Seema Srivastava , Manish Kumar Sharma
, Manish Kumar Sharma and Sharey
and Sharey
Department of Zoology, Reproductive Physiology Lab, University of Rajasthan, Jaipur-302004, Rajasthan, India.
Corresponding Author E-mail: drseemaa07@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2573
Abstract
Pubertal development of testis requires stringent regulations and precise expansion of germ cells. Reactive oxygen species (ROS) are involved in regulation and proliferation of spermatogonia. Bisphenol A (BPA) is well known for the induction of oxidative stress in testicular tissues leading to major adversities including reduced fertility in male. In the present study, BPA led response of antioxidants was assessed during critical period of pubertal maturation of testis. Four groups of Wistar albino rats were formed containing 15 animals each; Group I-Control, Group II-administered with 5 mg/kg/d BPA, Group III- administered with 25 mg/kg/d BPA and Group IV- administered with 50 mg/kg/d BPA. These groups were further bifurcated into three distinct periods of exposure i.e. 42-63 PND, 42-91 PND and 42-105 PND, containing 5 animals each. Level of CAT, SOD, GSH, GPx, and LPO was analysed. Linear regression for individual antioxidants and Pearson’s correlation between antioxidants were applied for age-wise analysis of variance. Results showed that during first three weeks (i.e. 42-63 PND) of BPA administration were better tolerated, irrespective of doses. Later intervals indicated significant decline (p<0.05) in the activity and level of antioxidants. Relatedness between variables of antioxidants following BPA exposure and control were extremely low (R2<0.1) indicating differential activity. Nonetheless, between antioxidants strong strength of association (r>0.9) was evident. Although initial toleration against BPA’s oxidative insult was evident, it could not be sustained following 91 or 105 PND. Extremely lower activity of antioxidant at later stage suggested potential delay in testicular maturity.
Keywords
Bisphenol A; Oxidative stress; Pubertal growth; Testicular maturation
Download this article as:| Copy the following to cite this article: Srivastava S, Sharma M. K, Sharey S. Variation in the Oxidative Status of Testicular Tissues During Critical Pubertal Age Under Influence of Bisphenol A. Biomed Pharmacol J 2022;15(4). |
| Copy the following to cite this URL: Srivastava S, Sharma M. K, Sharey S. Variation in the Oxidative Status of Testicular Tissues During Critical Pubertal Age Under Influence of Bisphenol A. Biomed Pharmacol J 2022;15(4). Available from: https://bit.ly/3DnuXrW |
Introduction
A study by Taberner et al.1 reported that arrival of puberty in groups of equally monitored minipigs differed significantly. Pubertal age varies within population, in females, age of puberty lies between 10-14 years, whereas, in male the range fall between 12-16 years. Spermatogenic development and availability of fully functional sperm in the epididymis are regarded as arrival of sexual maturity in male. This critical phenomenon differs from individual to individual. Differentiation in arrival of puberty depends on various factors, such as; genetic, environment, diet, nutrition, socioeconomic, chemical exposure, etc.2-3.
Reactive oxygen species (ROS) were initially regarded as toxic by-products of metabolism as it causes lethal damages to protein, DNA and lipids4-5. However, the dynamic role of oxidative radical or ROSs is way beyond causing damage to the cellular functioning. Without doubt, it causes sever damages to the cell and cellular function, nonetheless, it also plays important role in anti-inflammatory responses, iron homeostasis, cell proliferation, survival, differentiation and metabolism6. ROSs act as primary and secondary messengers to facilitate or impede cell growth7. Normal continuous spermatogenesis is a highly complex cellular growth and maturation process, thus, stringent regulation of cellular growth in testis is extremely important. Since gonadal tissues are rich in unsaturated fatty acid and goes through high number of cell divisions, overexpression of ROSs can cause severe damage to germ cell proliferation8. Thus, an advance antioxidant system regulates the metabolism of ROS in the testicular tissues. Standard growth of testis is dependent on perfect homeostasis between generation of ROSs and functioning of antioxidant system.
Cellular damages inflicted by oxidative stress have been associated with age in multiple tissues9-11. A study by Rey et al.12 reported that volume of seminiferous tubules increases significantly during puberty. Subsequently, number of spermatogonia, spermatocytes, and spermatids also increases dramatically. Likewise, at the pre-pubertal age number of spermatogenic degeneration is higher than pubertal and post-pubertal age12. Degenerative changes in testicular tissues have been reported in pre-pubertal13 and old age14. Since testicular cells go through significant changes during pre-pubertal, pubertal and post-pubertal age, ROSs may play important role in regulation of germ cell development and sperm maturation. Bisphenol A (BPA) is a known reproductive toxicant, it has been associated with dose dependent increase in oxidative stress in testicular tissues15. In the present study, differential pattern of BPA induced oxidative stress during pre-pubertal, pubertal, and post-pubertal age was investigated.
Materials and methods
Test materials
Bisphenol A (CAS no. 80-05-7) of 99% purity was purchased from Sigma Aldrich (MO, USA). Double distilled water (ddH2O) was used as both sham and vehicle. BPA solution made for administration was kept in dark at room temperature. Before administration and during resting solution was stirred continuously.
Experimental animals
Experiments carried out in this study were strictly followed according to guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals16. Rattus norvegicus (commonly known as Wistar albino rats) of age 42 postnatal days (PND) weighed in range of 100-110 g were used in the present study. Animals were maintained under equal day and night schedule (12 h:12 h) at the departmental facility. Polypropylene cages of size 43×27×15 cm were labelled appropriately and housed with randomly selected animals. These animals were provided with pellet diet, drinking water was made available ad libitum.
Ethical approval
Animals were maintained in the departmental facility under appropriated supervision of veterinary expert. Experiments and protocols were also approved by the Institutional Animal Ethics Committee (IAEC).
Experiment design
Animals were divided in three groups based on daily doses of BPA administration i.e. 5 mg, 25 mg, and 50 mg/kg body weight. According to Long and Evans17 rats reach puberty at around 50 PND, this is a significant event, during which first wave of spermatogenesis in testis and first functional sperm in the epididymis appear. These groups were further divided in three sub-groups based on age i.e. peri-adolescent (42-63 PND), adolescent (42-91 PND), and adult (42-105 PND). Therefore, BPA was administered daily from 42 PND to 63 PND, 91 PND, and 105 PND. Table 1 shows the doses of BPA and age-wise distribution of animals in the groups and sub-groups. A parallel sham control group was used, animals of these groups were administered with vehicle only. At termination of each schedule animals from each group were sacrificed for further investigation.
Table 1: Details of investigated groups with respective specifications.
| Groups | Specifications | |
| Group I (n=15) | a. | Sham control – Vehicle treated for 3 weeks from 42-63 PND (n=5) |
| b. | Sham control – Vehicle treated for 7 weeks from 42-91 PND (n=5) | |
| c. | Sham control – Vehicle treated for 9 weeks from 42-105 PND (n=5) | |
| Group II (n=15) | a. | Oral administration of 5 mg BPA/kg/body weight/day for 3 weeks from 42-63 PND (n=5) |
| b. | Oral administration of 5 mg BPA/kg/body weight/day for 7 weeks from 42-91 PND (n=5) | |
| c. | Oral administration of 5 mg BPA/kg/body weight/day for 9 weeks 42-105 PND (n=5) | |
| Group III (n=15) | a. | Oral administration of 25 mg BPA/kg/body weight/day for 3 weeks from 42-63 PND (n=5) |
| b. | Oral administration of 25 mg BPA/kg/body weight/day for 7 weeks from 42-91 PND (n=5) | |
| c. | Oral administration of 25 mg BPA/kg/body weight/day for 9 weeks 42-105 PND (n=5) | |
| Group IV (n=15) | a. | Oral administration of 50 mg BPA/kg/body weight/day for 3 weeks from 42-63 PND (n=5) |
| b. | Oral administration of 50 mg BPA/kg/body weight/day for 7 weeks from 42-91 PND (n=5) | |
| c. | Oral administration of 50 mg BPA/kg/body weight/day for 9 weeks 42-105 PND (n=5) | |
Antioxidative enzyme assay
Individual antioxidant enzymes were examined in testicular tissues for each group and sub-groups to observe pattern of differentiation in activity and levels. Observations were presented according to age-wise period of exposure for each group and simultaneously compared with the respective sham control.
Catalase
Activity of catalase was determined according to Aebi18. Briefly, rate of hydrogen peroxide (H2O2) decomposition was enumerated to estimate utilization of catalase. To measure H2O2 decomposition, a 475 ml of phosphate buffer (pH 7) was nicely mixed with 25 ml of H2O and 250 ml of H2O2. This mixture was allowed to settle in as control. On the other side, equal amount of phosphate buffer was mixed with 25 ml of testicular tissue homogenate and equal amount of H2O2 was allowed to react. Measurement of rate of decomposition from both experiments were estimated spectrophotometrically at 240 nm. Activity of enzyme was measured in U/mg protein, where ‘U’ abbreviated for mmol H2O2 decomposed every minute.
Superoxide dismutase
Activity of Superoxide dismutase (SOD) was estimated by Marklund and Marklund19 method. Based on the ability of the enzyme to inhibit the autoxidation of pyrogallol SOD activity was determined. A 2.85 g of Tris and 1.11 g of EDTA-Na2 were dissolved in 1 l of distilled water. A 0.252 g of pyrogallol was dissolved in a solution of 0.6 ml of concentrated hydrochloric acid diluted in 1 l of distilled water. Spectrophotometer was adjusted to read zero using Tris-EDTA buffer. Control and sample test tubes were prepared then pipetted into test tubes. Absorption was read at the wavelength of 420 nm against Tris-EDTA buffer at zero time and after 1 minute of the addition of pyrogallol.
Glutathione
Glutathione (GSH) was estimated according to the method explained by Hissin and Hilf20. Briefly, a 250 mg testicular tissue pellet was suspended in 25% metaphosphoric acid and potassium phosphate buffer (pH 8). The above solution was sonicated for 10 min. This solution was further centrifuged at 30000 × g for 30 min, later supernatant was stored at 0 °C until further use. A 0.2 ml of supernatant was incubated with 1.7 ml phosphate-EDTA buffer (pH 8) and 0.1 ml of fluorescence reagent 0-phthaldiaaldehyde (1 mg/ml) for 15 min. Following which GSH was determined by fluorescence spectrophotometer at 420 nm excited at 350 nm.
Glutathione peroxidase
Glutathione peroxidase (GPx) was measured in testicular tissues based on method explained by Wood21. Homogenate of testicular tissues were centrifuged at 14000 × g for 25 minutes, following which supernatant was collected. A 700 ml of phosphate buffer (pH 7) was poured into 1 ml cuvette, to this 25 ml of glutathione reductase, 25 ml of sodium azide, 50 ml of NADPH and 50 ml of sample was added. Mixture was allowed to settle at 25 °C, once solution is equilibrated H2O2 was added to the solution and absorbance was noted for 5 min at 1 min interval for change of NADPH into NADP. The respective absorbance was registered and estimated activity was represented in NADPH oxidized/min/mg protein.
Lipid peroxidation
Lipid peroxidation levels were measured by the thiobarbituric acid (TBA) reaction by the method of Ohkawa et al.22. This method was used to measure spectrophotometrically the color produced by the reaction of TBA with malondialdehyde (MDA) at 532 nm. Thiobarbituric acid reactive substances (TBARS) levels were measured using a commercial assay as the NWLSS TM Malondialdehyde Assay according to the manufacturer’s instructions. Tissue supernatant (50 μl) were added to test tubes containing 2 μl of butylated hydroxytoluene (BHT) in methanol. Next, 50 μl of acid reagent (1 M phosphoric acid) was added and finally 50 μl of TBA solution was added. The tubes were mixed vigorously and incubated for 60 min at 60°C. The mixture was centrifuged at 10,000 × g for 3 min. The supernatant was put into wells on a microplate in aliquots of 75 μl, and its absorbance was measured with a BIO RAD Benchmark Plus plate reader at 532 nm. TBARS levels were expressed as n mol/mg protein in testis.
Statistical Analysis
Numeric values of results were represented in mean±standard error (SE). Values were considered significant, highly significant, extremely significant when p<0.05, p<0.01, and p<0.001, respectively. Graph used in this study were plotted with MS-EXCEL version 16.44 (Redmond, WA, US). Linear forecast for the variation in activities and levels of antioxidants was examined through regression plotting (R2). These variations were further analysed by observing spread of values in quadrants. Pearson’s correlation test was applied to observe strength of association between antioxidants.
Results
Activity of catalase
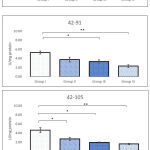
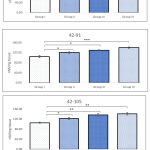
When compared with sham control there was no alteration in the activity of CAT in BPA administered animals, exposed between 42-63 PND. However, significant reduction in the activity was observed in Group III (p=0.015) and Group IV (p=0.003) following BPA exposure between 42-91 PND. Similarly, significant decline in the activity of CAT was observed in all groups (Group II-p=0.010; Group II-p=0.011; Group III-p=0.009) treated with BPA between 42-105 PND when compared with sham control. Maximum decline in activity was recorded in Group IV which was close to 40% compared to sham control. Regardless of doses of BPA, results of CAT activity showed no change following 3 weeks of administration (42-63 PND). While, doses of BPA played direct role in declination of activity of CAT in animals exposed between 42-91 and 42-105 PND. Response of CAT against BPA in testicular tissues indicated age related differentiation (Figure 1).
Activity of superoxide dismutase
Activity of SOD in testicular tissues indicated that exposure of BPA causes immense stress on testis. Although unobservable alteration was noted in Group II and III following 3 weeks exposure between 42-63 PND, all remaining groups at investigated age-wise duration of exposure indicated significant decline in the activity. Maximum decline in activity was observed in those animals exposed for longer duration i.e. 42-105 PND. Regardless of strength of doses decline in activity of SOD was within range of 50-60% comparing to observed activity during 42-63 PND. It was also noted that BPA had a dose dependent impact on activity of SOD, as decline in activity of test groups was highly related to respective doses (Figure 2).
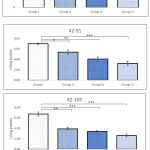
Level of reduced glutathione
Exposure of BPA between 42-63, 42-91, and 42-105 PND indicated differential levels of GSH in testicular tissue. Results showed significant decline in level of GSH in all groups at all investigated period of exposure, except for groups II and III administered between 42-63 PND, when compared against sham control. Following 3, 7 and 9 weeks of BPA exposure between 42-63, 42-91, and 42-105 PND, maximum decline in level of GSH was noted in Group IV. There was clear indication of bi-directional influence of both age-wise and dose-wise BPA exposure. Level of GSH observed in Group II following 42-105 PND of exposure was equivalent to 42-91 PND of exposure in Group III. Likewise, level of GSH noted in Group III following 42-105 PND of exposure was comparable to Group IV following 42-63 PND of exposure (Figure 3).
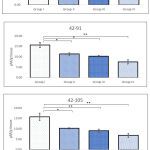
Activity of glutathione peroxidase
Groups administered with BPA for longer duration i.e. 42-91 and 42-105 PND indicated extremely lower activity of GPx when compared with sham control. For instance, activity of GPx in Group III animals those were exposed between 42-91 PND and Group IV animals exposed between 42-91 and 42-105 PND were measured as 1.87±0.23, 1.68±0.38, 1.32±0.19 nmol/min/mg protein, respectively. Whereas, activity of GPx in Group I was noted in range of 3-4 nmol/min/mg protein (Figure 4). No alteration in the activity of GPx was observed in Group II animals administered with BPA between 42-63 PND. Regardless of period of administration, groups III and IV showed significant decline in testicular activity of GPx.
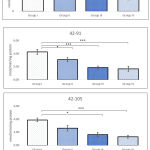
Lipid peroxidation
Administration of BPA in test groups showed extremely significant increase in the level of lipid peroxidation. Group IV was highly affected by the administration, which showed nearly 25-40% increase in the level of LPO. Level of LPO in Group IV was noted as 128.73±4.67, 139.86±2.79, 141.14±4.63 nM/mg tissue following 42-63, 42-91, and 42-105 PND, respectively. Whereas, the same in Group I was noted as 107.30±3.42, 104.13±4.13, 105.43±2.37 nM/mg tissue, respectively. Level of LPO in testicular tissues of Group III also indicated 20% increase in animals administered with BPA during 42-63 PND, likewise, 27% and 35% increase was observed in animals exposed between 42-91 and 42-105 PND (Figure 5). Within test groups (i.e. groups II-IV) also increase in level of LPO was noted. Following 42-63 PND of administration of BPA, at least 6% increase in LPO was noticed in Group III comparing to Group II, whereas, Group IV sowed 10% increase. Likewise, nearly 8% increase in LPO was observed in Group III animals following administration of BPA between period of 42-91, which further increase to 15% in Group IV comparing to Group II (Figure 5). Interestingly, following administration of BPA during 42-105 PND showed 11% increase in Group III and 15% in Group IV, when compared with respective Group II.
Linear regression forecast of variation in activity/level of antioxidant
Linear forecast for activity of catalase in testicular tissues based on variations posed by BPA administration indicated three distinct behaviours. Regression plot indicated convergence of variations of test groups (II-IV) at mid-lower range of activity of sham control. However, at higher range the variation appeared to distinctly diverge from each other, where Group II showed weak positive relatedness (R2=0.084), Group III indicated least relatedness (R2=0.0088), and Group IV showed weak but slightly stronger positive relatedness (R2=0.122) than Group II. Group IV and II reflected opposite relation to each other when compared against sham control (Group I). When values of catalase activity were segregated based on the maximum values of axis, it appeared that most values measured in test groups (II-IV) fell into lower (Q3) and higher (Q2) range of activity whereas, minimum number of values appeared in Q1 and Q4 of control (Figure 6).
Most values of SOD activities were found in Q2 and Q4, indicating BPA’s strong influence on the activity. However, values were still related to a greater extent with control (Group I), as more than 50% values fall into Q3 and Q2. Extremely weak relatedness between Group II and Group I was apparent (R2=0.029 × 10-3), likewise, weak relatedness (R2=0.005) was also apparent between Group IV and Group I. This indicated significant change in trend of variation in activity of SOD, particularly in groups II and IV. On the other hand, Group III although indicated weak similarity (R2=0.041) in variation but followed negative trend to that of the control group (Figure 6).
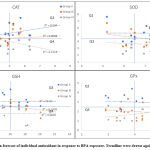 |
Figure 6: Linear regression forecast of individual antioxidant in response to BPA exposure. Trendline were drawn against observations of control. |
Relatedness between variations in activity of GSH in animals administered with BPA revealed similar trend to that of the SOD (Figure 6). Trendline for activity of SOD in Group II indicated nearly horizontal line comparable to the axis, indicating weak relationship (R2=0.072 × 10-3) between variables of Group II and Group I. Similar relationship was apparent with Group IV (R2=0.002 × 10-1), indicating an almost parallel trendline to that of the Group II. Alike variation recorded for SOD activity in testis, level of GSH also responded similarly. Weak but slightly sturdier relatedness (R2=0.164) was observed for Group III, however, the trend was negatively related to the control. Most values fell into upper quadrants (Q1 and Q2) indicating non-oriented relationship between variables of control and BPA exposed. Linearity forecast of variations observed for GPx activity indicated weak association of Group II (R2=0.002) and III (R2=0.024) (Figure 6). However, slightly better association was observed for activity in Group IV, nonetheless, it was still weak. Unlike GSH, most values under this category fell into lower quadrants.
Pearson’s correlation between variation in the activity of antioxidants
Activity of antioxidants were investigated for its association with each other. Cause and effect analysis indicated strong positive associations between all investigated antioxidants. Remarkably strongest positive strength of association was observed between GSH and GPx (r=0.963) followed by SOD and CAT (r=0.951) (Table 2). Nonetheless, lipid peroxidation showed strong but negative correlation with rest of the antioxidants. Highest association for LPO was observed with GPx (r=-0.982) and GSH (r=-0.980).
Discussion
Reactive oxygen species (ROS) has attracted researchers as novel signal mediators. Previous studies have agreed that ROSs play important role in cellular growth, differentiation, progression, and death23-24. Self-renewal and differentiation of spermatogonial cells goes through multiple cyclic waves of spermatogenesis, leading to high rate of oxygen consumption and generation of ROSs25. Previous study reported that ROSs may have beneficial role in germ cell development through meiosis to mature sperms26. BPA has been associated with multiple disorders and diseases and debated for the underlying mechanism to explain its dynamic adversities27. In last one decade much of the focus given to understand principal behind BPA’s action. It was revealed that ROSs generated by BPA exposure have significant role in associated adversities28-30.
Since ROS’s have significant role in proliferation and differentiation of spermatogonial stem cells, BPA’s underlying mechanism of action can interfere substantially when the testis is most vulnerable. Pubertal age requires stringent control over spermatogenesis to achieve sexual maturity at appropriate timing. Many studies have noted BPA’s role in dysfunctional and low count of sperms, testicular atrophy, disorientation of germ cells and infertility in men31. In the present study differential pattern in the activity of antioxidants was analysed in testicular tissues particularly during critical period of pre-pubertal, pubertal, and post-pubertal age, to establish its potential role in male reproduction.
Catalase breaks down hydrogen peroxide in to water and oxygen. Increase in activity of CAT also indicate increase in the level of hydrogen peroxide in target tissues. Significant reduction in the activity of CAT was observed in testicular tissues following 7 weeks and 9 weeks of BPA administration. This observation was in accordance with earlier studies32-33. However, this study also noted no significant alteration in the activity of CAT following first three weeks of administration of BPA, irrespective of doses. This period of administration also happens to be the most critical period of pubertal age in rats, also regarded as peri-adolescence34. In general, 3-week administration of BPA in adult rats causes significant reduction in activity of CAT in multiple tissues35-37. Unaltered activity of testicular CAT to the BPA induced oxidative insult is remarkable. A study by Siervo et al.38 reported that sleep restriction in rats during critical period of sexual maturation led to increase in lipid peroxidation causing negative effect on testicular tissues. This study also portrays that common stress such as sleep deprivation can affect level of oxidative stress in the testis during peripuberty. No significant change in activity of CAT despite administration of 5, 10, 50 mg/kg BPA for 3-weeks indicate either sufficient availability of CAT in the testicular cells or irresponsive to peroxidation. Previous study has reported improvement in antioxidation during pubertal age39. Selective modification of amino acid side-chain affects the state of enzyme from being chemical or catalytic in nature40. Catalase is a metalloenzyme, in absence of metal cation its activity is broadly affected41. Sertoli cells are responsible for bioavailability of metal ions in the testicular microenvironment42. Previous studies reported that during puberty Sertoli cells get elongated and tight junctions are complete between two cells, leading to secretion of seminiferous fluid containing metal ions43. It is possible that BPA exposure may have delayed maturation of Sertoli cells causing lower availability of metal cation and thus making CAT activity immune from BPA induced oxidative insult.
SOD catalyse dismutation of superoxide radicals into oxygen and hydrogen peroxide, thus, an extremely important antioxidant against BPA induced ROSs. Unlike CAT, activity of SOD declined significantly following administration of 50 mg/kg BPA (Group IV) for three weeks between 42-63 PND. However, during the same period decline in activity of groups II and III was not significant comparing to control. A study by Sahoo and Roy44, reported that alteration in antioxidant system before pubertal maturation affects spermatogenesis and steroidogenesis, marked by reduced germ cell count, decrease testosterone, and reduced diameter of seminiferous tubules. BPA administration between 42-91 PND and 42-105 PND showed significant decline in all three test groups (5, 10, and 50 mg/kg BPA). These observations were typical, as many previous studies have noted similar results45-46. Lower decline in activity of testicular SOD in animals exposed with 5 and 10 mg/kg BPA for 42-63 PND comparing to 42-91 and 42-105 PND could be due to sufficient availability of SOD in the testis.
GSH and GPx are participants of the glutathione cycle, where GSH reversibly convert into oxidized glutathione (GSSG) by degrading hydrogen peroxide into water. This reaction is catalysed by GPx, therefore, level of GPx decides activity of GSH in case of an oxidative insult. In the present study level of GSH and activity of GPx declined invariably in BPA exposed animals, irrespective of doses or period of exposure. Decline in level of GSH was expected as available GSH could have been utilized during earlier exposure, specifically, before exhaustion of GPx and presumably no recycling of GSSG. Though, Group IV showed significant decline in level of GSH in testicular tissue following 42-63 PND BPA exposure, level of GSH in groups II and III was not statistically significant. Similarly, groups III and IV showed significant decline in activity of GPx but Group II was found within control range. It indicated that lower dose of BPA exposure during pre-pubertal age has better toleration against induced oxidative stress. Under influence of BPA, decrease in testicular GSH level and GPx activity is not infrequent, previous studies have noted similar results47-49. Nonetheless, differential activity during early pubertal age is unique. Similar results for SOD and CAT reflected that there could be an underlying mechanism that either provides additional support to antioxidant defence system or modulate BPA’s action on testicular tissues in such a way that differed before and after puberty.
Results of lipid peroxidation in this study corroborated with results for activity of other antioxidants. Since effect of BPA exposure between 42-91 PND and 42-105 PND on investigated antioxidants were typical, exposure between 42-63 PND is of specific importance. Level of LPO in groups III and IV following 3 weeks of BPA administration was similar to that of the 7 weeks. It implicates that level of oxidative insult in response to BPA administration during pre-pubertal age was similar to that of the pubertal age. It also involves differential response of antioxidant defence system against BPA exposure specifically during testicular maturation.
Trend and linear forecast of antioxidants under influence of BPA administration during period of investigation was assessed to explore deviation against sham control. Unique trends were observed for variables in activity of CAT and GPx. Activity of CAT in Group II indicated weak but positive relationship with Group I whereas, negative relationship with Group IV. It indicated dose related dependency in activity of catalase, however, lack of relatedness in Group III ensured no direct impact of BPA. Previous studies have noted direct impact of BPA exposure on antioxidants enzymes50-51, however, activity of antioxidants during pubertal age seem to deflect from conventional response. Where most observed values for CAT activity were found in low range with respect to control, activity of SOD was found at high range. Activity of SOD in all three test groups during investigated periods reflected low relatedness with control. Despite significant reduction in activity of both SOD and CAT, specifically following 42-91 PND and 42-105 PND, the relationship between variables were too weak to relate with Group I. Similar trend was observed in level of GSH and activity of GPx. Extremely weak relatedness in variables of both enzymes revealed independent behaviour during pre-pubertal, pubertal and post-pubertal age. It also reflected differential activity for equal doses of BPA following each investigated interval. Generation of ROSs during initial spermatogenesis varies substantially based on regulation of spermatogenic germ cells52. During spermatogenesis, role of ROSs is indispensable, as it plays important role in regulation of proliferation and maturation of spermatogonia26. Based on results of this study, it appears that though BPA induces oxidative stress of various degree in the testis, there are possibly multiple other interlinking factors at play. These factors may be associated with initial pre-pubertal and pubertal changes in testis.
During an oxidative insult, antioxidants perform in a highly organised coordination. For instance, SOD dismutate superoxide into hydrogen peroxide, which further degraded by CAT and/or GPx. The actuality of this coordination can be established by relating level of variations in the activity or levels of antioxidant against common oxidative stress. The coordination and synchronization of antioxidant enzyme prevents cellular damages and vasoactive agents53. Interestingly, results of this study showed strong strength of associations between performances of antioxidants. Despite independent variables for activities of antioxidants in response to BPA administration, the variations in activity of individual antioxidant enzyme were still strongly related. It conveys that despite indulgence of two independent factors (pubertal changes and BPA induced oxidative stress) the coordination between individual antioxidant enzymes were intact. Which implicates that decline in activity of antioxidants were due to excessive generation of ROSs and not due collapse of antioxidant defence system. Testicular maturation is a complex process that requires precise expansion of germ cells, triggering of androgens, regulation of hypothalamic-pituitary-testicular axis, and development of seminiferous tubules. BPA exposed rats have been noted with testicular atrophy54 and reduced size48, its administration at critical period of development may delay maturation by many weeks. Initial toleration against BPA (i.e. following 42-63 PND) could be due to advance preparation for major spermatogenic event. Later responses of antioxidants (i.e. following 42-91 PND and 42-105 PND) suggested that pubertal exposure may deteriorate testicular functions beyond typical adversities caused following adult exposure.
Conclusion
In conclusion, this study revealed differential activity of antioxidant enzymes in response to BPA at critical age of puberty in rats. Study noted better toleration against variable doses BPA during peri-adolescence, while significant reduction in activity was observed at later stage. Response of antioxidants to BPA induced oxidative insult during initial 3 weeks was not conventional. Despite major pubertal histological alterations in testis and BPA induced oxidative stress, activity of antioxidants was largely coordinated. Based on results of this study, it was presumed that pubertal testis by default requires a distinctive rigorous balance of ROSs and antioxidants in preparation of initial spermatogenic wave, thus, exposure of BPA at this stage can delay testicular maturation.
Acknowledgement
Authors are thankful to UGC, New Delhi, for financial assistance provided by UGC-NET JRF fellowship and Department of Zoology, the University of Rajasthan, for providing necessary facilities.
Conflict of Interest
There are no conflict of interest.
Funding Sources
There is no funding Sources.
References
- Taberner E, Navratil N, Jasmin B, Salerno M, Grambo B, Althouse GC. Pubertal age based on testicular and epididymal histology in Göttingen minipigs. Theriogenology., 86(9): 2091-2095 (2016).
- Matsuo N, Anzo M, Sato S, Ogata T, Kamimaki T. Testicular volume in Japanese boys up to the age of 15 years. Eur J Pediatr., 159(11): 843-5 (2000).
- Flaws JA, Sharara FI, Silbergeld EK, Hirshfield AN. 50 – Environmental Exposures and Women’s Reproductive Health, Editor(s): Marlene B. Goldman, Maureen C. Hatch, Women and Health, Academic Press, pp. 625-633 (2000). ISBN 9780122881459, https://doi.org/10.1016/B978-012288145-9/50058-9.
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol., 55: 373–399 (2004).
- Foyer CH, Noctor G. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell., 17: 1866–1875 (2005).
- Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012 May;24(5):981-90. doi: 10.1016/j.cellsig.2012.01.008. Epub 2012 Jan 20. PMID: 22286106; PMCID: PMC3454471.
- Dennery PA. Effects of oxidative stress on embryonic development. Birth Defects Res C Embryo Today., 81(3): 155-62 (2007).
- Chen C, Ouyang WY, Grigura V, Zhou Q, Carnes K, Lim H, et al. ERM is required For transcriptional control of the spermatogonial stem cell niche. Nature., 436(7053): 1030–4 (2005).
- Driver AS, Kodavanti PR, Mundy WR. Age-related changes in reactive oxygen species production in rat brain homogenates. Neurotoxicol Teratol., 22(2): 175-81(2000).
- Rizvi F, Preston CC, Emelyanova L, Yousufuddin M, Viqar M, Dakwar O, et al. Effects of Aging on Cardiac Oxidative Stress and Transcriptional Changes in Pathways of Reactive Oxygen Species Generation and Clearance. J Am Heart Assoc., 10(16): (2021) e019948. doi: 10.1161/JAHA.120.019948.
- Lam YT. Critical Roles of Reactive Oxygen Species in Age-Related Impairment in Ischemia-Induced Neovascularization by Regulating Stem and Progenitor Cell Function. Oxidative Medicine and Cellular Longevity. 2016: 7095901. https://doi.org/10.1155/2016/7095901
- Rey RA, Campo SM, Bedecarrás P, Nagle CA, Chemes HE. Is infancy a quiescent period of testicular development? Histological, morphometric, and functional study of the seminiferous tubules of the cebus monkey from birth to the end of puberty. J Clin Endocrinol Metab., 76(5): 1325-31(1993).
- Asadi N, Bahmani M, Kheradmand A, Rafieian-Kopaei M. The Impact of Oxidative Stress on Testicular Function and the Role of Antioxidants in Improving it: A Review. J Clin Diagn Res., 11(5): IE01-IE05 (2017).
- Syntin P, Chen H, Zirkin BR, Robaire B. Gene expression in Brown Norway rat Leydig cells: effects of age and of age-related germ cell loss. Endocrinology., 142: 5277–5285(2001).
- Liu Y, Wu Y, Qin G, Chen Y, Wang X, Lin Q. Bioaccumulation and reproductive toxicity of bisphenol A in male-pregnant seahorse (Hippocampus erectus) at environmentally relevant concentrations. Science of The Total Environment., 753: 141805 (2021). ISSN 0048-9697,
- Guidelines on the regulation of scientific experiments on animals. New Delhi: Ministry of Environment and Forests, CPCSEA Standard Operating Procedures for Institutional Animals Ethics Committee (IAEC). (2010):
- Long JA, Evans AM. On the attainment of sexual maturity and the character of the first estrous cycle in the rat. ci., 18: 244 (1920).
- Aebi H. Catalase in Bergmeyer Hans Ulrich, 5th Edition, “Methods of Enzymatic Analysis”, Academic Press Incorporated, New York, USA, pp 273-278 (1974).
- Marklund S, Marklund G. Involvement of the superoxide anion radical in the autooxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem., 47: 469-474 (1974):.
- Hissin PJ, Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem., 74: 214-226 (1976).
- Wood JL In: Fishman, W.H. (Ed.), In: Metabolic Conjugation and Metabolic Hydrolysis, vol. II. Academic Press, New York. 61-299 (1970).
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem., 95: 351-358 (1979).
- Zhang H, Gomez AM, Wang X, Yan Y, Zheng M, Cheng H. ROS regulation of microdomain Ca2+ signalling at the dyads. Cardiovascular Research. 98(2): 248–258 (2013).
- Sena LA, Chandel NS Physiological roles of mitochondrial reactive oxygen species. Molecular Cell., 48(2): 158–166 (2012).
- Aitken RJ, Smith TB, Jobling MS, Baker MA, DeIuliis GN. Oxidative stress and male reproductive health. Asian J Androl., 16(1): 31–8 (2014).
- Shi Y, Buffenstein R, Pulliam DA, Van Remmen H. Comparative studies of oxidative stress and mitochondrial function in aging. Integr Comp Biol., 50(5): 869-79 (2010).
- Gassman NR. Induction of oxidative stress by bisphenol A and its pleiotropic effects. Environ Mol Mutagen., 58(2): 60-71 (2017).
- Schug TT, Heindel JJ, Camacho L, Delclos KB, Howard P, Johnson AF, et al. A new approach to synergize academic and guideline-compliant research: the CLARITY-BPA research program. Reprod Toxicol., 40: 35–40 (2013):.
- Seachrist DD, Bonk KW, Ho SM, Prins GS, Soto AM, Keri RA. A review of the carcinogenic potential of bisphenol A. Reprod Toxicol., 59: 167–182 (2016).
- Gassman NR, Wilson SH. Bisphenol A and Nongenotoxic Drivers of Cancer. In: Waters M, Hughes C, (Eds.). Translational Toxicology and Therapeutics: Windows of Developmental Susceptibility in Reproduction and Cancer. John Wiley & Sons, Inc; (2016).
- Castellini C, Totaro M, Parisi A, D’Andrea S, Lucente L, Cordeschi G, et al. Bisphenol A and Male Fertility: Myths and Realities. Front Endocrinol (Lausanne)., 11: 353 (2020):.
- El-Beshbishy HA, Aly HA, El-Shafey M. Lipoic acid mitigates bisphenol A-induced testicular mitochondrial toxicity in rats. Toxicol Ind Health., 29(10): 875-87 (2013).
- Tiwari D, Vanage G. Bisphenol A Induces Oxidative Stress in Bone Marrow Cells, Lymphocytes, and Reproductive Organs of Holtzman Rats. Int J Toxicol., 36(2): 142-152 (2017).
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol., 16(2): 83-109 (1983).
- Amraoui W, Adjabi N, Bououza F, Boumendjel M, Taibi F, Boumendjel A, et al. Modulatory Role of Selenium and Vitamin E, Natural Antioxidants, against Bisphenol A-Induced Oxidative Stress in Wistar Albinos Rats. Toxicol Res., 34(3): 231-239 (2018).
- Hassan ZK, Elobeid MA, Virk P, Omer SA, ElAmin M, Daghestani MH. Bisphenol A Induces Hepatotoxicity through Oxidative Stress in Rat Model. Oxidative Medicine and Cellular Longevity., 2012: 194829 (2012). https://doi.org/10.1155/2012/194829
- AbdEl-Gwaad HMS, El-Wahab HMFA, Mohamed EAK et al. Modulatory effect of dry orange (citrus sinensis) peel powder on bisphenol A-induced hepatic and splenic toxicity in rats. JoBAZ., 81: 49 (2020).
- Siervo GEML, Ogo FM, Staurengo-Ferrari L, Anselmo-Franci JA, Cunha FQ, Cecchini R, Guarnier FA, et al. Sleep restriction during peripuberty unbalances sexual hormones and testicular cytokines in rats. Biol Reprod., 100(1): 112-122 (2019).
- Paltoglou G, Fatouros IG, Valsamakis G, Schoina M, Avloniti A, Chatzinikolaou A, et al. Antioxidation improves in puberty in normal weight and obese boys, in positive association with exercise-stimulated growth hormone secretion. Pediatr Res., 78(2): 158-64 (2015).
- Vallee BL, Robert JP Williams. Metalloenzymes: The Entatic Nature of their Active Sites. Proceedings of the National Academy of Sciences of the United States of America., 59(2): 498–505 (1968).
- Plaunt AJ, de Lourdes Betancourt-Mendiola M, Smith BD. 4.17 – Biomolecule Recognition Using Transition Metal Ions and Hydrogen Bonding, Editor(s): Jerry L. Atwood, Comprehensive Supramolecular Chemistry II, Elsevier, pp 575-591 (2017), ISBN 9780128031995, https://doi.org/10.1016/B978-0-12-409547-2.12576-4.
- Shi JF, Li YK, Ren K, Xie YJ, Yin WD, Mo ZC. Characterization of cholesterol metabolism in Sertoli cells and spermatogenesis (Review). Mol Med Rep. 17(1): 705-713 (2018).
- Petersen C, Soder O. The sertoli cell–a hormonal target and ‘super’ nurse for germ cells that determines testicular size. Horm Res., 66(4): 153-61 (2006).
- Sahoo DK, Roy A. Compromised Rat Testicular Antioxidant Defence System by Hypothyroidism before Puberty. Int J Endocrinol., 2012:637825 (2012).
- Olujimi O, Ayoola R, Olayinka O, Dosumu O, Rotimi S, Aladesida A. Evaluation of antioxidant enzymes performances and DNA damage induced by bisphenol A and diisobutylphthalate in Hyperiodrilus africanus-earthworms. Emerging Contaminants., 6: 1-9 (2020).
- Santiago J, Silva JV, Santos MAS, Fardilha M. Fighting Bisphenol A-Induced Male Infertility: The Power of Antioxidants. Antioxidants (Basel)., 10(2): 289 (2021). doi: 10.3390/antiox10020289.
- Wang J, Chen C, Jiang Z, Wang M, Jiang H, Zhang X. Protective effect of Cordyceps militaris extract against bisphenol A induced reproductive damage. Syst Biol Reprod Med., 62: 249–257 (2016).
- Olukole SG, Lanipekun DO, Ola-Davies EO, Oke BO. Maternal exposure to environmentally relevant doses of bisphenol A causes reproductive dysfunction in F1 adult male rats: Protective role of melatonin. Environ Sci Pollut Res., 26: 28940–28950 (2019).
- Alboghobeish S, Mahdavinia M, Zeidooni L, Samimi A, Oroojan AA, Alizadeh S, Dehghani, MA, et al. Efficiency of naringin against reproductive toxicity and testicular damages induced by bisphenol A in rats. Iran J Basic Med Sci., 22: 315–523 (2019).
- Othman AI, Edrees GM, El-Missiry MA, Ali DA, Aboel-Nour M, Dabdoub BR. Melatonin controlled apoptosis and protected the testes and sperm quality against bisphenol A-induced oxidative toxicity. Toxicol Ind Health., 32(9): 1537-49 (2016).
- Kabuto H, Amakawa M, Shishibori T. Exposure to bisphenol A during embryonic/fetal life and infancy increases oxidative injury and causes underdevelopment of the brain and testis in mice. Life Sci., 74(24): 2931-40 (2004).
- Zhao GQ, Deng K, Labosky PA, Liaw L, Hogan BLM. The gene encoding bone morphogenetic protein 8B is required for the initiation and maintenance of spermatogenesis in the mouse. Genes Dev., 10: 1657–1669 (1996).
- Sáez GT, Están-Capell N. Antioxidant Enzymes. In: Schwab M. (eds) Encyclopedia of Cancer. Springer, Berlin, Heidelberg. (2014). https://doi.org/10.1007/978-3-662-46875-3_7210
- Manfo FP, Jubendradass R, Nantia EA, Moundipa PF, Mathur PP. Adverse effects of bisphenol A on male reproductive function. Rev Environ Contam Toxicol., 228: 57-82 (2014).