Walid Abu Rayyan1*, Wafa Abu Haza1*, Nesrin Seder2, O'la Al-Fawares1 and Abdul Fattah Salah Fararjeh1
1Department of Medical Laboratory Analysis Faculty of Science, Al-Balqa Applied University, Al-salt, Jordan.
2Department of Pharmaceutical Chemistry and Pharmacognosy, Faculty of Pharmacy, Applied Science Private University, Amman, Jordan.
Corresponding Author E-mail: walid.aburayyan@bau.edu.jo
DOI : https://dx.doi.org/10.13005/bpj/2522
Abstract
Wuhan, China, substantially is the epicenter of the COVID-19 pandemic in December 2019. Coronavirus, the confounder virus, a zoonotic in origin was the causative agent of the disseminated disease worldwide. Structural similarities and convergence points were demonstrated between the coronavirus, SARS, and MERS viruses. Aberrantly, a subset of patients developed a serious acute respiratory distress syndrome or diffuse alveolar injury whereas the rest of the patients encountered mild or no symptoms. The pathological clinical laboratory findings are not only critical in the diagnosis of the COVID-19 infection, on the contrary, but they are also crucial in the prognostic predictions about disease prognosis and therapeutic response. This review aims to give some historical context for the pandemic, demonstrate the laboratory's important role in the screening of COVID-19 infection, and review the current phase of biomarker examination in COVID-19 infection, focusing on markers derived directly from the hematological laboratory, reflecting the implications of COVID-19 on the hematological system and coagulation pathways. In conclusion, there is a direct significant correlation between infection severity, the death rate in COVID-19 patients, and the low number of either WBCs or a high number of WBCs with a low number of lymphocytes.
Keywords
COVID-19; Coagulation; D-Dimer; Hematology; Lymphopenia
Download this article as:| Copy the following to cite this article: Rayyan W. A, Haza W. A, Seder N, Al-Fawares O, Fararjeh A. F. S. The Implications of COVID-19 Infection on Hematologic Parameters and Coagulation Activity: A Systematic Review. Biomed Pharmacol J 2022;15(4). |
| Copy the following to cite this URL: Rayyan W. A, Haza W. A, Seder N, Al-Fawares O, Fararjeh A. F. S. The Implications of COVID-19 Infection on Hematologic Parameters and Coagulation Activity: A Systematic Review. Biomed Pharmacol J 2022;15(4). Available from: https://bit.ly/3OvPXRh |
Introduction
The pandemic of a severe acute respiratory syndrome (ARDS) aroused by coronavirus type 2 (SARS-CoV-2) continues to demonstrate diagnostic and therapeutic challenges 1,2. The first reported case of the emerged infection was discovered in December 2019 in Wuhan, China 3. On February 11, 2020, World Health Organization (WHO) officially named the disease coronavirus disease 2019 (COVID-19). On March 11, 2020, WHO declared COVID-19 as a pandemic 4,5. Consequently, on 18 September 2022, there are currently 608 million confirmed cases in 228 countries and territories, with more than 6.5 million deaths worldwide6.
A reliable and accurate diagnosis of COVID-19 is indispensable for the pivotal purposes of diagnosing newly infected patients and containment of the virus’s massive dissemination. Real-time reverse transcriptase-polymerase chain reaction (RT-qPCR), which is used all over the world, is the gold standard molecular-based test for the identification of COVID-19 in clinical samples from patients with COVID-19-compatible symptoms 7–9. Pathogen identification via RT-qPCR is common because it is rapid duration, high sensitivity, specificity, and additionally the affordability since PCR test cost is almost $51 10. The test was accomplished by extracting extremely small amounts of RNA from respiratory tract samples such as nasopharyngeal swab/oropharyngeal, tracheal aspirate, sputum, and bronchoalveolar lavage 9,11–20. Wang et al. highlighted in a study of 1070 samples from 205 patients with COVID-19 in China that the sensitivity of the test is 32%, 63%, 72%, and 93% in the oropharyngeal swab, nasopharyngeal swab, sputum, and bronchoalveolar lavage, respectively 21.
The clinical manifestations of COVID-19 infections were most commonly incorporated with pneumonia and acute respiratory distress syndrome 22,23. There is a scientific agreement on the role of COVID-19 in exerting a multisystem disease in adults and old ages 2425. Controversially, young patients with COVID-19 infections showed asymptomatic or mild-to-moderate illnesses which were rarely fatal 26,27. Remarkably, several cases of young patients developed severe symptoms that emanate from death as an end-stage 28. One of the primary symptoms of COVID-19 is hypoxia and a multi-systemic illness that may be exacerbated by the viral proteins’ modification of Hb. The alteration in Hb conformational shape reduces the fraction of completely functioning Hb in oxygen transportation 29. In a study of 508 patients conducted by Al-Balas et al., the most commonly reported symptoms in both medically free and medically ill individuals were dry cough, nonspecific malaise, and fever. The average length of hospital stay was shorter in medically free patients than in medically ill patients, and it was shorter in asymptomatic patients than in symptomatic patients 30,31. There was a statistically significant link between the presence of long-term illnesses and the onset of symptoms in COVID-19 patients 30. Noticeably, intensive care was needed more frequently and hospitalization for longer periods for children with the multisystem inflammatory syndrome (MIS-C) 32,33.
The clinical findings of the complete blood cells (CBC) and coagulopathy were indispensable tests for the prognostic activity of the disease prognosis in COVID-19 patients. For instance, a massive high platelet count in the presence of systemic immunological and coagulation activity is a prognostic of a significant compensatory platelet generation response in these patients 34. A study conducted on 449 severe COVID-19 patients by Srivastava et al., revealed that fibrinogen and D-dimer degradation products are significantly increased in COVID-19 infections. This escalation is proportionally linked to hypercoagulability throughout the body and repetitive venous thromboembolic complications 35. Mortality rates among COVID-19 patients are found to be directly correlated to the elevation in D-dimer level 36. COVID-19 causes not only microvascular thrombotic disorders but also arterial thrombotic events such as strokes and ischemic limbs 37.
Controversially, mild thrombocytopenia is noticed significantly in COVID-19 patients. The cause is attributed to two conditions; firstly, an increase in platelet consumption and, secondly, a decrease in platelet production 38. Eventually, COVID-19 patients rarely develop disseminated intravascular coagulopathy (DIC) or life-threatening bleeding complications during the infection 39. The immune system’s cells, specifically lymphocytes, might be affected 40. Lymphopenia may develop from virus-induced destruction of lymphocytes (especially T lymphocytes) and reduction of lymphocyte proliferation, and recovered lymphocytes may be a prediction of eventual recovery 41. Eventually, we conducted the current literature review on hematological diversities and coagulopathy in COVID-19 infection, which may help health professionals in COVID-19 management, we have reviewed over 150 relevant articles in the area of the pathological association between Covid-19 infections and coagulopathy disorders published either in Clarivate database or Scopus database.
The Impact of Covid-19 on Hematological Cellular Elements
Platelets
Platelets, also known as thrombocytes, are small nucleated cellular fragments (2-4 μm) that originated from megakaryocytes and are released into the bloodstream at maturation. These spherical fragments have a short lifespan of only 8 or 9 days. They act as the first line of defense against vascular injuries and thrombosis by stimulating a cellular response 42. As already reviewed by M. Yang et al. in the 2003 SARS epidemic, thrombocytopenia affected 20-55% of patients 43. Meanwhile, a rebound in thrombocyte count was also reported by Giannis et al. after the infection clearance 44. Based on Wong et al. patients’ encountered acquired thrombocytopenia due to the outbreak of SARS-Cov-1 exposed to a higher risk of mortality 45. Concomitantly, thrombocytopenia was reported in Middle East Respiratory Syndrome (MERS) infections, another respiratory infection caused by an etiological agent from the coronaviridea family. Additionally, Wool & Miller et al. designated a relevant proportion of 5% to 41.7% of people infected with COVID-19 developed thrombocytopenia, even though, the decrease in thrombocyte number was mild (with counts often falling within the range of 100 to 150 109/L). Thrombocytopenia was significantly proportional to the severity of the disease 46, nine studies in China 47–55 with 1779 samples- of COVID-19 patients administrated by Lippi et al. revealed that platelets count of individuals with severe disease is only 23 109/L to 31 109/L lower than that of patients with the non-severe disease56. This moderate thrombocytopenia has been identified in a proportion of 58-95% of severely infected cases of COVID-19 57. On the other hand, patients with COVID-19 rarely developed a blended condition of immune thrombocytopenic purpura and severe thrombocytopenia at the same time 58.
Erythrocytes and hemoglobin
Coincided with the emergence of science, red blood cells, also known as RBCs, were designated exclusively as carriers of oxygen and nutrients to the various tissues throughout the body 59. Recent empirical evidence suggests that RBCs have a substantial role in versatile physiological functions, such as controlling systemic nitric oxide metabolism, blood rheology, redox regulation, and viscosity 60. In mature RBCs, the nucleus and organelles are abandoned to ameliorate RBCs structure to pertain hemoglobin (Hb), the primary protein responsible for oxygen transport, so that the RBCs can execute their role in oxygen transportation more efficiently 61,62.
Additionally, RBCs are performing a feat role in the microcirculation since they act as sensors of local hypoxia 63. A study published by Favaron et al. noticed an improved oxygen extraction capability in the microcirculation of COVID-19 patients with severe hypoxia. This was found to be a result of increased RBC availability 64. However, several studies have demonstrated that patients with COVID-19 have modifications in RBC membrane metabolism and structure 65–69. In addition, due to COVID-19 impacts on Hb, COVID-19 has been evaluated as a possible source of acquired acute porphyria. It has been demonstrated that raising Hb F levels in critically sick COVID-19 patients may slow disease progression, reduce morbidity, and improve survival. 70. Commensurately, Russo et al., highlighted the usage of umbilical fetal blood transfusion on COVID-19 patients providing objective confirmation of the role of Hb beta chains in the development of COVID-1971. On the bases of wenzhong & hualan revealed that coronavirus can interact with protoporphyrin IX via the spike protein as same as various other viruses 72. Hemoglobin beta chains, specifically ORF 8, and viral surface glycoproteins show a high affinity to allosteric binding 73. Liu et al. identified several viral proteins (ORF3a, ORF8a, orf1ab, ORF7a, and ORF10) as possible ligands for the binding of hemoglobin’s 1-beta chains. This binding induces Hb denaturation that hampers viral replication by preventing the cell fusion of COVID-19 through spike protein (as found in other viruses) 74. High-performance liquid chromatography analysis of the serum of 134 COVID-19 patients by San Juan et al., revealed an abundant accumulation of the byproducts of uroporphyrin I, metabolite coproporphyrin III, and coproporphyrin I 75. According to Shoenfeld et al., COVID-19 and Hb would interact in two locations: erythrocytes, where the virus is delivered intracellularly via the link between Band-3 and spike proteins, and the bone marrow, in which the virus binds with nascent erythroblasts via CD147 and CD2676. While in erythroblasts the presence of nuclear material would facilitate viral reproduction and, in this scenario, restrict the normal circulation of red blood cells from the spleen to the bloodstream, causing anemia, this is not the case at the erythrocyte level, where the virus penetrates the red blood cell and interacts with the Hb molecule but its replication is blocked by the absence of a nucleus 77. Anai et al., in a study, revealed that elevated glycosylated Hb levels raise CD147 expression, which in turn raises the likelihood of further problems 78. Several investigations in individuals with severe COVID-19 disease have reported lower Hb levels, but there is no experimental data to suggest a modification of the oxygen dissociation curve at this time.
Leukocyte
Leukocytosis, a high white blood cell count (WBC) is indicative of an active immune response in COVID-19 infections 79. A Retrospective study of the clinical characteristics of 52 COVID-19 patients demonstrated that asymptomatic people may have been exposed to the virus at a much earlier stage or that they may have compromised IgM production 80. Complete blood counts demonstrated that asymptomatic patients had higher counts of eosinophils, lymphocytes, and basophils than symptomatic patients, according to these result Han et al. shows that a high number of WBC, especially lymphocytes, is directly linked to a seropositive COVID-19 patient even though there is an absence of symptoms 80.
Compiled data for COVID-19 patients with elevated WBC counts showed that the patients were more likely to develop severe illness and ultimately pass away 81–83. Meanwhile, another study has designated a direct significant correlation between infection severity, the death rate in COVID-19 patients, and the low number of either WBCs or a high number of WBCs with a low number of lymphocytes 84.
L. Yang et al. have established in a meta-analysis study a link between lymphopenia and death rate in COVID-19 patients 85. Regardless of the severity of the baseline disease a descriptive study of 99 cases of COVID-19 in Wuhan, China revealed that lymphocytes were considerably lower on admission and remained lower during hospitalization in non-survivors, but increased following therapy in survivors 86. Both human macrophages and monocytes express ACE2, making them susceptible to COVID-19infections activate and transcribe proinflammatory genes 87. Fascinatingly, COVID-19 infection appears to dramatically down-regulate the expression of ACE2 in peripheral blood (PB) monocytes. This may be a secondary result of viral binding, we still don’t know if this decrease in ACE2 receptor activity is a proxy for viremia 88. In addition, ACE2 expression was detected on CD169+ and CD68+ macrophages in the lymph nodes and spleen of COVID-19 patients, suggesting that the COVID-19 virus may specifically target ACE2-positive myeloid cells in these organs 89. The red pulp of the spleen is a common location for infected CD169+ macrophages. Furthermore, positive tests for viral nucleocapsid protein antigens were more common in macrophage-dense regions of the lymph node periphery 90. Previous research by Honke et al. has shown that CD169+ macrophages, due to their resistance to type I IFN-dependent activation, are in charge of maintaining a steady state of viral replication in aid of immune development. This suggests that COVID-19 could enter spleens and lymph nodes after infecting CD169+ macrophages 91. However according to J. Wang et al., there may be more viral replication sites in the body as a result of this, and it may also reduce immunity 79.
In addition, demographic characteristics such as smoking and blood groups affect the levels of immunoglobulin, Cross-sectional research of 412 Jordanians conducted between September 2021 and January 2022 demonstrated that the enzyme-linked immunosorbent assay approach was used to test total IgG antibodies against COVID-1992. The seroprevalence of IgG in the study’s population was 81.8%, with a mean of 15.17 IU/ml. 45.4% of the positive participants had already been infected with COVID-19, while the rest of the study population received vaccination doses 92.
Effect of COVID-19 pandemic on hematopoietic organs (bone marrow and spleen)
In the majority of cases, Hematopoietic stem and progenitor cells (HSPCs) in the bone marrow are responsible for producing a variety of peripheral immune cell subpopulations 93. There have been some debates about whether COVID-19 affects the HSPC niche directly or indirectly. Research by Zheng et al. conducted immunofluorescence on 33 types of tissues and found that the receptor-binding subdomain 1 of the spike protein of the COVID-19 (RBD-SD1) probe could interact with bone marrow cells without the need for ACE2. However, it has been observed, that COVID-19 causes a rise in ACE2 expression in human bone marrow primary cells 94. In Elahi et al. study, the authors concluded that human bone marrow cells may be susceptible to infection with COVID-1995. In concomitant, various stages of specification of human stem cells were used in the experiments of Kucia et al., that has revealed ACE2 and transmembrane serine protease 2 (TMPRSS2) are expressed at the gene and protein levels in a variety of human stem cell lineages, including CD34+CD133+linCD45- cells, which may develop into endothelial progenitor cells (EPCs) and Hematopoietic stem cells (HSCs), CD34+LinCD45+ HSCs, and CD34+CD133+KDR+CD31+EPCs 96. In addition, pyroptosis in HSCs was found to be triggered by the viral spike protein, which activates the NLrp3 inflammasome 96. In a series of COVID-19 patients, Varga et al. showed endothelial cell involvement across vascular beds of diverse organs, their results proved further evidence that COVID-19infections promote endothelial cell damage in COVID-19 patients by targeting ACE2 expressers 97. This finding is also supported by the fact that ACE2 is shown to be present in a large number of hematopoietic progenitor cells (HPCs) and up to 65% of HSCs deduced from human cord blood 9899.
Debliquis et al. demonstrated that three severely ill COVID-19 patients had an increase in plasma cells, pleomorphic megakaryocytes, macrophages, and hemophagocytosis in their bone marrow aspirates 100. According to Rapkiewicz et al., increased numbers of megakaryocytes with morphology indicative of active platelet synthesis were discovered in the bone marrow, and electron microscopy of megakaryocytes in bone marrow also revealed the presence of extremely rare virions101.
However, in comparison to the lung, heart, and intestines, the spleen has lower levels of ACE-2 receptor expression 102.
On the bases of Feng et al. study, six COVID-19 patients have examined postmortem, and ACE-2 was located in the red pulp and medulla of lymph nodes, indicating that it was expressed in these tissues. In addition, CD169 and CD68 macrophages in the lymph nodes and spleen expressed ACE-2 receptors 103. The spleen’s red pulp, and only rarely its white pulp, was found to contain viral nucleocapsid antigens. Lymphocyte apoptosis could be triggered by IL-6 secreted by virus-infected macrophages 103. Pathological abnormalities in the spleen of 10 patients with COVID-19 were evaluated by Xu et al., 104 who found a reduction in T and B lymphocytes, a shrinking and atrophy of lymphoid follicles, a thinning of the white pulp, and an infiltration of neutrophils and plasma cells. In the spleen, Yao et al discovered a decline in lymphocyte count and evidence of cell degeneration/necrosis 105.
The implication of COVID-19 Infection on Thrombus Formation
Thrombosis contributes significantly to the devastating consequences of COVID-19 infections such as myocardial infarction and acute respiratory distress syndrome. Fard et al. declared that several pathological modifications such as endothelial cell injury, plaque formation, and oxygen demand injuries are encountered following COVID-19 infection 106.
Congruently, Choudhury & Mukherjee et al. reported hyperactivity of platelets in extremely sick COVID-19 patients, indicating that platelets may play a vital role in the disease progression 107. Additionally, they revealed that platelets can interact with pathogens through a variety of receptors, including NOD-like receptors, Toll-like receptors (TLRs), the C-type lectin receptor family, and glycoproteins (GPs), including GPαIIbβ3 and GPIbα 107.
Summarizing that platelet activation and thrombosis are directly mediated by platelet TLRs and NOD2. Furthermore, recent in silico studies by Choudhury and Mukherjee et al., demonstrated that the spike protein of COVID-19 could interact with TLRs, particularly TLR-4108. Gorog et al. hypothesized putative signaling pathways responsible for the activity of platelets that could be the cause of recurrent thrombosis in COVID-19 patients 109.
Girardin et al. proposed a provision concerning the critical role of platelets in hemostasis, thrombosis, and immune response. Additionally, they highlighted Nucleotide Binding Oligomerization Domain Containing 2 (NOD2) activation in COVID-19 infections implication on the pathophysiology, thrombotic consequences, and treatment of inflammation in COVID-19 disease 110.
Furthermore, P-selectin, also known as CD62P, is an integral protein that serves as a cell adhesion molecule on the surfaces of activated endothelial cells and active platelets, allowing them to adhere to neutrophils and monocytes 111.
Interestingly, Larsen et al. in a study demonstrated that P-selectin stimulates monocytes to secret tissue factor (TF), the primary activator of the extrinsic coagulation cascade 112. Noticeably, P-selectin-mediated recruitment of leukocytes into the lungs occurs during ARDS, and that infusion of anti-P-selectin (a monoclonal antibody) reduces the severity of ARDS 113. According to Mulligan et al. and Neri et al. studies, soluble P-selectin levels are increased in ARDS cases compared to the control group, as well as in non-survivors compared to survivors 114,115. Klok et al., in a study of 184 patients with confirmed cases of COVID-19 pneumonia who were being treated in the intensive care unit (ICU), 23 had passed away (13%), 22 had been successfully discharged (12%), and 139 (76%) were still being treated there as of April 5, 2020. Patients were given at least the minimum necessary doses of thromboprophylaxis 116. Their study revealed that COVID-19 patients are more likely to have problems with blood clots, and P-selectin may play a role in starting intravascular coagulation 116.
In a normal scenario, platelets are responsible for preserving the integrity of the alveolar capillaries. However, in pathologic situations, platelets may be a contributing factor in the development of lung injury 117118. Furthermore, according to Zarbock et al., platelet-leukocyte aggregation and platelet-endothelial interactions have a role in the pathophysiology of acute lung damage 119. Hottz et al. demonstrated that in the case of viral infections, interactions with leukocytes, thrombocytopenia, and platelet secretion can all have either protective or harmful effects on the immune system 120.
A retrospective cohort study of a total of 383 COVID-19 patients at Wuhan’s Central Hospital was assessed by Liu et al., together with their respective clinical and laboratory data, to determine a definitive outcome by March 1, 2020. Those patients showed an increase in platelets per 50×109/L was related to a 40% reduction in mortality 121. Their study concluded that the thrombocytopenia rate in COVID-19 patients is predicted to be 5-41.7%, with a moderate form (100-150 x109/L) being the most common 121. A meta-analysis of 7,613 COVID-19 patients by Jiang et al. 122 revealed that platelet counts were observed to be significantly lower in severe and non-survivor patients as compared to those in non-severe and survivors, respectively, Figure 1123.
As a result of Q. Li et al., 2020 studies, low platelet count in COVID-19 patients may be due to platelet consumption and associated with an increased risk of mortality. However, it has not been established as a predictive factor for this disease’s mortality 124. According to Thach et al., patients who have a temporal tendency to a lowered platelet count may have a devastating thrombotic complication, and the lower platelet counts are also connected to an increased mortality rate 125.
Furthermore, Vice et al. research revealed that hypoxia and sepsis can independently enhance platelet aggregation via the release of von Willebrand factor, which is elevated in the entire blood of COVID-19 patients in the intensive care unit (ICU) 126. On the bases of Langer et al. and Yau et al. studies, the significant activation of coagulation in severe COVID-19 infection is most likely connected to the persistent inflammatory response caused by the virus’s strong cytokine production 127,128. However increased levels of D-dimers, thrombocytopenia, a prolonged prothrombin time (PT), and an activated partial thromboplastin time (aPTT) are all indicative of the severity of the disease and have been linked to a poor prognosis and a higher mortality rate in COVID-19 patients 127,128.
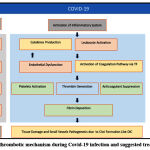 |
Figure 1: Possible thrombotic mechanism during Covid-19 infection and suggested treatment strategies129 |
According to Sui et al., showed that 119 COVID-19 patients, were hospitalized in the intensive care unit. A total of 67.2% of patients (80/119) had abnormally high levels of fibrinogen 130. However, fibrinogen levels were linked to disease severity and inflammatory markers, but not to the cardiac injury biomarker high sensitivity troponin I. The study concludes that patients with COVID-19, especially those with severe disease, had increased levels of fibrinogen which means fibrinogen levels were high in all patients at entry 130. Patients with COVID-19 who have elevated fibrinogen levels also tend to have increased inflammation, and more severe illness and require hospitalization to the intensive care unit 130. Additionally, Ranucci et al. concluded that fibrinogen, D-dimer, and IL-6 levels were evaluated in COVID-19 patients with ARDS who required mechanical ventilation 131. Tang et al. found the fact that increased IL-6 levels were associated with increased fibrinogen levels demonstrated and confirmed the relationship between procoagulant and inflammation alterations; all patients had raised IL-6 levels on admission 132.
Concomitantly, in a study of 1,099 Chinese patients with COVID-19, Guan et al. showed that 260/560 (46%) had an increased D-dimer (>0.5 mg/L) 133. According to the findings of Han et al., patients with COVID-19 have higher levels of D-dimer and fibrinogen than healthy controls. Also, significant elevations in fibrinogen degradation products (FDP) were observed 134. While the fibrinogen level remained high, the levels of D-dimers and FDP continued to rise with the severity of COVID-19 (Figure 2) 134.
Additionally, elevated D-dimer levels at admission, as well as increasing D-dimer levels throughout time, are both related to an increase in COVID-19 mortality. So patients who develop septic shock and septic physiology are at an increased risk of death. Patients who acquire DIC, even if it develops in the absence of sepsis, are also at an increased risk of death 135. The mechanisms that cause coagulation in COVID-19infection are not clear and confounder at this time, but the scientific society confirms an augmented role for inflammatory responses in the devastating cellular injuries rather than viral features.
Patients diagnosed with COVID-19 pneumonia showed coagulation abnormalities, namely, an escalation in levels of fibrinogen and D-dimer combined with mild thrombocytopenia36. Higher mortality rates were proportionally correlated with elevated D-dimer levels. Additionally, PT and aPTT times were abnormally short in COVID-19 patients which can lead to activation of clot formation events 121. Consequently, shortened aPTT is directly linked to elevated levels in the acute-phase protein Factor VIII (FVIII) 44. Meanwhile, in patients with severely advanced states, a condition mimicking DIC can develop especially in conditions of prolonged PT and aPTT readings 135. Moreover, D-dimer levels are aberrantly elevated in an extraordinary ratio with no correlation with any abnormalities detected in the PT/INR, aPTT, fibrinogen level, or platelet count nor these findings are unusual for DIC 130. Dissimilar to the pattern demonstrated in conventional DIC caused by traumatic origin or bacterial sepsis, COVID-19 patients show a minimal prolongation in aPTT and/or PT 44, and thrombocytopenia is mild (a platelet count of 100–150 ×109 /L) 58. The coagulopathy associated with COVID-19- extensively illustrates the spectrum of coagulation stages related to COVID-19 patients. Three stages have been demonstrated for COVID-19-associated coagulopathy: stage 1 D-dimer levels are extremely elevated, in stage 2 the elevated D-dimer levels are combined with mild thrombocytopenia and mild prolongation in PT/INR and aPTT, and eventually, stage 3 show classic DIC scenario 44.
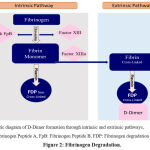 |
Figure 2: Fibrinogen Degradation. |
Eventually, Tsoupras et al., declared that COVID-19 coagulopathy is not been directly linked to severe bleeding as demonstrated in RNA-type viruses related to hemorrhagic symptoms, such as Ebola and some other hemorrhagic fever viruses136.
Conclusion
COVID-19 is a causative agent of a severe acute respiratory syndrome. Several cases of young patients developed severe symptoms that emanate from death as an end-stage. There is a statistically significant link between the presence of long-term illnesses and the onset of symptoms in COVID-19 patients. Patients who encountered acquired thrombocytopenia due to the outbreak of SARS-Cov-1 were exposed to a higher risk of mortality. Thrombocytopenia was significantly proportional to the severity of the disease COVID-19 and Hb would interact in two locations: erythrocytes, where the virus is delivered intracellularly via the link between Band-3 and spike proteins, and the bone marrow. Complete blood counts demonstrated that asymptomatic patients had higher counts of eosinophils, lymphocytes, and basophils than symptomatic patients. There is a direct significant correlation between infection severity, the death rate in COVID-19 patients, and the low number of either WBCs or a high number of WBCs with a low number of lymphocytes. Additionally, fibrinogen levels were linked to disease severity and inflammatory markers, but not to the cardiac injury biomarker high sensitivity troponin I. Inflammatory markers such as fibrinogen, D-dimer, and IL-6 levels showed evaluations in COVID-19 patients with ARDS who required mechanical ventilation. Eventually, significant elevations in fibrinogen degradation products were reported in severe patients, concomitantly with high levels of fibrinogen and D-dimers that continued to rise with the severity of COVID-19.
Acknowledgment
The authors would like to thank M Mansour Albalbaki for assistance with data collection and extraction.
References
- Coronavirus disease (COVID-19). Accessed September 26, 2022. https://www.who.int/emergencies/diseases/novel-coronavirus-2019?adgroupsurvey={adgroupsurvey}&gclid=EAIaIQobChMI7Zizjs2y-gIV4ZBoCR0-6wHJEAAYASAAEgKQFPD_BwE
- Vishnu KN, Kumar Uppala P, Vangoori Y, Rao Gudhanti SNK. A Review on Covid 19 Pandemic and its Global Effects. Asian Journal of Pharmaceutical Research. Published online November 27, 2021:242-246. doi:10.52711/2231-5691.2021.00042
CrossRef - WHO Coronavirus (COVID-19) Dashboard | WHO Coronavirus (COVID-19) Dashboard With Vaccination Data. Accessed December 19, 2021. https://covid19.who.int/
- Naming the coronavirus disease (COVID-19) and the virus that causes it. Accessed September 26, 2022. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it
- Naresh B v. A review of the 2019 novel coronavirus (covid-19) pandemic. Asian Journal of Pharmaceutical Research. 2020;10(3):233. doi:10.5958/2231-5691.2020.00040.4
CrossRef - Coronavirus disease (COVID-19). Accessed September 27, 2022. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- Raytthatha N, Shah I, Vyas J, Upadhyay U. An Informative Review on screening of COVID-19 (SARS-COVID-II). Research Journal of Pharmaceutical Dosage Forms and Technology. Published online August 21, 2021:259-265. doi:10.52711/0975-4377.2021.00043
CrossRef - Seder N, Abu Bakar M H, & Abu Rayyan W S. Transcriptome Analysis of Pseudomonas aeruginosa Biofilm Following the Exposure to Malaysian Stingless Bee Honey. Advances and applications in bioinformatics and chemistry. 2021: 14, 1–11. https://doi.org/10.2147/AABC.S292143.
CrossRef - Walid Abu Rayyan. High-throughput RNA extraction method for P. aeruginosa and S. pyogenes biofilms. J Appl Pharm Sci. 2023;13(00):001-010.
- CMS. Medicare Administrative Contractor (MAC) COVID-19 Test Pricing.
- Khiabani K, Amirzade-Iranaq MH. Are saliva and deep throat sputum as reliable as common respiratory specimens for SARS-CoV-2 detection? A systematic review and meta-analysis. Am J Infect Control. 2021;49(9):1165-1176. doi:10.1016/J.AJIC.2021.03.008
CrossRef - Kiro VV, Singh P, Srivastav S, et al. SARS-CoV-2 Rapid Antigen Detection in Respiratory and Nonrespiratory Specimens in COVID-19 Patients. J Lab Physicians. 2022;14(3). doi:10.1055/S-0042-1742415
CrossRef - Mohammadi A, Esmaeilzadeh E, Li Y, Bosch RJ, Li JZ. SARS-CoV-2 detection in different respiratory sites: A systematic review and meta-analysis. EBioMedicine. 2020;59:102903. doi:10.1016/J.EBIOM.2020.102903
CrossRef - Sethuraman N, Jeremiah SS, Ryo A. Interpreting Diagnostic Tests for SARS-CoV-2. JAMA. 2020;323(22):2249-2251. doi:10.1001/JAMA.2020.8259
CrossRef - Singhal T. A Review of Coronavirus Disease-2019 (COVID-19). Indian J Pediatr. 2020;87(4):281-286. doi:10.1007/S12098-020-03263-6
CrossRef - Zhang N, Wang L, Deng X, et al. Recent advances in the detection of respiratory virus infection in humans. J Med Virol. 2020;92(4):408-417. doi:10.1002/JMV.25674
CrossRef - Seder N, Rayyan WA, Dayyih WA, Al-Natour MA, Hilmi ABM. Phytochemical Investigation, Comparison and Characterization Study of Malaysian Stingless Bee Honey versus Jordanian Honey by LC-MS/MS: doi. org/10.26538/tjnpr/v5i9. 12. Tropical Journal of Natural Product Research (TJNPR). 2021;5(9):1597-1605.
CrossRef - W. Abu Rayyan A. M. Al-Jaafreh W. Abu Dayyih M. Bustami S. Salem N. Seder K. Schröppel AS. The Role of Glutamine-Rich Region of Candida Albicans Tec1p in Mediating Morphological Transition and Invasive Growth. International Journal of Medical and Health Sciences. 2019;13(4):151-158.
- Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25(3):2000045. doi:10.2807/1560-7917.ES.2020.25.3.2000045/CITE/PLAINTEXT
CrossRef - Al-Shdefat R, Al-Ani I, Tamimi L, Awad R, Rayyan WA, Dayyih WA. Development and Validation of a Stability-Indicating HPLC-DAD Method for the Determination of Canagliflozin and Metformin Simultaneously in Combination Dosage Form. Pharm Chem J. 2021;55(4):402-409.
CrossRef - Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020;323(18):1843-1844. doi:10.1001/JAMA.2020.3786
CrossRef - Nair B, Mathew J. Mental health and psychosocial wellbeing during Covid-19. International Journal of Nursing Education and Research. Published online December 1, 2021:488-490. doi:10.52711/2454-2660.2021.00114
CrossRef - Dubey SR. Legal and Ethical Concerns in Critical Care Nursing to Covid-19. International Journal of Nursing Education and Research. Published online July 1, 2021:367-369. doi:10.52711/2454-2660.2021.00085
CrossRef - Dewangan V, Sahu R, Satapathy T, Roy A. The exploring of current development status and the unusual symptoms of coronavirus pandemic (Covid-19). RESEARCH JOURNAL OF PHARMACOLOGY AND PHARMACODYNAMICS. 2020;12(4):172-176. doi:10.5958/2321-5836.2020.00031.2
CrossRef - Patel P, Decuir J, Abrams J, Campbell AP, Godfred-Cato S, Belay ED. Clinical Characteristics of Multisystem Inflammatory Syndrome in Adults: A Systematic Review. JAMA Netw Open. 2021;4(9):e2126456-e2126456. doi:10.1001/JAMANETWORKOPEN.2021.26456
CrossRef - Davies P, Evans C, Kanthimathinathan HK, et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study. Lancet Child Adolesc Health. 2020;4(9):669-677. doi:10.1016/S2352-4642(20)30215-7
CrossRef - Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N Engl J Med. 2020;383(4):334-346. doi:10.1056/NEJMOA2021680
CrossRef - Singh-Grewal D, Lucas R, McCarthy K, et al. Update on the COVID-19-associated inflammatory syndrome in children and adolescents; paediatric inflammatory multisystem syndrome-temporally associated with SARS-CoV-2. J Paediatr Child Health. 2020;56(8):1173-1177. doi:10.1111/JPC.15049
CrossRef - Motta I, Bou-Fakhredin R, Taher AT, Cappellini MD. Beta Thalassemia: New Therapeutic Options Beyond Transfusion and Iron Chelation. Drugs. 2020;80(11):1053-1063. doi:10.1007/S40265-020-01341-9
CrossRef - Al-Balas M, Al-Balas HI, Alqassieh R, et al. Clinical Features of COVID-19 Patients in Jordan: A Study of 508 Patients. Open Respir Med J. 2021;15(1):28-34. doi:10.2174/1874306402115010028
CrossRef - Dawood AA. Using Remdesivir and Dexamethasone for Treatment of SARS-CoV-2 Shortens the patient’s stay in the Hospital. Asian Journal of Pharmaceutical Research. Published online May 11, 2021:138-140. doi:10.52711/2231-5691.2021.00026
CrossRef - Ding Y, Yan H, Guo W. Clinical Characteristics of Children With COVID-19: A Meta-Analysis. Front Pediatr. 2020;8. doi:10.3389/FPED.2020.00431
CrossRef - Cui X, Zhao Z, Zhang T, et al. A systematic review and meta-analysis of children with coronavirus disease 2019 (COVID-19). J Med Virol. 2021;93(2):1057-1069. doi:10.1002/JMV.26398
CrossRef - Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58(7):1021-1028. doi:10.1515/CCLM-2020-0369
CrossRef - Srivastava S, Garg I, Bansal A, Kumar B. COVID-19 infection and thrombosis. Clin Chim Acta. 2020;510:344. doi:10.1016/J.CCA.2020.07.046
CrossRef - Lippi G, Favaloro EJ. D-dimer is Associated with Severity of Coronavirus Disease 2019: A Pooled Analysis. Thromb Haemost. 2020;120(5):876-877. doi:10.1055/S-0040-1709650
CrossRef - Eljilany I, Elzouki AN. D-dimer, fibrinogen, and il-6 in covid-19 patients with suspected venous thromboembolism: A narrative review. Vasc Health Risk Manag. 2020;16:455-462. doi:10.2147/VHRM.S280962
CrossRef - Abu-Farha M, Al-Sabah S, Hammad MM, et al. Prognostic Genetic Markers for Thrombosis in COVID-19 Patients: A Focused Analysis on D-Dimer, Homocysteine and Thromboembolism. Front Pharmacol. 2020;11. doi:10.3389/FPHAR.2020.587451/FULL
CrossRef - Hottz ED, Azevedo-Quintanilha IG, Palhinha L, et al. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COV19. Blood. 2020;136(11):1330-1341. doi:10.1182/blood.2020007252
CrossRef - Rahman A, Niloofa R, Jayarajah U, de Mel S, Abeysuriya V, Seneviratne SL. Hematological Abnormalities in COVID-19: A Narrative Review. Am J Trop Med Hyg. 2021;104(4):1188. doi:10.4269/AJTMH.20-1536
CrossRef - Chen R, Sang L, Jiang M, et al. Longitudinal hematologic and immunologic variations associated with the progression of COVID-19 patients in China. J Allergy Clin Immunol. 2020;146(1):89-100. doi:10.1016/J.JACI.2020.05.003
CrossRef - Rohlfing AK, Rath D, Geisler T, Gawaz M, Thieme G, Kg V. Platelets and COVID-19. Hamostaseologie. 2021;41:379-385. doi:10.1055/a-1581-4355
CrossRef - Yang M, Ng MHL, Li CK, et al. Thrombopoietin levels increased in patients with severe acute respiratory syndrome. Thromb Res. 2008;122(4):473. doi:10.1016/J.THROMRES.2007.12.021
CrossRef - Giannis D, Ziogas IA, Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. Journal of Clinical Virology. 2020;127:104362. doi:10.1016/J.JCV.2020.104362
CrossRef - Wong RSM, Wu A, To KF, et al. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. BMJ. 2003;326(7403):1358-1362. doi:10.1136/BMJ.326.7403.1358
CrossRef - Wool GD, Miller JL. The Impact of COVID-19 Disease on Platelets and Coagulation. Pathobiology. 2021;88(1):15-27. doi:10.1159/000512007
CrossRef - Guan W jie, Ni Z yi, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. New England Journal of Medicine. 2020;382(18):1708-1720. doi:10.1056/NEJMOA2002032/SUPPL_FILE/NEJMOA2002032_DISCLOSURES.PDF
CrossRef - Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. doi:10.1016/S0140-6736(20)30183-5
CrossRef - Liu W, Tao ZW, Wang L, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl). 2020;133(9):1032-1038. doi:10.1097/CM9.0000000000000775
CrossRef - Liu Y, Yang Y, Zhang C, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Science China Life Sciences 2020 63:3. 2020;63(3):364-374. doi:10.1007/S11427-020-1643-8
CrossRef - Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846-848. doi:10.1007/S00134-020-05991-X/FIGURES/1
CrossRef - Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. doi:10.1001/JAMA.2020.1585
CrossRef - Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475-481. doi:10.1016/S2213-2600(20)30079-5
CrossRef - Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore. JAMA. 2020;323(15):1488-1494. doi:10.1001/JAMA.2020.3204
CrossRef - Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395(10229):1054-1062. doi:10.1016/S0140-6736(20)30566-3
CrossRef - Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin Chim Acta. 2020;506:145-148. doi:10.1016/J.CCA.2020.03.022
CrossRef - Thachil J. What do monitoring platelet counts in COVID-19 teach us? J Thromb Haemost. 2020;18(8):2071-2072. doi:10.1111/JTH.14879
CrossRef - Bomhof G, Mutsaers PGNJ, Leebeek FWG, et al. COVID-19-associated immune thrombocytopenia. Br J Haematol. 2020;190(2):e61-e64. doi:10.1111/BJH.16850
CrossRef - Monod J, Wyman J, Changeux JP. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965;12(1):88-118. doi:10.1016/S0022-2836(65)80285-6
CrossRef - Kuhn V, Diederich L, Keller TCS, et al. Red Blood Cell Function and Dysfunction: Redox Regulation, Nitric Oxide Metabolism, Anemia. Antioxid Redox Signal. 2017;26(13):718. doi:10.1089/ARS.2016.6954
CrossRef - Moras M, Lefevre SD, Ostuni MA. From Erythroblasts to Mature Red Blood Cells: Organelle Clearance in Mammals. Front Physiol. 2017;8(DEC):1076. doi:10.3389/FPHYS.2017.01076
CrossRef - Hendrickson JE, Tormey CA. The RBC as a Target of Damage. Pathobiology of Human Disease: A Dynamic Encyclopedia of Disease Mechanisms. Published online January 1, 2014:3068-3080. doi:10.1016/B978-0-12-386456-7.06203-1
CrossRef - Piagnerelli M, Boudjeltia KZ, Brohee D, et al. Alterations of red blood cell shape and sialic acid membrane content in septic patients. Crit Care Med. 2003;31(8):2156-2162. doi:10.1097/01.CCM.0000079608.00875.14
CrossRef - Favaron E, Ince C, Hilty MP, et al. Capillary Leukocytes, Microaggregates, and the Response to Hypoxemia in the Microcirculation of Coronavirus Disease 2019 Patients. Crit Care Med. 2021;49(4):661. doi:10.1097/CCM.0000000000004862
CrossRef - Thomas T, Stefanoni D, Dzieciatkowska M, et al. Evidence of Structural Protein Damage and Membrane Lipid Remodeling in Red Blood Cells from COVID-19 Patients. J Proteome Res. 2020;19(11):4455-4469. doi:10.1021/ACS.JPROTEOME.0C00606
CrossRef - Renoux C, Fort R, Nader E, et al. Impact of COVID-19 on red blood cell rheology. Br J Haematol. 2021;192(4):e108-e111. doi:10.1111/BJH.17306
CrossRef - Lam LKM, Reilly JP, Rux AH, et al. Erythrocytes identify complement activation in patients with COVID-19. Am J Physiol Lung Cell Mol Physiol. 2021;321(2):L485-L489. doi:10.1152/AJPLUNG.00231.2021
CrossRef - Piagnerelli M, Vanderelst J, Rousseau A, et al. Red Blood Cell Shape and Deformability in Patients With COVID-19 Acute Respiratory Distress Syndrome. Front Physiol. 2022;13:333. doi:10.3389/FPHYS.2022.849910/BIBTEX
CrossRef - Schapkaitz E, de Jager T, Levy B. The characteristic peripheral blood morphological features of hospitalized patients infected with COVID-19. Int J Lab Hematol. 2021;43(3):e130-e134. doi:10.1111/IJLH.13417
CrossRef - Majeed A, Ashraf Shajar M, St Z, Maather A, Maazer A. Is hemoglobin the missing link in the pathogenesis of COVID-19? Anaesthesia, Pain & Intensive Care. 2020;24(1):9-12. doi:10.35975/APIC.V24I1.1216
CrossRef - Russo A, Tellone E, Barreca D, Ficarra S, Laganà G. Implication of COVID-19 on Erythrocytes Functionality: Red Blood Cell Biochemical Implications and Morpho-Functional Aspects. Int J Mol Sci. 2022;23(4):2171. doi:10.3390/IJMS23042171
CrossRef - wenzhong liu, hualan L. COVID-19:Attacks the 1-Beta Chain of Hemoglobin and Captures the Porphyrin to Inhibit Human Heme Metabolism. Published online July 13, 2020. doi:10.26434/CHEMRXIV.11938173.V9
CrossRef - Lechuga GC, Souza-Silva F, Sacramento CQ, et al. SARS-CoV-2 Proteins Bind to Hemoglobin and Its Metabolites. Int J Mol Sci. 2021;22(16). doi:10.3390/IJMS22169035
CrossRef - COVID-19:Attacks the 1-Beta Chain of Hemoglobin and Captures the Porphyrin to Inhibit Human Heme Metabolism | Request PDF. Accessed September 18, 2022. https://www.researchgate.net/publication/350035223_COVID-19Attacks_the_1-Beta_Chain_of_Hemoglobin_and_ Captures_the_Porphyrin_ to_Inhibit_Human_Heme_Metabolism
- San Juan I, Bruzzone C, Bizkarguenaga M, et al. Abnormal concentration of porphyrins in serum from COVID‐19 patients. Br J Haematol. 2020;190(5):e265. doi:10.1111/BJH.17060
CrossRef - Shoenfeld Y. Corona (COVID-19) time musings: Our involvement in COVID-19 pathogenesis, diagnosis, treatment and vaccine planning. Autoimmun Rev. 2020;19(6). doi:10.1016/J.AUTREV.2020.102538
CrossRef - de Domenico I, Ward DM, Kaplan J. Hepcidin regulation: ironing out the details. J Clin Invest. 2007;117(7):1755-1758. doi:10.1172/JCI32701
CrossRef - Anai M, Akaike K, Iwagoe H, et al. Decrease in hemoglobin level predicts increased risk for severe respiratory failure in COVID-19 patients with pneumonia. Respir Investig. 2021;59(2):187-193. doi:10.1016/J.RESINV.2020.10.009
CrossRef - Wang J, Jiang M, Chen X, Montaner LJ. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J Leukoc Biol. 2020;108(1):17-41. doi:10.1002/JLB.3COVR0520-272R
CrossRef - Han H, Xu Z, Cheng X, et al. Descriptive, Retrospective Study of the Clinical Characteristics of Asymptomatic COVID-19 Patients. mSphere. 2020;5(5). doi:10.1128/MSPHERE.00922-20/SUPPL_FILE/MSPHERE.00922-20-SF003.PDF
CrossRef - Kumar R, Chawla A, Gaganpreet, Diksha. A valuable insight to the novel deadly covid-19: A review. Research Journal of Pharmacology and Pharmacodynamics. 2020;12(3):111. doi:10.5958/2321-5836.2020.00021.X
CrossRef - Favaron E, Ince C, Hilty MP, et al. Capillary Leukocytes, Microaggregates, and the Response to Hypoxemia in the Microcirculation of Coronavirus Disease 2019 Patients. Crit Care Med. 2021;49(4):661. doi:10.1097/CCM.0000000000004862
CrossRef - Talukdar M, Dasgupta S, Osta M. Neutrophil to Lymphocyte Ratio in Hospitalized COVID-19 Patients – A Study in a Tertiary Care Covid Centre in Eastern India. Biomedical and Pharmacology Journal. 2022;15(1):321-325. doi:10.13005/bpj/2370
CrossRef - Zhu B, Feng X, Jiang C, et al. Correlation between white blood cell count at admission and mortality in COVID-19 patients: a retrospective study. BMC Infect Dis. 2021;21(1):1-5. doi:10.1186/S12879-021-06277-3/FIGURES/2
CrossRef - Yang L, Jin J, Luo W, Gan Y, Chen B, Li W. Risk factors for predicting mortality of COVID-19 patients: A systematic review and meta-analysis. PLoS One. 2020;15(11). doi:10.1371/JOURNAL.PONE.0243124
CrossRef - Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513. doi:10.1016/S0140-6736(20)30211-7
CrossRef - Zhou Y, Fu B, Zheng X, et al. Aberrant pathogenic GM-CSF+ T cells and inflammatory CD14+CD16+ monocytes in severe pulmonary syndrome patients of a new coronavirus. bioRxiv. Published online February 20, 2020:2020.02.12.945576. doi:10.1101/2020.02.12.945576
CrossRef - Yang X, Dai T, Zhou X, et al. Analysis of adaptive immune cell populations and phenotypes in the patients infected by SARS-CoV-2. medRxiv. Published online December 21, 2020:2020.03.23.20040675. doi:10.1101/2020.03.23.20040675
CrossRef - Zhang D, Guo R, Lei L, et al. COVID-19 infection induces readily detectable morphological and inflammation-related phenotypic changes in peripheral blood monocytes, the severity of which correlate with patient outcome. medRxiv. Published online March 26, 2020:2020.03.24.20042655. doi:10.1101/2020.03.24.20042655
CrossRef - Feng Z, Diao B, Wang R, et al. The Novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Directly Decimates Human Spleens and Lymph Nodes. medRxiv. Published online March 31, 2020:2020.03.27.20045427. doi:10.1101/2020.03.27.20045427
CrossRef - Honke N, Shaabani N, Cadeddu G, et al. Enforced viral replication activates adaptive immunity and is essential for the control of a cytopathic virus. Nat Immunol. 2011;13(1):51-57. doi:10.1038/NI.2169
CrossRef - Rayyan WA. Seroprevalence of SARS-CoV-2 antibodies among Jordanian citizens: A cross-sectional study of the demographic and clinical factors that ameliorate serum IgG concentration. J Appl Pharm Sci. 2022; 12(11): 151-156. doi:10.7324/JAPS.2022.121116
CrossRef - Jaiswal S, Weissman IL. Hematopoietic stem and progenitor cells and the inflammatory response. Ann N Y Acad Sci. 2009;1174:118-121. doi:10.1111/J.1749-6632.2009.04930.X
CrossRef - Zheng B, Yuan M, Ma Q, et al. Landscape of SARS-CoV-2 spike protein-interacting cells in human tissues. Int Immunopharmacol. 2021;95:107567. doi:10.1016/J.INTIMP.2021.107567
CrossRef - Elahi S. Hematopoietic responses to SARS-CoV-2 infection. Cellular and Molecular Life Sciences. 2022;79(3):187. doi:10.1007/S00018-022-04220-6
CrossRef - Kucia M, Bujko K, Ciechanowicz A, et al. The ACE2 Receptor for COVID-19 Entry Is Expressed on the Surface of Hematopoietic Stem/Progenitor Cells and Endothelial Progenitors As Well As Their Precursor Cells and Becomes Activated in Nlrp3 Inflammasome-Dependent Manner By Virus Spike Protein – a Potential Pathway Leading to a Cytokine Storm. Blood. 2020;136(Supplement 1):8-8. doi:10.1182/BLOOD-2020-137083
CrossRef - Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. The Lancet. 2020;395(10234):1417-1418. doi:10.1016/S0140-6736(20)30937-5
CrossRef - Ropa J, Cooper S, Capitano ML, Van’t Hof W, Broxmeyer HE. Human Hematopoietic Stem, Progenitor, and Immune Cells Respond Ex Vivo to SARS-CoV-2 Spike Protein. Stem Cell Rev Rep. 2021;17(1):253-265. doi:10.1007/S12015-020-10056-Z/FIGURES/5
CrossRef - Elahi S. Hematopoietic responses to SARS-CoV-2 infection. Cellular and Molecular Life Sciences. 2022;79:187. doi:10.1007/s00018-022-04220-6
CrossRef - Debliquis A, Harzallah I, Mootien JY, et al. Haemophagocytosis in bone marrow aspirates in patients with COVID‐19. Br J Haematol. 2020;190(2):e70. doi:10.1111/BJH.16860
CrossRef - Rapkiewicz A v., Mai X, Carsons SE, et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series. EClinicalMedicine. 2020;24:100434. doi:10.1016/J.ECLINM.2020.100434
CrossRef - Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631. doi:10.1002/PATH.1570
CrossRef - Feng Z, Diao B, Wang R, et al. The Novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Directly Decimates Human Spleens and Lymph Nodes. medRxiv. Published online March 31, 2020:2020.03.27.20045427. doi:10.1101/2020.03.27.20045427
CrossRef - Xia X, Xiaona C, Huaxiong P, et al. [Pathological changes of the spleen in ten patients with coronavirus disease 2019(COVID-19) by postmortem needle autopsy]. Zhonghua Bing Li Xue Za Zhi. 2020;49(6):576-582. doi:10.3760/CMA.J.CN112151-20200401-00278
- Xiaohong Y, Tingyuan L, Zhicheng H, et al. [A pathological report of three COVID-19 cases by minimal invasive autopsies]. Zhonghua Bing Li Xue Za Zhi. 2020;49(5):411-417. doi:10.3760/CMA.J.CN112151-20200312-00193
- Fard MB, Fard SB, Ramazi S, Atashi A, Eslamifar Z. Thrombosis in COVID-19 infection: Role of platelet activation-mediated immunity. Thrombosis Journal 2021 19:1. 2021;19(1):1-11. doi:10.1186/S12959-021-00311-9
CrossRef - Choudhury A, Mukherjee S. In silico studies on the comparative characterization of the interactions of SARS-CoV-2 spike glycoprotein with ACE-2 receptor homologs and human TLRs. J Med Virol. 2020;92(10):2105-2113. doi:10.1002/JMV.25987
CrossRef - Choudhury A, Mukherjee S. In silico studies on the comparative characterization of the interactions of SARS-CoV-2 spike glycoprotein with ACE-2 receptor homologs and human TLRs. J Med Virol. 2020;92(10):2105-2113. doi:10.1002/JMV.25987
CrossRef - Gorog DA, Storey RF, Gurbel PA, et al. Current and novel biomarkers of thrombotic risk in COVID-19: a Consensus Statement from the International COVID-19 Thrombosis Biomarkers Colloquium. doi:10.1038/s41569-021-00665-7
CrossRef - Girardin SE, Boneca IG, Viala J, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278(11):8869-8872. doi:10.1074/JBC.C200651200
CrossRef - The role of P-selectin in experimental colitis as determined by antibody immunoblockade and genetically deficient mice – PubMed. Accessed September 3, 2022. https://pubmed.ncbi.nlm.nih.gov/12101263/
- Larsen E, Celi A, Gilbert GE, et al. PADGEM protein: a receptor that mediates the interaction of activated platelets with neutrophils and monocytes. Cell. 1989;59(2):305-312. doi:10.1016/0092-8674(89)90292-4
CrossRef - Celi A, Pellegrini G, Lorenzet R, et al. P-selectin induces the expression of tissue factor on monocytes. Proc Natl Acad Sci U S A. 1994;91(19):8767-8771. doi:10.1073/PNAS.91.19.8767
CrossRef - Mulligan MS, Polley MJ, Bayer RJ, Nunn MF, Paulson JC, Ward PA. Neutrophil-dependent acute lung injury. Requirement for P-selectin (GMP-140). J Clin Invest. 1992;90(4):1600-1607. doi:10.1172/JCI116029
CrossRef - Neri T, Nieri D, Celi A. P-selectin blockade in COVID-19-related ARDS. https://doi.org/101152/ajplung002022020. 2020;318(6):L1237-L1238. doi:10.1152/AJPLUNG.00202.2020
CrossRef - Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. doi:10.1016/J.THROMRES.2020.04.013
CrossRef - Rokade M, Khandagale P. Coronavirus disease: A review of a new threat to public health. Asian Journal of Pharmaceutical Research. 2020;10(3):241. doi:10.5958/2231-5691.2020.00042.8
CrossRef - Weyrich AS, Zimmerman GA. Platelets in lung biology. Annu Rev Physiol. 2013;75:569-591. doi:10.1146/ANNUREV-PHYSIOL-030212-183752
CrossRef - Zarbock A, Singbartl K, Ley K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J Clin Invest. 2006;116(12):3211-3219. doi:10.1172/JCI29499
CrossRef - Hottz ED, Bozza FA, Bozza PT. Platelets in Immune Response to Virus and Immunopathology of Viral Infections. Front Med (Lausanne). 2018;5(APR). doi:10.3389/FMED.2018.00121
CrossRef - Liu Y, Sun W, Guo Y, et al. Association between platelet parameters and mortality in coronavirus disease 2019: Retrospective cohort study. Platelets. 2020;31(4):490-496. doi:10.1080/09537104.2020.1754383
CrossRef - Jiang SQ, Huang QF, Xie WM, Lv C, Quan XQ. The association between severe COVID-19 and low platelet count: evidence from 31 observational studies involving 7613 participants. Br J Haematol. 2020;190(1):e29-e33. doi:10.1111/BJH.16817
CrossRef - Wool GD, Miller JL. The Impact of COVID-19 Disease on Platelets and Coagulation. Pathobiology. 2021;88(1):15-27. doi:10.1159/000512007
CrossRef - Li Q, Cao Y, Chen L, et al. Hematological features of persons with COVID-19. Leukemia. 2020;34(8):2163-2172. doi:10.1038/S41375-020-0910-1
CrossRef - Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023-1026. doi:10.1111/JTH.14810
CrossRef - Viecca M, Radovanovic D, Forleo GB, Santus P. Enhanced platelet inhibition treatment improves hypoxemia in patients with severe Covid-19 and hypercoagulability. A case control, proof of concept study. Pharmacol Res. 2020;158. doi:10.1016/J.PHRS.2020.104950
CrossRef - Yau JW, Teoh H, Verma S. Endothelial cell control of thrombosis. BMC Cardiovasc Disord. 2015;15(1). doi:10.1186/S12872-015-0124-Z
CrossRef - Langer F, Kluge S, Klamroth R, Oldenburg J. Coagulopathy in COVID-19 and Its Implication for Safe and Efficacious Thromboprophylaxis. Hamostaseologie. 2020;40(3):264-269. doi:10.1055/A-1178-3551
CrossRef - Srivastava S, Garg I, Bansal A, Kumar B. COVID-19 infection and thrombosis. Clin Chim Acta. 2020;510:344. doi:10.1016/J.CCA.2020.07.046
CrossRef - Sui J, Noubouossie DF, Gandotra S, Cao L. Elevated Plasma Fibrinogen Is Associated With Excessive Inflammation and Disease Severity in COVID-19 Patients. Front Cell Infect Microbiol. 2021;11. doi:10.3389/FCIMB.2021.734005
CrossRef - Ranucci M, Ballotta A, di Dedda U, et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020;18(7):1747-1751. doi:10.1111/JTH.14854
CrossRef - Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844-847. doi:10.1111/JTH.14768
CrossRef - Guan W jie, Ni Z yi, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. New England Journal of Medicine. 2020;382(18):1708-1720. doi:10.1056/NEJMOA2002032/SUPPL_FILE/NEJMOA2002032_DISCLOSURES.PDF
CrossRef - Han H, Yang L, Liu R, et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58(7):1116-1120. doi:10.1515/CCLM-2020-0188/MACHINEREADABLECITATION/RIS
CrossRef - Viral hemorrhagic fever–a vascular disease? – PubMed. Accessed September 3, 2022. https://pubmed.ncbi.nlm.nih.gov/12783108/
- Tsoupras A, Lordan R, Zabetakis I. Thrombosis and COVID-19: The Potential Role of Nutrition. Front Nutr. 2020;7:177. doi:10.3389/FNUT.2020.583080/XML/NLM
CrossRef








