Olaniru B. Olumide1,2 , Adoga I. Godwin2
, Adoga I. Godwin2 , Johnson O. Titilayo2
, Johnson O. Titilayo2 , Isichei O. Christian3
, Isichei O. Christian3 , Nkereuwem S. Etukudoh4
, Nkereuwem S. Etukudoh4 , Obeta M. Uchejeso4,5*
, Obeta M. Uchejeso4,5* , Selowo T. Temitope1
, Selowo T. Temitope1 , Sulagna Dutta6
, Sulagna Dutta6  and Pallav Sengupta7*
and Pallav Sengupta7*
1Department of Chemical Pathology, Jos University Teaching Hospital Jos, Jos, 930241, Plateau, Nigeria
2Department of Biochemistry, Faculty of Basic Medical Sciences, University of Jos, Jos, 930001, Plateau, Nigeria
3Department of Chemical Pathology, Faculty of Clinical Sciences, University of Jos, Jos, 930001, Plateau, Nigeria
4Department of Chemical Pathology, Federal School of Medical Laboratory Science Jos, Jos, 930241, Plateau State, Nigeria
5Department of Human Physiology, University of Jos, Jos, 930001, Plateau, Nigeria
6School of Medical Sciences, Bharath Institute of Higher Education and Research (BIHER), Chennai, Tamil Nadu, India
7Physiology Unit, Department of Biomedical Sciences, College of Medicine, Gulf Medical University, Ajman, UAE
Corresponding Author E-mail: pallav_cu@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2585
Abstract
Background: Anti-Müllerian hormone (AMH) is a Sertoli cell-derived glycoprotein that mediates regression of Müllerian duct in male embryos. The present study aims to evaluate the diagnostic efficacy of serum AMH in the detection of oligozoospermia and non-obstructive azoospermia (NOA) in a homogenous population of Nigerian men. Methods: This case-controlled prospective study was conducted on eighty male subjects (aged 18-45 years), at the Jos University Teaching Hospital, Nigeria. Subjects were classified as control (n=30), oligozoospermic (n=27) and non-obstructive azoospermia (NOA; n=23) (World Health Organization, 2010). Serum concentrations of various hormones were measured. Statistical analyses were performed using MedCalc. (v.19.5.1, Ostend, Belgium). Results: Serum AMH levels did not differ significantly among the study groups (P>0.05). Serum levels of testosterone were significantly lower, while serum FSH levels were significantly higher in the infertile groups than the control (P<0.000001). Serum LH levels were significantly higher in the NOA men (P<0.000001), while oligozoospermic men showed no significant difference, compared to control. Receiver operating characteristics (ROC) curve analysis depicted the same cut-off value (≤1.7 ng/ml) of serum AMH for oligozoospermia and NOA with low sensitivity and moderate specificity. Conclusion: The findings suggest that serum AMH is not a potent stand-alone marker of NOA or oligozoospermia among Nigerian men.
Keywords
Anti-Müllerian Hormone; Azoospermia; Oligozoospermia; Sertoli Cells; Spermatogenesis; Testosterone
Download this article as:| Copy the following to cite this article: Olumide O. B, Godwin A. I, Titilayo J. O, Christian I. O, Etukudoh N. S, Uchejeso O. M, Temitope S. T, Dutta S, Sengupta P. Assessment of Serum Anti-Müllerian Hormone (AMH) as an Independent Marker for Oligozoospermia and Non-Obstructive Azoospermia in Infertile Nigerian Men. Biomed Pharmacol J 2023;16(1). |
| Copy the following to cite this URL: Olumide O. B, Godwin A. I, Titilayo J. O, Christian I. O, Etukudoh N. S, Uchejeso O. M, Temitope S. T, Dutta S, Sengupta P. Assessment of Serum Anti-Müllerian Hormone (AMH) as an Independent Marker for Oligozoospermia and Non-Obstructive Azoospermia in Infertile Nigerian Men. Biomed Pharmacol J 2023;16(1). Available from: https://bit.ly/3Hi2i9H |
Introduction
Anti-Müllerian Hormone (AMH) is a Sertoli cell-derived dimeric glycoprotein that acts a pivotal role in the development of male reproductive tract 1. It induces regression of the Müllerian ducts during male fetal development 2. AMH is the earliest Sertoli cell-specific protein which is produced by the testes throughout life. It begins to be produced by the testicles as early as the ninth week of pregnancy and continues to be secreted at a high level until puberty 3,4. The concentration of AMH in the blood drops substantially during puberty and remains at extremely low levels throughout adulthood 5. AMH has also been found to regulate the proliferation of Leydig cells as well as their steroidogenic function 6. It inhibits production of pre-pubertal progenitor Leydig cells and prevent regeneration of Leydig cells after chemical ablation 6,7.
In the recent decades, AMH has gained much attention in male fertility research. There are conflicting reports on whether seminal or serum AMH can serve as a potential marker for disrupted spermatogenesis in infertile male 8,9. Oligozoospermia and azoospermia are common male reproductive diseases with varied serum AMH levels 10. It has been reported that infertile men with oligozoospermia have lower serum AMH concentrations than control men. Moreover, the same study has suggested that serum AMH is a superior marker for male factor infertility to seminal AMH 11. Another study revealed no significant difference in blood AMH between fertile men and men with low sperm counts, but discovered a link between serum AMH, sperm counts, follicle stimulating hormone (FSH), and free testosterone. 12. These studies had small sample sizes and varied underlying reasons of reproductive issues. Another study, a retrospective analysis of 199 well-characterized men with normal or reduced sperm concentration, found that serum AMH levels correlated negatively with FSH and positively with testicular volume and sperm concentration in men with maldescended testes. 13.
Considering the suggested merit of serum AMH in detection of male fertility impairment, on the other hand, the lack of consensus whether AMH can serve as an independent serum marker of male infertility, the present study objects to examine the diagnostic efficacy of serum AMH in detection of oligozoospermia and NOA in a homogenous population of Nigerian men.
Materials and Methods
Study setting and Patients
This prospective, analytical study was conducted in the Departments of Chemical Pathology and Obstetrics and Gynecology of Jos University Teaching Hospital (JUTH), Jos, Plateau State, North Central Nigeria. The duration of the study was 15 months (April 2016 to April 2017). Ethical clearance was obtained from research and ethical committee of Jos University Teaching Hospital, Jos Plateau State, Nigeria (JUTH/DCS/ADM/127/XIX/6332) following the World Medical Association’s Helsinki Declaration on Human Subject Research. A total of 50 diagnosed infertile male patients, aged between 18 and 45 years (having a fertile female partner) of which 27 were with oligozoospermia and 23 with non-obstructive azoospermia (NOA) attended the Infertility Unit of Department of Obstetrics and Gynecology, JUTH and 30 age-matched fertile control men participated in this prospective case-controlled study. All participants provided written informed consent. Any etiologies connected to physiological abnormalities, e.g. varicocele, as established by physical examination according to the Dublin grading system 14, (b) males with chronic conditions (diabetes, hypertension, etc. ), (c) men taking antioxidants, anabolic steroids, hormones, or reproductive treatments, and (d) men with autoimmune diseases. The study also excluded data on female infertility. The body mass index (BMI) was estimated by the formula: BMI = weight (kg)/(Height in m)2 15.
Semen analysis
Patients included in this study had seminal fluid analysed according to the World Health Organization (WHO) Manual (fifth edition) [16]. Masturbation was used to obtain sperm specimens after a period of 3 – 5 days of sexual abstinence, and following liquefaction, a sperm concentration assay was done to evaluate the sperm concentration in the specimen. Normal values for sperm concentration were ≥15×106/ml, patients who had less than 15×106/ml were considered oligozoospermic and patients with no spermatozoa are found in the sediment of a centrifuged sample were considered as azoospermic patients.
Serum hormone assays
Five milliliters of peripheral venous blood were collected from each participant. Blood samples were collected in plain tubes, allowed to clot and then centrifuged at 1500g for five minutes. The serum was separated then frozen at -20oC until the time of analysis. The hormonal analyses were done by Enzyme Linked Immunosorbent Assay (ELISA) for follicle stimulating hormone (FSH) (Monobind Inc., Lake Forest, California, USA), total testosterone (Monobind, Lake Forest, California, USA), luteinizing hormone (LH) (Monobind, Lake Forest, California, USA), and AMH (Monobind, Lake Forest, California, USA).
Statistical analyses
Data were analysed with MedCalc Statistical software (v.19.5.1, Ostend, Belgium). The Kolmogorov-Smirnov normality test was used to check the distribution of the samples. Comparison of the seminal and hormonal parameters among control, oligozoospermic and NOA groups, was by the non-parametric Kruskal-Wallis test. If significant differences were found, Dunn’s multiple comparison post-hoc test was used. Spearman rank correlation was used to detect the association of variable parameters in oligozoospermic and NOA patients. Receiver operator characteristic (ROC) analysis was with AMH as continuous variables and sperm concentration as the categorical variables to obtain and compare the area under the curves (AUCs), sensitivities, specificities, Youden’s indices and cut-off values. For all comparisons, P-Values <0.05 were considered statistically significant.
Results
The present study included a total of 80 subjects, out of which 33.75% (27 subjects) were recorded with oligozoospermia, 28.75% (23 subjects) with NOA, whereas and 37.5% (30 subjects) were healthy fertile men. Table 1 presents the age, BMI and seminal parameters of the respondents. Figure 1 shows the serum hormonal levels in control, oligozoospermic and NOA groups. The three groups had similar serum AMH levels (P>0.05). Serum FSH levels were significantly higher in the infertile groups than the control (P<0.000001). Serum LH levels were significantly higher in the NOA men (P<0.000001), while oligozoospermic men showed no significant difference, as compared to control.
Table 1: Age, BMI and semen parameters of healthy and infertile subjects.
| Age | BMI (kg/m2) | Semen Volume (ml) | Sperm Concentration (106/ml) | Sperm Motility (%) | Sperm Morphology (%) | |
| Control | 31.40±6.891 | 25.22±3.825 | 4.527±0.613 | 52.67±5.768 | 63.10±6.840 | 36.73±3.373 |
| Oligozoospermia | 31.26±6.377 | 25.72±4.517 | 3.722±0.612 | 5.626±2.616a | 33.37±6.362a | 26.93±4.094a |
| NOA | 30.57±7.668 | 26.10±4.259 | – | – | – | – |
Data are expressed as Mean±SD, avs Control, P<0.05
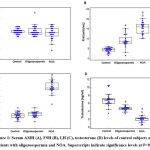 |
Figure 1: Serum AMH (A), FSH (B), LH (C), testosterone (D) levels of control subjects and patients with oligozoospermia and NOA. Superscripts indicate significance levels at P<0.05 |
The findings of this investigation revealed that there was no statistically significant relationship between blood AMH concentration and serum levels of FSH, LH, and testosterone in the control, oligozoospermia, and NOA groups. In addition, there was no statistically significant relationship between serum AMH and the semen parameters in either in the control or infertile groups (Table 2).
Table 2: Correlation of serum AMH with semen parameters and serum hormonal levels in control, oligozoospermic and NOA men.
| Semen Volume2 | Sperm Concentration3 | Sperm morphology4 | Sperm motility4 | Testosterone1 | LH1 | FSH1 | |
| Control
AMH1 |
-0.221 (0.24) | -0.354 (0.055) | -0.227 (0.22) | 0.218 (0.24) | -0.019 (0.91) | 0.111 (0.56) | 0.316 (0.08) |
| Oligozoospermia
AMH1 |
-0.078 (0.69) | -0.146 (0.46) | -0.247 (0.21) | -0.249 (0.21) | 0.134 (0.50) | 0.046 (0.82) | 0.103 (0.60) |
| NOA
AMH1 |
– | – | – | – | -0.110 (0.61) | 0.019 (0.93) | -0.05 (0.82) |
Data are expressed as r (p), 1ng/ml, 2ml, 3106/ml, 4%
The comparison of ROC curves of AMH for prediction of oligozoospermia and NOA are presented in Figure 2. The ROC curve analysis for AMH in predicting oligozoospermia showed AUCs (CI 95%) of 0.536 (0.399 to 0.670) with the cutoff value of ≤1.7 ng/ml, sensitivity of 29.60 and specificity of 83.3. The AUCs (CI 95%) for NOA were 0.578 (0.435 to 0.713) with the cutoff value of ≤1.7 ng/ml, sensitivity of 39.13 and specificity of 83.33 (Figure 2).
 |
Figure 2: (A) ROC curves of AMH in the prediction of oligozoospermia (n=27) and (B) azoospermia (n=23). |
Discussion
In a time when there is a declining trend in male fertility across the globe 17 with most of the cases being idiopathic, it is essential to bring forth some simple clinically reliable biomarkers for early detection of male infertility. Lately, there have been contradictory reports on the diagnostic value of serum or seminal AMH as simple markers of impaired spermatogenesis and semen parameters in men with NOA and oligozoospermia 11-13,18-20. Africa is not an exception among the continents with reduction in male fertility over the last decades 21, but there is a lack of studies to suggest investigating the efficacy of serum AMH in detection of fertility complications in African men. This study has included a homogeneous cohort of idiopathic infertile Nigerian men with either oligozoospermia or NOA; and investigated whether serum AMH can serve as an independent serum marker for these conditions.
Several studies have been reported the possibilities of serum AMH as a predictor of spermatogenesis in men 18,22. The present study observed that the serum levels of AMH did not differ significantly among the study groups that included NOA, oligozoospermic and fertile men. Although it is known that Sertoli cells secrete AMH into the seminiferous tubules, the rationale of studying the serum AMH in the present study was that seminal AMH levels may be influenced by the activities of seminal proteases. This also may explain why there is undetectable seminal AMH concentrations even in some fertile donors 22. Moreover, immature Sertoli cells cannot produce AMH into seminiferous tubules via the apical layer; instead, secretion occurs via the basal layer into the interstitium. In infertile males, substantial spermatogenic impairment in human seminiferous tubules is related with a prepubertal Sertoli cell population 23, so more likely to be released into the circulation and detectable in serum. There are studies that revealed that serum AMH were significantly lower in infertile or subfertile men as compared to fertile control 11,24,25. Thus, circulating AMH content was assumed to be a superior marker of Sertoli cell maturity and spermatogenesis. However, in our study, it may be due to the small sample size that no significant differences in the serum levels of AMH could be detected among the study groups. Alike the present observation, there are several studies that reported no significant changes in the serum AMH levels in infertile/subfertile and fertile men 12,13,26,27. A largescale study in the Nigerian population should be carried out to validate our observation. Moreover, testicular biopsies in infertile male patients can also further confirm whether AMH could be specific in detecting the type of infertility in men. Another reason for persistent serum AMH levels in all the study groups may be that in most of the NOA or oligozoospermic patients, the functions Sertoli cells and interstitial cells are not completely lost 28.
The reproductive hormones, namely the FSH, LH and testosterone are the key endocrine regulators of male reproductive functions, while there are several other hormones that can crosstalk with these prime hormones 29. The present investigation found that both infertile groups had considerably greater FSH serum levels than earlier studies on diverse study populations 30,31 and LH level was significantly higher in the NOA group 30,31, as compared to the control, while there was significant reduction in the serum levels of testosterone in the infertile men as compared to the fertile control 32. Though AMH levels are unrelated to gonadotropin regulation, they have been linked to sperm count and FSH levels 12. However, in this study, we found no significant correlation of serum AMH with the reproductive hormones (Table 2). Moreover, it is known that testicular AMH production is regulated by androgens, but serum levels of testosterone do not inevitably reflect the concentration of intratesticular androgen and Sertoli cells are regulated by autocrine actions of local androgen rather than by the serum testosterone 33. Thus, the non-significant correlation of AMH with serum testosterone observed in our study (Table 2) may be relevant in justifying that association between these two hormones are not very essential for prediction of testicular functions.
There is no confirmatory evidence on the diagnostic value of serum AMH and so far, the limited evidence negates the potential of either seminal or serum AMH as a stand-alone marker for prediction of NOA or oligozoospermia 34,35. Studies by Aksglaede et al. (2018) had also suggested that serum AMH is not a predictor of impaired semen quality in infertile men 36. Moreover, there are studies that suggest that serum AMH may not serve as predictor of sperm recovery in azoospermic men 26,37. In the present study, we used ROC curves to indicate that AMH is not a good predictor of the incidence of NOA or oligozoospermia among the study patients. We used continuous variables such as AMH and categorical factors such as sperm concentration to illustrate this. The findings revealed that cut-off values of ≤1.7ng/ml of AMH were effective in predicting both NOA and oligozoospermia, with poor sensitivity and intermediate specificity in both cases in the study.
Conclusion
The present study is the first ever evaluation of the predictive efficacy of serum AMH for infertile men in Nigeria, presented with NOA and oligozoospermia. The results showed that serum AMH may not serve as a single predictor of NOA or oligozoospermia. This is a notable scientific revelation that directs future studies in unveiling other endogenous factors, which together with serum AMH may emerge as predictors of the male infertility subtypes. The study also aims to encourage further research considering the seminal plasma concentration of AMH including larger sample size. It is thereby suggested that precise prediction of infertility or subfertility in men is warranted by using a multivariate model including all the male reproductive hormones that demonstrate the complex regulation of testicular microenvironment.
Author Contributions: OBO, AIG, and JOT conceptualized the study; OBO, AIG, JOT, and NSE did bench work; OBO, AIG, JOT, IOC, NSE, OMU, STT, SD, PS did literature search and initial manuscript writing; OBO, AIG, JOT, OMU, SD, PS, SRC did final editing and approved for final publication.
Ethical Approval: Ethical clearance was obtained from research and ethical committee of Jos University Teaching Hospital, Jos Plateau State, Nigeria (JUTH/DCS/ADM/127/XIX/6332)
Conflict of Interest
There are no conflict of interest
Funding Sources
There is no funding sources.
References
- Silva, M.S.; Giacobini, P. New insights into anti-müllerian hormone role in the hypothalamic–pituitary–gonadal axis and neuroendocrine development. Cellular and Molecular Life Sci 2021, 78, 1-16.
CrossRef - Altincik, A.; Karaca, F.; Onay, H. Persistent müllerian duct syndrome: A novel mutation in the anti-müllerian hormone gene. Hormones 2017, 16, 205-208.
CrossRef - Josso, N.; Legeai, L.; Forest, M.G.; Chaussain, J.L.; Brauner, R. An enzyme linked immunoassay for anti-müllerian hormone: A new tool for the evaluation of testicular function in infants and children. J Clin Endocrinol Metab 1990, 70, 23-27.
CrossRef - McCredie, S.; Ledger, W.; Venetis, C.A. Anti-müllerian hormone kinetics in pregnancy and post-partum: A systematic review. Reprod BioMed Online 2017, 34, 522-533.
CrossRef - Garrel, G.; Racine, C.; L’Hôte, D.; Denoyelle, C.; Guigon, C.J.; di Clemente, N.; Cohen-Tannoudji, J. Anti-müllerian hormone: A new actor of sexual dimorphism in pituitary gonadotrope activity before puberty. Sci Reports 2016, 6, 1-11.
CrossRef - Edelsztein, N.Y.; Grinspon, R.P.; Schteingart, H.F.; Rey, R.A. Anti-müllerian hormone as a marker of steroid and gonadotropin action in the testis of children and adolescents with disorders of the gonadal axis. Int J Pediat Endocrinol 2016, 2016, 1-10.
CrossRef - Salva, A.; Hardy, M.P.; Wu, X.-f.; Sottas, C.M.; MacLaughlin, D.T.; Donahoe, P.K.; Lee, M.M. Müllerian-inhibiting substance inhibits rat leydig cell regeneration after ethylene dimethanesulphonate ablation. Biol Reprod 2004, 70, 600-607.
CrossRef - Kong, X.; Ye, Z.; Chen, Y.; Zhao, H.; Tu, J.; Meng, T.; Xiong, C.; Li, H.; Gong, Y.; Zheng, L. Clinical application value of inhibin b alone or in combination with other hormone indicators in subfertile men with different spermatogenesis status: A study of 324 chinese men. J Clin Lab Ana 2021, 35, e23882.
CrossRef - Benderradji, H.; Prasivoravong, J.; Marcelli, F.; Barbotin, A.-L.; Catteau-Jonard, S.; Marchetti, C.; Guittard, C.; Puech, P.; Mitchell, V.; Rigot, J.-M. Contribution of serum anti-müllerian hormone in the management of azoospermia and the prediction of testicular sperm retrieval outcomes: A study of 155 adult men. Basic Clin Androl 2021, 31, 1-12.
CrossRef - Xu, H.-Y.; Zhang, H.-X.; Xiao, Z.; Qiao, J.; Li, R. Regulation of anti-müllerian hormone (amh) in males and the associations of serum amh with the disorders of male fertility. Asian J Androl 2019, 21, 109.
CrossRef - Al‐Qahtani, A.; Muttukrishna, S.; Appasamy, M.; Johns, J.; Cranfield, M.; Visser, J.; Themmen, A.; Groome, N. Development of a sensitive enzyme immunoassay for anti‐müllerian hormone and the evaluation of potential clinical applications in males and females. Clin Endocrinol 2005, 63, 267-273.
CrossRef - Appasamy, M.; Muttukrishna, S.; Pizzey, A.; Ozturk, O.; Groome, N.; Serhal, P.; Jauniaux, E. Relationship between male reproductive hormones, sperm DNA damage and markers of oxidative stress in infertility. Reprod Biomed Online 2007, 14, 159-165.
CrossRef - Tüttelmann, F.; Dykstra, N.; Themmen, A.P.; Visser, J.A.; Nieschlag, E.; Simoni, M. Anti-müllerian hormone in men with normal and reduced sperm concentration and men with maldescended testes. Fertil Steril 2009, 91, 1812-1819.
CrossRef - Hirsh, A.; Cameron, K.; Tyler, J.; Simpson, J.; Pryor, J. The doppler assessment of varicoceles and internal spermatic vein reflux in infertile men. Brit J Urol 1980, 52, 50-56.
CrossRef - World Health Organization, W.H. World health organization bmi classification. World Health Organization 2020.
- World Health Organization, W.H. Who laboratory manual for the examination and processing of human semen. World Health Organization 2010.
- Sengupta, P.; Dutta, S.; Krajewska-Kulak, E. The disappearing sperms: Analysis of reports published between 1980 and 2015. Am J Men’s Health 2017, 11, 1279-1304.
CrossRef - Fujisawa, M.; Yamasaki, T.; Okada, H.; Kamidono, S. The significance of anti-müllerian hormone concentration in seminal plasma for spermatogenesis. Hum Reprod 2002, 17, 968-970.
CrossRef - Alfano, M.; Ventimiglia, E.; Locatelli, I.; Capogrosso, P.; Cazzaniga, W.; Pederzoli, F.; Frego, N.; Matloob, R.; Saccà, A.; Pagliardini, L. Anti-mullerian hormone-to-testosterone ratio is predictive of positive sperm retrieval in men with idiopathic non-obstructive azoospermia. Sci Reports 2017, 7, 1-9.
CrossRef - Xiong, Z.; Ye, Z.; Tu, J.; Meng, T.-Q.; Ren, N. Serum anti-müllerian hormone level for differential diagnosis of obstructive and non-obstructive azoospermia. Nat J Androl 2019, 25, 823-827.
- Sengupta, P.; Nwagha, U.; Dutta, S.; Krajewska-Kulak, E.; Izuka, E. Evidence for decreasing sperm count in african population from 1965 to 2015. Afr Health Sci 2017, 17, 418-427.
CrossRef - Fenichel, P.; Rey, R.; Poggioli, S.; Donzeau, M.; Chevallier, D.; Pointis, G. Anti-mullerian hormone as a seminal marker for spermatogenesis in non-obstructive azoospermia. Hum Reprod 1999, 14, 2020-2024.
CrossRef - Steger, K.; Rey, R.; Kliesch, S.; Louis, F.; Schleicher, G.; Bergmann, M. Immunohistochemical detection of immature sertoli cell markers in testicular tissue of infertile adult men: A preliminary study. Int J Androl 1996, 19, 122-128.
CrossRef - Muttukrishna, S.; Yussoff, H.; Naidu, M.; Barua, J.; Arambage, K.; Suharjono, H.; Sathanandan, M. Serum anti-müllerian hormone and inhibin b in disorders of spermatogenesis. Fertil Steril 2007, 88, 516-518.
CrossRef - Goulis, D.G.; Polychronou, P.; Mikos, T.; Grimbizis, G.; Gerou, S.; Pavlidou, V.; Papanikolaou, A.; Tarlatzis, B.C.; Bontis, I.N.; Papadimas, I. Serum inhibin-b and follicle stimulating hormone as predictors of the presence of sperm in testicular fine needle aspirate in men with azoospermia. Hormones 2008, 7, 140-147.
CrossRef - Isikoglu, M.; Ozgur, K.; Oehninger, S.; Ozdem, S.; Seleker, M. Serum anti-müllerian hormone levels do not predict the efficiency of testicular sperm retrieval in men with non-obstructive azoospermia. Gynecol Endocrinol 2006, 22, 256-260.
CrossRef - Nayyfe, H.A.; Calapoglu, M.; Ozmen, I. Investigation the relationship between spermatogenesis and the levels of some hormones in a sample of infertile iraqi males with azoospermia and oligospermia. Iraqi J Sci 2018, 1378-1386.
- Schlegel, P.N. Testicular sperm extraction: Microdissection improves sperm yield with minimal tissue excision. Hum Reprod 1999, 14, 131-135.
CrossRef - Dutta, S.; Sengupta, P.; Muhamad, S. Male reproductive hormones and semen quality. Asian Pac J Reprod 2019, 8, 189.
CrossRef - Babu, S.R.; Sadhnani, M.; Swarna, M.; Padmavathi, P.; Reddy, P. Evaluation of fsh, lh and testosterone levels in different subgroups of infertile males. Indian J Clin Biochem 2004, 19, 45-49.
CrossRef - Koşar, P.A.; Özçelik, N.; Koşar, A. Cytogenetic abnormalities detected in patients with non-obstructive azoospermia and severe oligozoospermia. J Assist Reprod Genet 2010, 27, 17-21.
CrossRef - Huang, I.-S.; Huang, W.J.; Lin, A.T. Distinguishing non-obstructive azoospermia from obstructive azoospermia in taiwanese patients by hormone profile and testis size. J Chin Med Assoc 2018, 81, 531-535.
CrossRef - Rey, R.; Lukas-Croisier, C.; Lasala, C.; Bedecarrás, P. Amh/mis: What we know already about the gene, the protein and its regulation. Mol Cell Endocrinol 2003, 211, 21-31.
CrossRef - Toulis, K.A.; Iliadou, P.K.; Venetis, C.A.; Tsametis, C.; Tarlatzis, B.C.; Papadimas, I.; Goulis, D.G. Inhibin b and anti-müllerian hormone as markers of persistent spermatogenesis in men with non-obstructive azoospermia: A meta-analysis of diagnostic accuracy studies. Hum Reprod Update 2010, 16, 713-724.
CrossRef - La Marca, A.; Sighinolfi, G.; Radi, D.; Argento, C.; Baraldi, E.; Artenisio, A.C.; Stabile, G.; Volpe, A. Anti-müllerian hormone (amh) as a predictive marker in assisted reproductive technology (art). Hum Reprod Update 2010, 16, 113-130.
CrossRef - Aksglaede, L.; Olesen, I.; Carlsen, E.; Petersen, J.; Juul, A.; Jørgensen, N. Serum concentration of anti‐müllerian hormone is not associated with semen quality. Andrology 2018, 6, 286-292.
CrossRef - Goulis, D.G.; Tsametis, C.; Iliadou, P.K.; Polychronou, P.; Kantartzi, P.-D.; Tarlatzis, B.C.; Bontis, I.N.; Papadimas, I. Serum inhibin b and anti-müllerian hormone are not superior to follicle-stimulating hormone as predictors of the presence of sperm in testicular fine-needle aspiration in men with azoospermia. Fertil Steril 2009, 91, 1279-1284.








