Muhammad Hilman Azzam1 , Nisa Fauziah2,3,4
, Nisa Fauziah2,3,4 and Hesti Lina Wiraswati2,3,4*
and Hesti Lina Wiraswati2,3,4*
1Post Graduate Student of Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia.
2Advance Biomedical Laboratory, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia.
3Department of Biomedical Science, Faculty of Medicine, Universitas Padjadjaran, 45363, Jatinangor, Jawa Barat, Indonesia.
4Oncology and Stem Cells Working Group, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia.
Corresponding Author E-mail: hesti.lina@unpad.ac.id
DOI : https://dx.doi.org/10.13005/bpj/2564
Abstract
Cancer treatment still has challenges from its expense, side effect, and survival rate. One of the actions to improve this is searching for new anticancer agents. Medicinal plants are a candidate source since they have traditionally been used to treat illness. Phytochemicals of medicinal plants play a significant role in exhibiting anticancer effects. Literature studies of the phytochemicals of existing medicinal plants can be a clue to finding out the potential other plants whose studies are still limited, such as Breynia cernua, a plant with anticancer effects used traditionally. This study will provide information on the phytochemicals effect of medicinal plants or other compounds against cancer and their anticancer mechanisms. The agents are collected based on their compound's group, and each group's anticancer mechanism is resumed. The results showed that phytochemicals (flavonoids, alkaloids, saponins, quinone, tannins, and terpenoids) affect cancer cell through variant mechanism; induction of apoptosis, inhibition of cell growth, inhibition of cell migration, and induction of autophagic pathway. Most of the studies used methanol extracts, and most showed very strong toxicity to cancer cells. For further study, we suggest using isolated compounds from methanol, ethanol, or N-hexane extracts of Breynia cernua to get better anticancer activity, especially compounds belonging to the flavonoid or quinone group.
Keywords
Breynia cernua; Cancer; Flavonoids; Medicinal plants; Phytochemicals
Download this article as:| Copy the following to cite this article: Azzam M. H, Fauziah N, Wiraswati H. L, The Anticancer Effect of Phytochemicals and Potential of Breynia cernua: An overview. Biomed Pharmacol J 2022;15(4). |
| Copy the following to cite this URL: Azzam M. H, Fauziah N, Wiraswati H. L, The Anticancer Effect of Phytochemicals and Potential of Breynia cernua: An overview. Biomed Pharmacol J 2022;15(4). Available from: https://bit.ly/3VhShxD |
Introduction
Cancer has accounted for 9.5 million deaths worldwide and ranks 2nd in the world’s leading cause of death.1,2 Some of the challenges in cancer treatment such as high cost, unfavorable side effects (e.g. fatigue, soreness, nausea, vomit, tumor lysis syndrome, cardiac arrest, disturb life quality, anxiety or depressive symptoms), and low survival rates in poor countries up to 60%.3–10 Seeing this, the research to develop cancer treatment still need to be done. One of the action is to find new candidates as adjuvant or even new drug candidates. According to WHO, 80% of world’s population uses traditional medicine to treat disease.5 One of the source of traditional medicine is a plant that can be utilized directly or by extracting it.7 National Cancer Institute (NCI) noted that 3,000 of 35,000 species were identified to have anticancer activity.11
The plants’ phytochemicals exhibit cancer cell proliferation inhibitory activity, cell cycle arrest, or induce apoptotic cell death.12 The vinca alkaloid is one example of plant-derived anticancer agent in clinical use.11 Besides alkaloids, several group compounds reported having anticancer potential, including flavonoids, saponins, quinone, tannins, and terpenoids.12–16 One of the medicinal plants that contain those phytochemicals is Breynia cernua which also has a history of traditional use as a medicine for cough, swelling, wound, and soreness.17–19 There is also a clinical study showing this plant’s cytotoxicity against MCF-7 cancer cell line.17 Furthermore, this plant also grows well in an extreme areas such as coal reclamation, so it has the potential to be cultivated easily as a source of medicinal raw materials.20 The study aims to obtain information about the anticancer effect of the mentioned phytochemicals and to observe the anticancer potential of Breynia cernua from its latest studies to have a better development of this plant in the future.
Material and methods
The search strategy uses several keywords combined with boolean operators in online databases, as stated in table 1. We included original articles published before 2016 about flavonoids, alkaloids, saponins, quinone, tannins, and terpenoids against cancer, whether in English or Indonesian. The same applies to the Breynia cernua’s species, except the year of publication is not limited because the study of Breynia cernua is scarce. (Table 2).
Table 1: Keywords and Boolean Operators of Phytochemicals
| Keywords | Database |
| (“flavonoids” OR “alkaloids” OR “saponins” OR “quinone” OR “terpenoids” OR “tannins”) AND (“anticancer” OR “antitumor” OR “antineoplastic”) | Google Scholar |
| Sciencedirect | |
| Medline | |
| Springer Links | |
| Cochrane | |
| (“flavonoid” OR “alkaloid” OR “saponin” OR “kuinon” OR “terpenoid” OR “tannin”) AND (“antikanker” OR “antitumor” OR “antineoplastik”) | Google Scholar Indonesia |
| Portal Garuda |
Table 2: Keywords and Boolean Operators of Breynia cernua
| Keywords | Database |
| (“breynia cernua” OR “ironstone range” OR “coffee bush”) AND (“anticancer” OR “antitumor” OR “antineoplastic”) | Google Scholar |
| Sciencedirect | |
| Medline | |
| Springer Links | |
| Cochrane | |
| (”breynia cernua” OR “katuk hutan” OR “sugi-sugi”) AND (“antikanker” OR “antitumor” OR “antineoplastik”) | Google Scholar Indonesia |
| Portal Garuda |
Results and Discussion
From the previous methodology, forty-seven articles have been obtained, as described in figure 1. Thirteen original articles used samples classified as flavonoids, fifteen as alkaloids, twelve as saponins, two as quinone, three as tannins, and two as terpenoids.
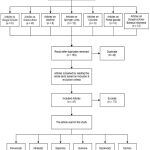 |
Figure 1: PRISMA flow diagram of results. |
Most studies use cancer cell lines (in vitro), and a few use animal models. Most articles use the compound (isolated compound, synthesized compound, or purchased compound) as a sample, and only a few use fraction or extraction. There are twenty-one types of cancer cell lines used to evaluate the toxicity of these agents. In addition, some of them investigated the anticancer mechanism. (Table 3).
 |
Table 3: Results from studies on Phytochemicals |
Phytochemical and It’s anticancer effect
Plants produce non-nutritive chemical compounds called phytochemicals or secondary metabolites.68 Until now, more than a thousand plant species, including Breynia cernua, have been identified with anticancer potential.17,69 Phytochemicals had anti-tumor, such as induced apoptosis, inhibiting cell growth, or inhibiting cell migration.70 Three types of cell death are involved in this study; apoptosis, necrosis, or autophagy.71 Necrosis is morphologically characterized by cell swelling, organelle dysfunction, and cell lysis.71 Autophagy is a process that begins with the formation of autophagosomes.71 Apoptosis is characterized by cell shrinkage, nuclear condensation, nuclear fragmentation, dynamic membrane blebbing, and loss of adhesion to neighbors or extracellular matrix through intrinsic or extrinsic pathway.71 There are various descriptions regarding the anticancer mechanism of samples in this study, most of which lead to the intrinsic pathway of apoptosis.
Flavonoids
Flavonoids are a class of plant secondary metabolites that have a polyphenolic structure.72 This phytochemical is found in many fruits and vegetables and has broad biological activities.72 In this study, two samples used crude extracts, one used fraction, and the rest used pure compounds obtained by isolation, synthesis, or purchase. Gunawan Indrayanto et al. showed that most of the samples were considered (IC50 value is below 200 μM or 100 μg/mL), and there was only one sample above that value, morin-7-sulphate sodium.24,73 The compound with the strongest cytotoxic value is oncamex with IC50 value 0.4150 μM, which is considered to have very strong activity.30 Oncamex is a second-generation analog of AO-1530 (myricetin-based flavonoid).30 The mechanism of oncamex involved several pathways; involvement of caspase protein, production of superoxide, and altered gene expression that related to cell cycle and apoptosis.30 Interestingly, strong cytotoxicity was also demonstrated by crude extract of Dodonaea viscosa.33
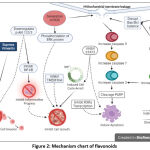 |
Figure 2: Mechanism chart of flavonoids |
This study indicated that the anticancer activity of flavonoids is related to inhibiting cell invasion and cell growth or inducing apoptosis. The generation of ROS seems to start the process of apoptosis, which then triggers mitochondrial membrane leakage and activates the caspase-dependent intrinsic pathway of apoptosis.22,25,26,28,30,32 Some studies also proposed that mitochondrial membrane leakage is caused by disruption of Bax/Bcl balance, which is triggered by disabling the binding of P53 protein with E6 or EGFR protein.29,34,41,42 Another study showed the involvement of caspase-8, indicating that an extrinsic apoptosis pathway may also happen.66 Inhibition of some proteins (RXRa transcription and STAT3) also leads to apoptosis, although the detailed mechanism needs more investigation.22,31 STAT3 has been shown to regulate several genes involved in the cell cycle; therefore, its inhibition may lead to the induction of cell cycle arrest and will inhibit the growth of cancer cells.22 Inhibition of cell growth may also be indirectly caused by factors such as inhibition of TMEM16A, Phosphorylation of ERK protein caused by ROS generation, and inhibition of inflammation.21,24 Suppression of vimentin and MMP-9 also appears to correlate to cell invasion inhibition.24 Briefly, most of the compounds used in this study indicate that flavonoids play a role against cancer cells, either in crude extracts form (such as crude extract of Dodonaea viscosa) or pure compounds (such as oncamex, hesperetin, or galangin).21,27,30,33 Twelve of thirteen articles proposed an anticancer mechanism; induced apoptosis, inhibited cell growth or migration, as depicted in figure 2.
Alkaloids
Alkaloids are secondary metabolites that usually consist of basic nitrogen atoms.74 In these articles on alkaloids, most studies evaluated pure compounds obtained by isolation, synthesis, or purchased, the rest using crude extract. Gunawan Indrayanto et al. showed that most of the agents had good activity against cancer cells, except two compounds, vincamine and crebanine.35,44,73 The strongest cytotoxic activity was demonstrated by Loonamycins A (obtained from bacteria, Nocardiopsis flavescens) with IC50 value of 31.4 nM.37,73 The second strongest is the Khasuanine A (obtained from plant, Melodinus khasianus) with IC50 value of 0.74 ± 0.03 μM.34,73 Eight of fifteen articles proposed an anticancer mechanism; leads to induction of apoptosis, inhibition of cell growth, and induction of autophagic pathway, as depicted in figure 3. Khasuanine A appears to induce an intrinsic apoptotic pathway involving several proteins such as Bcl-2, caspase 3, and P53.34,37 These studies showed that alkaloids have good cytotoxicity towards cancer cells in the form of crude extracts such as in ethanol extract of Solanum blumei Nees ex Blume and pure compounds khasuanine A of Melodinus khasianus.
Figure 3 shows alkaloids against cancer cells by inhibiting cell growth, inducing autophagic pathways, and inducing apoptosis. Apoptosis pathway in a caspase-dependent manner seems related to stress oxidative affected by ROS generation. In addition, the redox stress may cause depolarization of mitochondrial membrane potential, resulting in mitochondrial membrane leakage. Then the release of cytochrome-c from mitochondria will initiate intrinsic apoptosis by activating caspase 7 and caspase 3.34–36,38,41,42 ROS also appears to mediate the autophagic pathway. However, the detailed mechanism needs to be investigated.38 It also appears that the decrease of Bcl-2, caused by increasing P53, also contributes to mitochondrial membrane leakage, activating caspase-3, resulting in the intrinsic pathway of apoptosis.34,41 HMGB1 reported promotes cancer cell growth; therefore, inhibition in this will lead to inhibition of cell growth.39
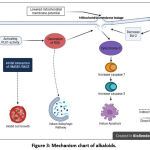 |
Figure 3: Mechanism chart of alkaloids. |
Saponins
Saponins are a groups of natural plant products which have a form of glycosides of triterpenes and steroids.75 These studies evaluated the cytotoxic effect of pure compounds, crude extracts, fraction, or crude saponins. Most of them were active except fraction Dia-80 from Astragalus glycyphyllos L, saponin-containing fractions 1 – 6 from Astragalus glycyphyllos, and crude extract of organic culture Spirulina.52,53,60,73 The most potent compound is the Parquispiroside from leaves of Cestrum parqui with IC50 value of 3.3 ± 0.63 μM.55,73 The methanol extract of Zanthoxylum armatum also showed potent cytotoxicity with IC50 value of 0.87 ± 0.4 μg/mL.51,73 Interestingly, the methanol extract which was further processed into crude saponins was found to have lower activity against cancer cells (IC50 13.92 ± 3.6 g/ml).51 These findings indicate that the saponin group’s interaction with other compounds makes their work more effective against cancer.76
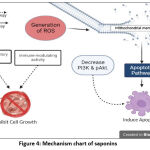 |
Figure 4: Mechanism chart of saponins. |
Eight of twelve articles in this study on saponins mentioned the anticancer mechanism of action. Overall, the anticancer agents of the saponins group involves an intrinsic pathway in induction of apoptosis and other routes, such as inhibiting cell growth. (Figure 4). Anticancer evaluation of one of the most potent agents, Paris saponin 1, showed changes in PI3K, pAKT, Bcl-2, Bax, caspase-3, and capsase-9.49 Therefore, Xinhai Zhu et al. proposed in their article that Paris saponins could target the PI3K/AKT pathway to activate the apoptotic pathway.49
Figure 4 shows that saponins have two effects on cancer cells; inhibit cell growth and induce apoptosis. ROS generation is reported to trigger mitochondrial membrane leakage.54 Further studies demonstrating mitochondrial membrane depolarization, the release of cytochrome c, and activation of caspase 9 and 3, can be done to prove the apoptotic pathway in a caspase-dependent manner. Saponin seems involved in decreasing PI3K/Akt in inducing apoptosis, although the mechanism has not been explained in detail.49 Nonetheless, inactivation of the PI3K/Akt signaling pathway has been reported to be involved in apoptosis via the generation of ROS.77 The contribution of PI3K/AKT in apoptosis is also associated with activating pro-apoptotic protein Bax.78 In addition, saponins were reported to be involved in cell growth inhibition which seems to be due to anti-inflammatory and immune modulating activities.50
Quinone
Quinone derivatives from natural products display various compounds that have been used as cytotoxic agents for anticancer therapy, such as daunorubicin, doxorubicin, geldanamycin, and mitomycin C.79–82 Quinones plays an important role in the aerobic metabolism of most cell because of its capacity on their one- or two-reduction to generate Reactive Oxygen Species (ROS), or its arilation capacity through quinone-thiol formation (Michael adduct formation).80,83–86 Based on these activities, many studies have used compounds from the quinone group to understand the role of proteins involved in fighting cancer.74,83,84,87–90 In addition, the researchers are looking for new anti-cancer candidates from the quinone group. In this study there are two articles that investigate the anticancer effects of quinones derived from medicinal plants. They investigated samples from the root of the plant species Maytenus ilicifolia; then they isolated Maylenin and 22-b-hydrodymaytenin compounds.61 Both compounds were considered to have very strong cytotoxicity.61,73 The other study did a direct synthesis to get fourteen derivatives; compounds 9h, 9k, 9i, and 9b exhibited very good anticancer activities.62 Further analysis showed that maylenin induced apoptosis with the involvement of caspase 3 and caspase 7 (figure 5).61 All of these results reinforce the potential of quinone as a source of new drug candidates.
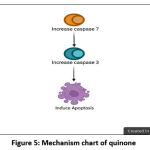 |
Figure 5: Mechanism chart of quinone. |
Figure 5 shows that the quinone group of medicinal plants appears to induce apoptosis in a caspase-dependent manner, involving activation of caspase 7 and caspase 3.61 Further studies using the pan-caspase inhibitor ZVAD-FMK (N-benzyloxy carbonyl-Val-Ala-Asp-fluoromethyl ketone) can be carried out for confirmation this pathway.91,92 Another important thing to do is to observe the morphological changes of apoptosis in cells treated with candidate anticancer compounds, such us the round-up of cells, apoptotic bodies formation, cell shrinkage, or chromatin condensation.93
Tannins
Tannins are a group of natural phenolic biomolecules that protect plants against fungi and insects.94 In this study, methanol extract of three plant species, Rubus niveus, Rubus Fairholmianus, and Rubus ellipticus showed very strong cytotoxicity.63,73 Another section with various solvents (such as ether, petroleum, methanol, hexane, and water) of Azadirachta indica A. Juss and Melia azedarach Linn.64 Both species exhibit lower anticancer activity with IC50 value more than 100 μg/mL.64,73 All studies did not mention the mechanism of action against cancer cells. Further purification is needed to get the active compound of methanol extract of the Rubus genus.
Terpenoids
Terpenoids are a vast group of natural compounds which form a major constituent of essential oil from plants and it can be classified according to the number of their isoprene unit.95 In this study, two samples used crude extract, one used fractions, and one used compound. In the first study, they isolated BC I compounds which is considered to have a moderate cytotoxicty 65,73 The second study investigate samples from the plant species Stachys pilifera; then, they proceed its extract and fraction.66 Both of them were considered to have moderate cytotoxicity66,73 The third study investigate samples from the fruit of the plant species Luffa echinata Roxb.67 They used LC50 as a cytotoxicity parameter and it is stated that the extracts showed a remarkable anticancer activity.67 Two of three articles proposed an anticancer mechanismwhich involve arrest of cell cycle phase, involvement of NO concentration and also involvement of caspase proteins, as stated in the figure 6.65,67 All of these results reinforce the potential of terpenoids to be an anticancer agents.
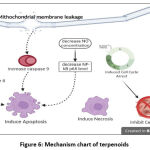 |
Figure 6: Mechanism chart of terpenoids. |
Figure 6 demonstrates that terpenoids inhibit cell growth and inducing apoptosis. Intrinsic and extrinsic apoptosis pathways have been reported to be affected by terpenoids, with the involvement of caspase 9 and caspase 8.66 There was also a decrease in the concentration of nitric oxide (NO), which caused a decrease in NF-kB p65 levels and could lead to the induction of apoptosis.66 In this pathway, terpenoids may activate ROS production because increased ROS levels seem to suppress NO synthase (NOS), reducing NO.96 NF-κB is commonly known to mediate cell proliferation and survival, which is also associated with apoptosis. In vitro studies identified that NF-κB inhibition is induces apoptosis in leukemic stem cells and intrinsic apoptotic pathways in leukemia cell lines.97,98 Nonetheless, other studies have also reported necrosis induction by terpenoids. Terpenoids are also reported to inactivate cell growth by inhibiting the cell cycle.65
The Potential of Medicinal Plant Breynia cernua
It was recorded that from 35,000 plant species studied by the National Cancer Institute (NCI), 3,000 species have anticancer activity.11 The compounds contained in this plant include flavonoids, alkaloids, saponins, quinones, tannins, terpenoids, and many more.12–16 These compounds were also detected in the Breynia cernua.17–19 Nasrul Wathan et al. revealed that Breynia cernua contains saponins, flavonoids, and quinones.18 Another study using Thin Layer Chromatography (TLC) and column chromatography indicated that this plant showed the presence of alkaloids, saponins, terpenoids, flavonoids, and tannins.17
Breynia cernua, also known as Katuk Hutan or Sugi-sugi, is a small shrub or tree with a height of 3 – 5 m.18,99,100 It has thin green leaves with prominent veins and red to purple berry-like fruits.18,100 This plant is found in tropical areas such as Indonesia (Java, Papua, or Kalimantan province), Philippines, East Malesia, Northern Australia and Solomons.18,100 It can be cultivated around the house but primarily grows under primary forest and also commonly found on the hills in secondary forest.100 This plant can grow in an area with reduced soil fertility, an ex-mining or reclamation land.20,101 Our preliminary study showed that Breynia cernua is one of the dominant medicinal plants growing in the coal reclamation area at Tanah Bumbu-South Kalimantan, Indonesia.20 According to the WHO catalog, this plant is traditional used to treat diseases.100 People used its leaves directly or with various methods (e.g., crush, bake, heat, or decoct the leaves) to relieve illnesses such as cough, soreness, ulcer, and fever.100 People from Jayapura and Timika also used this plant as an alternative breast and cervical cancer treatment.99
Two studies in this review showed that Breynia cernua has cytotoxic activity, as stated in table 4. In the first study, seven species, including Breynia cernua, were obtained from some public forest in West Papua Province, Manokwari; Merauke: Sentani; Serui and Jayapura City (Indonesia). 99 Their cytotoxicity was evaluated using in vitro Brine Shrimp Lethality Test (BSLT). The ethanol extract of Breynia cernua showed toxicity with a Lethal Concentration (LC50) value of 255.76 ppm.99 Second study obtained fresh leaves of Breynia cernua from Jayapura City, Papua (Indonesia).17 The study showed that the extract or fraction of Breynia cernua showed cytotoxic activity against MCF-7 tumor cell line, with the highest activity recorded in the n-hexane fraction.17
Table 4: Results from studies on phytochemicals.
| No | Part Of Plant | Sample Used | Results | Active Phytochemicals | Reference |
| 1 | Leaves | Ethanolic extracts | LC 50: 255.76 ppm | N/A | 99 |
| 2 | Leaves | Ethanolic extracts | IC50: 246,841 ppm | Alkaloids, Flavonoids, Terpenoids, Tannins | 17 |
| N-hexane fraction | IC50: 165,65 ppm | ||||
| Ethylacetate fraction | IC50: 562,57 ppm | ||||
| Water fraction | IC50: 713,78 ppm. |
Furthermore, the extract or fraction of B. cernua has cytotoxicity to cancer cells. Although research is still limited, phytochemical studies of medicinal plants provide clues that the phytochemicals contained in B. cernua have anticancer potential. Therefore, further studies need to be carried out, for example, purification of the n-hexane fraction to obtain the active compound. In addition, this plant has additional values, such as it can grow easily in infertile soil and also its history of medical use. The flavonoid or quinone group of Breynia cernua may play a role in causing cytotoxic effects on cancer cell lines. The most common mechanism that appear in this study were intrinsic pathway of apoptosis which involved caspase and other pro-apoptotic protein. This pathway might be the one that will appear in the further study about this plant towards cancer cell. There are also various pathway in inhibiting cancer cell proliferation, which one them might also be the mechanism of action of anticancer agents inside Breynia cernua.
Nanoparticles have been extensively studied to increase anticancer activity by strengthening the bioavailability.43,102 Anna Grebinyk et al. used berberine (Ber) with a carbon nanomaterial, C60 fullerenes (C60), and formed C60-Ber nano complexes.43 This complex amplified its toxic effect in a low concentration range and potentiated its effect in vivo.43 Ruma Baksi et al. also developed quercetin-containing chitosan. Nanoparticles (QCT-CS NPs) with enhanced encapsulation efficiency and sustained release property.102 This suggests that QCT-CS NPs have increased efficacy over free quercetin in reducing the tumor size of mice containing lung and breast tumor xenografts.102 Gold and silver are also widely used as nanoparticle study materials to increase the cytotoxic activity of natural products.
Conclusion
In this present study, phytochemicals has various anticancer effect towards cancer cell. The flavonoid group is involved in the induction of apoptosis through caspase activity, inhibition of cell growth, and inhibition of cell migration. The alkaloid group induces apoptosis in the intrinsic pathway, cell growth inhibition, and autophagic induction. The saponins group can target the PI3K/AKT pathway to activate intrinsic apoptotic. Anticancer properties of quinones lead to the generation of Reactive Oxygen Species (ROS) associated with apoptosis in a dependent or independent caspase manner. Few studies have discussed the potential of the tannin group as an anticancer candidate. Mechanism against cancer cells of terpenoid group involves cell cycle arrest, NO concentration, and caspase proteins. Seven of the twelve extracts used in this study were methanolic extracts, and most of them have very strong cytotoxicity. Meanwhile, no studies have been conducted on the methanol extract of Breynia cernua, so using this extract seems promising. In addition, nine of twenty-three samples with low IC50 (strong activity) were isolated or purchased compounds. Therefore, we recommend isolating compounds from methanol, ethanol, or N-hexane extracts of Breynia cernua to get better anticancer activity, especially compounds belonging to the flavonoid or quinone group. Furthermore, using nanoparticles such as carbon, chitosan, silver, or gold may increase the bioavailability of anticancer agents. In summary, medicinal plants with limited studies, such as Breynia cernua, deserve to be explored for their anticancer potential based on their history of traditional use and phytochemical content. Therefore, the use of methanol extract needs to be investigated, with the target of its phytochemical content being flavonoids or quinones.
Acknowledgement
The authors acknowledge the support of Faculty of Medicine Universitas Padjadjaran and the Directorate of Research, Community Service, and Innovation Universitas Padjadjaran.
Conflict of Interest
The authors declare that there is no conflict of interest.
Funding Source
This work is supported by the Internal Grant Program of the Directorate of Research, Community Service, and Innovation Universitas Padjadjaran.
Reference
- CDC. Deaths and Mortality [Internet]. 2021. Available from: https://www.cdc.gov/nchs/fastats/deaths.htm
- NCI. Cancer Statistics [Internet]. 2020. Available from: https://www.cancer.gov/about-cancer/understanding/statistics
- Roy PS, Saikia BJ. Cancer and cure : A critical analysis. 2021;(3):441–3.
- Park J, Look KA. Health Care Expenditure Burden of Cancer Care in the United States. 2019;
- Aiello P, Sharghi M, Mansourkhani SM, Ardekan AP, Jouybari L, Daraei N, et al. Review Article Medicinal Plants in the Prevention and Treatment of Colon Cancer. 2019;2019.
- Henry DH, Viswanathan HN, Elkin EP, Traina S, Wade S. Symptoms and treatment burden associated with cancer treatment : results from a cross-sectional national survey in the U . S . 2008;791–801.
- Jain S, Dwivedi J, Jain PK, Satpathy S, Patra A. Medicinal Plants for Treatment of Cancer : A Brief Review. :87–102.
- Williams SM, Killeen AA. Tumor Lysis Syndrome. 2019;143(March).
- Hanneke Poort, Jamie M. Jacobs, William F. Pirl, Jennifer S. Temel, Joseph A. Greer. Fatigue in Patients on Oral Targeted or Chemotherapy for Cancer and Associations with Anxiety, Depression, and Quality of Life. Heal Res Alliance. 2020;18(2):141–7.
- Ritchie MR and H. CANCER. Our world Data [Internet]. 2015; Available from: https://ourworldindata.org/cancer
- Desai AG, Qazi GN, Ganju RK, El-tamer M, Singh J. Medicinal Plants and Cancer Chemoprevention. NIH public access. 2014;9(7):581–91.
- Group EPF. Medicinal Plants : Their Use in Anticancer Treatment. 2015;6(10):4103–12.
- Xu X, Li T, Man C, Fong V, Chen X, Chen X, et al. Saponins from Chinese Medicines as Anticancer Agents. 2016;1–27.
- Saibu M, Sagar S, Green I, Ameer F, Meyer M. Evaluating the Cytotoxic Effects of Novel Quinone Compounds. Anticancer Res. 2014;34:4077–86.
- Cai Y, Zhang J, Chen NG, Shi Z, Qiu J, He C, et al. Recent Advances in Anticancer Activities and Drug Delivery Systems of Tannins. Med Res Rev. 2016;(0):1–37.
- Kamran S, Sinniah A, Abdulghani MAM, Alshawsh MA. Therapeutic Potential of Certain Terpenoids as Anticancer Agents : A Scoping Review. Cancers (Basel). 2022;14:1100.
- Dirgantara S, Tanjung RHR, Maury HK, Meiyanto E. Cytotoxic Activity and Phytochemical Analysis of Breynia cernua from Papua. Indones J Pharm Sci Technol. 2018;1(1):31–6.
- Fitriyanti, Wathan N, Gunawan. Kajian Farmakognostik Tumbuhan Sugi-Sugi ( Breynia cernua Muel . Arg .) Asal Amuntai Kalimantan Selatan. J Pharmascience. 2016;03(02):43–8.
- Useful Tropical Plants. Breynia cernua [Internet]. 2021. Available from: http://tropical.theferns.info/viewtropical.php?id=Breynia+cernua
- Supandi, Saputra YHE, Anwar C, Kinanto, Kodir RA, Kurnia D, et al. POTENTIAL OF RECLAMATION AREA OF COAL MINING SITES IN MEDICAL FIELD. Int J Adv Res Eng Technol. 2020;11(8):714–20.
- Zhang X, Li H, Zhang H, Liu Y, Huo L, Jia Z, et al. Inhibition of transmembrane member 16A calcium-activated chloride channels by natural flavonoids contributes to flavonoid anticancer effects. Br J Pharmacol. 2017;174(14):2334–45.
- Bi Y, Min M, Shen W, Liu Y. Genistein induced anticancer effects on pancreatic cancer cell lines involves mitochondrial apoptosis, G0/G1cell cycle arrest and regulation of STAT3 signalling pathway. Phytomedicine. 2018;39:10–6.
- Vrhovac I, Maduni J, Antunovi M, Parad M, Garaj-vrhovac V. Apigenin , a dietary flavonoid , induces apoptosis , DNA damage , and oxidative stress in human breast cancer MCF-7 and MDA MB-231 cells. Naunyn Schmiedebergs Arch Pharmacol. 2018;391:537–50.
- Li H, Zou T, Jia Q, Xia E, Cao W, Liu W, et al. Anticancer effects of morin-7-sulphate sodium , a flavonoid derivative , in mouse melanoma cells. Biomed Pharmacother. 2016;84:909–16.
- Martínez JJ, Naso LG, Pérez AL, Rizzi A, Ferrer EG, Williams PAM. Antioxidant and anticancer effects and bioavailability studies of the fl avonoid baicalin and its oxidovanadium ( IV ) complex. J Inorg Biochem. 2017;166:150–61.
- Wei S, Sun T, Du JIE, Zhang B, Xiang D, Li W. Xanthohumol , a prenylated flavonoid from Hops , exerts anticancer effects against gastric cancer in vitro. Oncol Rep. 2018;40(6):3213–22.
- Prakash S, Elavarasan N, Subashini K. Isolation of hesperetin – A flavonoid from Cordia sebestena flower extract through antioxidant assay guided method and its antibacterial, anticancer effect on cervical cancer via in vitro and in silico molecular docking studies. J Mol Struct. 2020;1207:127751.
- Rajendran P, Maheshwari U, Muthukrishnan A, Muthuswamy R, Anand K. Myricetin : versatile plant based flavonoid for cancer treatment by inducing cell cycle arrest and ROS – reliant mitochondria ‑ facilitated apoptosis in A549 lung cancer cells and in silico prediction. Mol Cell Biochem. 2020;476:57–68.
- Attari F, Arefian E, Keighobadi F, Abdollahi M, Farimani MM. Inhibitory effect of flavonoid xanthomicrol on triple-negative breast tumor via regulation of cancer-associated microRNAs. Phyther Res. 2020;35(4):1967–82.
- Martı C, Ward C, Turnbull AK, Mullen P, Cook G, Meehan J, et al. Antitumour activity of the novel flavonoid Oncamex in preclinical breast cancer models. Br J Cancer. 2016;114:905–16.
- Xu Q, Zhu D, Wang G, Lin T, Sun C, Ding R, et al. Phenolic glycosides and flavonoids with antioxidant and anticancer activities from Desmodium caudatum. Nat Prod Res. 2021;35(22):4534–41.
- Fakhoury VS, Pessoa ADS, Kazuko C, Pagnan AL, Silva G, Oliveira N De, et al. Evaluation of Myrcia bella in murine osteosarcoma cells : Effect of the extract and enriched fractions of tannins and flavonoids. Nat Prod Res. 2022;
- Ali Alzandi A et al. Phytochemical components, antioxidant and anticancer activity of 18 major medicinal plants in Albaha region, Saudi Arabia. Biocatal Agric Biotechnol. 2021;34.
- Zhou J, Feng J, Fang L. A novel monoterpenoid indole alkaloid with anticancer activity from Melodinus khasianus. Bioorg Med Chem Lett. 2017;27(4):893–6.
- Al-rashed S, Baker A, Sayeed S, Syed A, Bahkali AH, Elgorban AM, et al. Vincamine, a safe natural alkaloid, represents a novel anticancer agent. Bioorg Chem. 2021;107:104626.
- Qazzaz ME, Raja VJ, Lim K, Kam T, Lee JB, Gershkovich P, et al. In vitro anticancer properties and biological evaluation of novel natural alkaloid jerantinine b. Cancer Lett. 2016;370(2):185–97.
- Yang CL, Zhang B, Xue WW, Li W, Xu ZF, Shi J, et al. Discovery , Biosynthesis and Heterologous Production of Loonamycin , a Potent Anticancer indolocarbazole alkaloid. Org Lett. 2020;22(12):4665–9.
- Bakthavachalam V, Chinnapaka S, Samy ALPA, Munirathinam G. Neferine, an alkaloid from lotus seed embryo targets HeLa and SiHa cervical cancer cells via pro-oxidant anticancer mechanism. Phyther Res. 2020;34(9):2366–84.
- Inada M, Sato A, Shindo M, Yamamoto Y. Anticancer Non-narcotic Opium Alkaloid Papaverine. Anticancer Res. 2019;39(12):6743–50.
- Nurdin WB, Hasanuddin U. In Vitro Study of the Alkaloid Anticancer Compound From Makassar Medicinal Plants Boehmeria virgata Linn. Int J Pharm Sci Rev Res. 2018;13:77–81.
- Fang Z, Ren Y, Du S, Zhang M, Wang Y, Fang L. Melosuavine I , an apoptosis-inducing bisindole alkaloid from Melodinus suaveolens. Fitoterapia. 2019;133:175–9.
- Pal HC, Katiyar SK. Cryptolepine, a Plant Alkaloid, Inhibits the Growth of Non-Melanoma Skin Cancer Cells through Inhibition of Topoisomerase and Induction of DNA Damage. Molecules. 2016;21(12):1758.
- Grebinyk A, Prylutska S, Grebinyk S, Evstigneev M, Krysiuk I, Skaterna T, et al. Antitumor efficiency of the natural alkaloid berberine complexed with C60 fullerene in Lewis lung carcinoma in vitro and in vivo. Cancer Nanotechnol. 2021;12(24).
- Joshi S, Amgoth C, Narayana S, Padmavathi, Madhavi J, Satya K. Antioxidant and anticancer activities of an Aporphine alkaloid isolated from Alphonsea sclerocarpa. J Phytopharm. 2018;7(1):51–5.
- Saputri RD, Tjahjandarie TS, Tanjung M, Products N, Division OC, Airlangga U. Alkaloid Kuinolin Dari Melicope denhamii dan Uji Aktivitas Antikankernya. 2018;1(9):505–9.
- Toddalia R, Dan L, Aktivitas UJI. Isolasi Senyawa Alkaloid Turunan Furokuinolin Dari Ranting Toddalia asiatica L. dan Uji Aktivitas Antikanker. J Kim Ris. 2018;3(2):102–7.
- Saputri RD, Tjahjandarie TS, Tanjung M. Alkaloid Furokuinolin dan Asam Sinamat Ter-O-Geranilasi dari Kulit Batang Melicope hookeri T.G. Hartley. J Sains dan Kesehat. 2019;2(1):1–7.
- Simorangkir M, Silaban S, Surbakti R, Barus T, Simanjuntak P. Aktivitas Antikanker Ekstrak Etanolbuah Ranti Hitam (Solanum blumei Nees ex Blume) Terhadap Sel Leukimia L1210. Chim Nat Acta. 2017;5(1):31–5.
- Zhu X, Jiang H, Li J, Xu J, Fei Z. Anticancer Effects of Paris Saponins by Apoptosis and PI3K / AKT Pathway in Gefitinib- Resistant Non-Small Cell Lung Cancer. Med Sci Monit. 2016;22:1435–41.
- Georgieva A, Popov G, Shkondrov A, Toshkova R, Krasteva I, Kondeva-burdina M, et al. Antiproliferative and antitumour activity of saponins from Astragalus glycyphyllos on myeloid Graffi tumour. J Ethnopharmacol. 2021;267:113519.
- Alam F, Najum Q, Waheed A. Cytotoxic activity of extracts and crude saponins from Zanthoxylum armatum DC. against human breast (MCF-7, MDA-MB- 468) and colorectal (Caco-2) cancer cell lines. BMC Complement Altern Med. 2017;17(368).
- Mihaylova R, Shkondrov A, Aluani D, Ionkova I, Tzankova V, Krasteva I. In vitro antitumour and immunomodulating activity of saponins from Astragalus glycyphyllos. Biotechnol Biotechnol Equip. 2022;35(1):1948–55.
- Shkondrov A, Krasteva I, Ionkova I, Popova P, Zarev Y, Mihaylova R, et al. Production of saponins from in vitro cultures of Astragalus glycyphyllos and their antineoplastic activity. Biotechnol Biotechnol Equip. 2019;33(1):1413–8.
- Abdelrahman M, Ali H, El-sayed M, Tanaka S, Tran LP. Isolation and characterization of Cepa2, a natural alliospiroside A, from shallot (Allium cepa L. Aggregatum group) with anticancer activity. Plant Physiol Biochem. 2017;116:167–73.
- Mosad RR, Ali MH, Ibrahim MT, Shaaban HM, Emara M. New cytotoxic steroidal saponins from Cestrum parqui. Phytochem Lett. 2017;22:167–73.
- Fayyad RJ, Ali ANM, Dwaish AS, Al-Abboodi AKA. Anticancer Activity of Spirulina Platensis Methanolic Extracts Against L20B and MCF7 Human Cancer Cell Lines. Plant Arch. 2019;19:1419–26.
- Jaramillo-carmona S, Guillen-Bejarano R, Jimenez-Araujo A, Rodriguez-Arcos R, Lopez S. In vitro toxicity of asparagus saponins in distinct multidrug resistance colon cancer cells. Chem Biodivers. 2018;15(11).
- Ma Q, Jiang J, Yuan X, Qiu K, Zhu W. Comparative antitumor and anti-inflammatory effects of flavonoids, saponins, polysaccharides, essential oil, coumarin and alkaloids from Cirsium japonicum DC. Food Chem Toxicol. 2019;125:422–9.
- Hur W, Son SE, Kim SN, Seong GH. Bioelectrochemistry Cell-based electrochemical cytosensor for rapid and sensitive evaluation of the anticancer effects of saponin on human malignant melanoma cells. Bioelectrochemistry. 2021;140:107813.
- Sirait PS, Setyaningsih I, Tarman K. Anticancer Activity of Spirulina Cultivated in Walne and Organic Media. J Pengolah Has Perikan Indones. 2019;22.
- Hernandes C, Miguita L, Sales RO De, Silva EDP, Omori P, Mendonça R De, et al. Anticancer Activities of the Quinone-Methide Triterpenes Maytenin and 22-β-hydroxymaytenin Obtained from Cultivated Maytenus ilicifolia Roots Associated with Down-Regulation of miRNA-27a and miR-20a/miR-17-5p. Molecules. 2020;25(3):760.
- Payili N, Yennam S, Rekula SR, Naidu CG, Bobde Y, Ghosh B. Design , Synthesis , and Evaluation of the Anticancer Properties of Novel Quinone Bearing Carbamyl β-Lactam Hybrids. J Heterocycl Chem. 2018;
- Muniyandi K, George E, Sathyanarayanan S, George BP, Abrahamse H, Thamburaj S, et al. Phenolics , tannins , flavonoids and anthocyanins contents influenced antioxidant and anticancer activities of Rubus fruits from Western Ghats, India. Food Sci Hum Wellness. 2019;8(1):73–81.
- Malar TRJJ, Antonyswamy J, Vijayaraghavan P, Ock Y, Al-ghamdi AA, Elshikh MS, et al. In-vitro phytochemical and pharmacological bio-efficacy studies on Azadirachta indica A . Juss and Melia azedarach Linn for anticancer activity. Saudi J Biol Sci. 2020;27(2):682–8.
- Sallum LO, Siqueira VL, Custodio JMF, Borges M, Lima RS, Lacerda DPS. Molecular modeling of cytotoxic activity of a new terpenoid-like bischalcone. New J Chem. 2019;43:18451–60.
- Kokhdan EP, Sadeghi H, Ghafoori H, Sadeghi H. Cytotoxic effect of methanolic extract, alkaloid and terpenoid fractions of Stachys pilifera against HT-29 cell line. Res Pharm Sci. 2018;13(5):404–12.
- Patel SB, Ghane SG. Phyto-constituents profiling of Luffa echinata and in vitro assessment of antioxidant, anti-diabetic, anticancer and anti-acetylcholine esterase activities. Saudi J Biol Sci. 2021;28(7):3835–46.
- Yoo S, Kim K, Nam H, Lee D. Discovering Health Benefits of Phytochemicals with Integrated Analysis of the Molecular Network , Chemical Properties and Ethnopharmacological Evidence. Nutrients. 2018;10:1042.
- Ashraf MA. Phytochemicals as Potential Anticancer Drugs : Time to Ponder Nature ’ s Bounty. Biomed Res Intrernational. 2020;2020:7.
- Wang H, Khor TO, Shu L, Su Z, Fuentes F, Lee J-H, et al. Plants Against Cancer: A Review on Natural Phytochemicals in Preventing and Treating Cancers and Their Druggability. Anticancer Agents Med Chem. 2012;12(10):1281–305.
- Ouyang L, Shi Z, Zhao S, Wang F, Zhou T, Liu B, et al. Programmed cell death pathways in cancer : a review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012;(15):487–98.
- Kopustinskiene DM, Jakstas V, Savickas A, Bernatoniene J. Flavonoids as Anticancer Agents. 2020;(C):1–25.
- Indrayanto G, Putra GS, Suhud F. Validation of in-vitro bioassay methods : Application in herbal drug research. 1st ed. Prof. of Drug Substances, Excipients & Related Methodology. Elsevier Inc.; 2020. 1–35 p.
- Dey P, Kundu A, Kumar A, Gupta M, Lee MB, Bhakta T, et al. Recent Advances in Natural Products Analysis: Chapter 15 – Analysis of alkaloids (indole alkaloids, isoquinoline alkaloids, tropane alkaloids). Elsevier; 2020. 505–567 p.
- Mugford ST, Osbourn A. Saponin Synthesis and Function. Nat Public Heal Emerg Collect. 2012;405–24.
- Rasoanaivo P, Wright CW, Willcox ML, Gilbert B. Whole plant extracts versus single compounds for the treatment of malaria : synergy and positive interactions. Malar J. 2011;10(Suppl 1):S4.
- Liu Y, Shi C, He Z, Zhu F. Inhibition of PI3K/AKT signaling via ROS regulation is involved in Rhein-induced apoptosis and enhancement of oxaliplatin sensitivity in pancreatic cancer cells. Int J Biol Sci. 2021;17(2):589–602.
- Takino J, Sato T, Nagamine K, Hori T. The inhibition of Bax activation-induced apoptosis by RasGRP2 via R-Ras-PI3K-Akt signaling pathway in the endothelial cells. Sci Rep. 2019;9:16717.
- Sarewicz M, Osyczka A. Electronic connection between the quinone and cytochrome C redox pools and its role in regulation of mitochondrial electron transport and redox signaling. Physiol Rev. 2015;95:219–43.
- Colucci MA, Moody CJ, Couch GD, Moody C. Natural and synthetic quinones and their reduction by the quinone reductase enzyme NQO1 : from synthetic organic chemistry to compounds with anticancer potential. Org Biomol Chem. 2008;6(4):637–56.
- Thomson RH. Distribution of naturally occuring quinones. Pharm Weekbl. 1991;13:70–3.
- Thomson RH. Naturally occuring quinones. Elsevier; 2012.
- Kitano T, Yoda H, Tabata K, Miura M, Toriyama M, Motoshashi S, et al. Vitamin K3 analogs induce selective tumor cytotoxicity in neuroblastoma. Biol Pharm Bull. 2012;35:617–23.
- Nakaoka E, Tanaka S, Onda K, Sugiyama K, Hirano T. Effects of Vitamin K3 and K5 on Daunorubicin-resistant Human T Lymphoblastoid Leukemia Cells. Anticancer Res. 2015;35:6041–8.
- Wiraswati HL, Hangen E, Sanz AB. Apoptosis inducing factor (AIF) mediates lethal redox stress induced by menadione. Oncotarget. 2016;7(47).
- Wiraswati HL, Akhmaloka A, Martoprawiro M. Apoptosis Inducing Factor ( AIF ) Stabilizes Menadione- Conjugate Product in Programmed Cell Death. Int J PharmTech Res. 2017;10(4):237–45.
- Mustofa HN, Wiraswati HL, Ekawardhani S. Anticancer Activities of Saponins and Quinones Group through Oxidative Stress and Glycolysis Inhibition via in Silico Studies. Biomed Pharmacol J. 2020;13(June):999–1010.
- Wiraswati HL. 1 , 4 Benzoquinone Induced Apoptosis on MCF-7 Breast Cancer Cell Line. Int J Innov Sci Res Technol. 2019;4(12):689–92.
- Pardo GL, Zuccolotto F, Reis D, González-durruthy M, Delgado R, Vries RFD, et al. Rapanone, a naturally occurring benzoquinone, inhibits mitochondrial respiration and induces HepG2 cell death. Toxicol Vitr. 2020;63:104737.
- Duval R, Bui L, Mathieu C, Nian Q, Berthelet J, Xu X, et al. Benzoquinone, a leukemogenic metabolite of benzene, catalytically inhibits the protein tyrosine phosphatase PTPN2 and alters STAT1 signaling. J Biol Chem. 2019;294:12483–94.
- Susin S, Lorenzo H, Zamzami N, Marzo I. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397(6718):441–6.
- Mateo V, Lagneaux L, Bron D, Biron G. CD47 ligation induces caspase-independent cell death in chronic lymphocytic leukemia. Nature. 1999;5:1277–84.
- Kroemer G, Galluzi L, Vandenabeele P, Abrams J. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11.
- Szczurek A. Perspectives on Tannins. Biomolecules. 2021;11:442.
- Cox-georgian D, Ramadoss N, Dona C. Therapeutic and Medicinal Uses of Terpenes. Nat Public Heal Emerg Collect. 2019;333–59.
- N. Farrow K, Lakshminrusimha S, J. Reda W, Wedgewood S. Superoxide dismutase restores eNOS expression and function in resistance pulmonary arteries from neonatal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;295:L979–L987.
- L. Guzman M, J. Neering S, Upchurch D, Grimes B, S. Howard D. Nuclear factor-κB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood. 2001;98(8):2301–7.
- Dong Q-M, Ling C, Chen X, Zhao L. Inhibition of tumor necrosis factor-α enhances apoptosis induced by nuclear factor-κB inhibition in leukemia cells. Oncol Lett. 2015;10(6):3793–8.
- Dirgantara S, Tanjung RHR, Nurlatifah R, Meiyanto E. Cytotoxic Activity of Selected Medicinal Plants from Papua, Indonesia. BROMO. 2018;155–8.
- WHO. Breynia cernua (Poir.) Muell. Arg. In: Medicinal Plants in Papua New Guinea. 2009. p. 45–6.
- Quadros PD de, Zhalnina K, Davis-richardson AG, Drew JC, Menezes FB, Camargo FADO, et al. Coal mining practices reduce the microbial biomass , richness and diversity of soil. Appl Soil Ecol. 2015;
- Baksi R, Pratap D, Borse SP, Rana R, Sharma V. In vitro and in vivo anticancer efficacy potential of Quercetin loaded polymeric nanoparticles. Biomed Pharmacother. 2018;106(April):1513–26.








