercalcemia: report of a case treated with pamidronate. J Pediatr. 1997;130(5):828-831. doi:10.1016/s0022-3476(97)80030-3
CrossRef - Illidge TM, Hussey M, Godden CW. Malignant hypercalcaemia in pregnancy and antenatal administration of intravenous pamidronate. Clin Oncol (R Coll Radiol). 1996;8(4):257-258. doi:10.1016/s0936-6555(05)80667-3
CrossRef - Oryan A, Sahvieh S. Effects of bisphosphonates on osteoporosis: Focus on zoledronate. Life Sci. 2021;264:118681. doi:10.1016/j.lfs.2020.118681
CrossRef - Li M, Zhong M, Guan C. Bisphosphonates and risk of lung cancer: Protocol for a systematic review and meta-analysis. Medicine (Baltimore). 2021;100(1):e22839. doi:10.1097/MD.0000000000022839
CrossRef - Oliveira JR, Oliveira MF. Primary brain calcification in patients undergoing treatment with the biphosphonate alendronate. Sci Rep. 2016;6:22961. doi:10.1038/srep22961
CrossRef - Nicolatou-Galitis O, Schiødt M, Mendes RA, et al. Medication-related osteonecrosis of the jaw: definition and best practice for prevention, diagnosis, and treatment. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;127(2):117-135. doi:10.1016/j.oooo.2018.09.008
CrossRef - Tsolaki E, Bertazzo S. Pathological Mineralization: The Potential of Mineralomics. Materials (Basel). 2019;12(19):3126. doi:10.3390/ma12193126
CrossRef







