Nicolás Padilla-Raygoza1* , Edna Griselda Lara-Mares2
, Edna Griselda Lara-Mares2 , Efraín Navarro-Olivos3
, Efraín Navarro-Olivos3 , Jorge Lira-Gómez2
, Jorge Lira-Gómez2 , Gilberto Flores-Vargas1
, Gilberto Flores-Vargas1 , María de Jesús Gallardo-Luna1
, María de Jesús Gallardo-Luna1
1Department of Research and Technological Development, Directorate of Teaching and Research, Institute of Public Health from Guanajuato State, 36250 México
2Department of Teaching and Training, Directorate of Teaching and Research, Institute of Public Health from Guanajuato, 36250 México
3Directorate of Teaching and Research, Institute of Public Health from Guanajuato State, México
Corresponding Author E-mail: padillawarm@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2563
Abstract
Hypertensive disease is one of the main concerns during pregnancy. It is classified as gestational hypertension, preeclampsia, and eclampsia. The objective of this study was to analyze whether there is a relationship between gestational hypertension and high birth weight (≥3500 g) in newborns. It was designed a retrospective quantitative study, considering women admitted for obstetric resolution and their products treated in hospitals from Guanajuato State, Mexico, with hypertension developing after 20 weeks of pregnancy. The Chi-squared test was performed to show a relationship between gestational hypertension and preeclampsia-eclampsia with the newborn's birth weight. Then calculated the effect of gestational hypertension on birthweight by computing the corresponding Odds Ratios (OR). We also fitted a multivariate logistic regression model that included age group, marital status, number of previous pregnancies, and gestational age as covariates. It was computed the Pearson correlation and adjusted a linear regression model to identify the relationship between systolic and diastolic pressure with birthweight. The alpha value was fixed at .05 as a threshold for statistical significance. The number of reviewed registries was 1,675. For the variables age, gestational age at hospital admission, systolic and diastolic blood pressure, and birth weight of the products, there are differences between the groups of mothers (P<.05). For the logistic regression model just including gestational hypertension as covariable, the OR was 1.28 (95% CI=1.0 to 1.63). The gestational age plays a confounding role -other variables did not improve the model-. Systolic and diastolic blood pressure showed a statistically significant (P=0.2, P=0.048) low negative correlation (r=-0.07, r=-0.06) with birth weight for women with gestational hypertension. There is an effect between gestational hypertension and high birth weight, although the effect is confounded by gestational age at birth time. Linear regression between systolic-diastolic pressure and birthweight supports this association. The evidence is low and further studies are advisable.
Keywords
Birthweight; Eclampsia; Gestational Hypertension; Preeclampsia
Download this article as:| Copy the following to cite this article: Raygoza N. P, Mares E. G. L, Olivos E. N, Gómez J. L, Vargas G. F, Luna M. D. J. Relationship between Gestational Hypertension and High Birth Weight, in the State of Guanajuato, Mexico. Biomed Pharmacol J 2022;15(4). |
| Copy the following to cite this URL: Raygoza N. P, Mares E. G. L, Olivos E. N, Gómez J. L, Vargas G. F, Luna M. D. J. Relationship between Gestational Hypertension and High Birth Weight, in the State of Guanajuato, Mexico. Biomed Pharmacol J 2022;15(4). Available from: https://bit.ly/3iEXDom |
Introduction
Hypertensive disease in pregnancy is one of the chief causes of morbidity and mortality in all countries, with a higher burden in low-income countries1-3. It is classified as gestational hypertension, preeclampsia, and eclampsia4. Gestational hypertension is arterial hypertension that appears after week 20 of gestation, with blood pressure ≥140/90 mmHg. When it is accompanied by proteinuria, it is called preeclampsia5.
Preeclampsia, defined as arterial hypertension and maternal proteinuria, is a syndrome that is unique in pregnancy, affects between 5 and 10% of pregnant women, and continues to be the leading cause of maternal morbidity and mortality worldwide, related to adverse perinatal outcomes6,7. 25% of preeclampsia cases occur in Latin America and the Caribbean7; in Mexico, the incidence of preeclampsia is 47.3 per 100,000 newborns8. In Alberta, Canada, Xiong et al.8 found that 26% of infants born to mothers with preeclampsia or eclampsia had low birthweight (<2500 gr). Meanwhile, 10% of infants born to mothers with gestational hypertension were underweight.
It is considered that preeclampsia causes low birth weight and growth retardation in utero in 26% of the products, compared to 10.6% of low weight among products of mothers who presented gestational hypertension. However, as reported by Xiong et al.9, it has also been studied in the association between preeclampsia and high birth weight. They found a high birth weight in 7.3% of children born to mothers with gestational hypertension, 5% in children born to mothers with preeclampsia/eclampsia, and 5.6 % in those born to non-hypertensive mothers9.
Xiong et al.9, consider the theory of the pathophysiological process of preeclampsia. They propose that newborns have a low birth weight due to a reduction in uteroplacental flow. They also found that the high weight birth newborns proportion was lower among normotensive women than among women with preeclampsia and gestational hypertension. Then, the theory of decreased uteroplacental flow seems to be contradicted. So, they concluded that many mothers with preeclampsia might have typical or increased uteroplacental blood flow.
In Celaya, Mexico, Padilla et al.10, reported that 20.57% of newborns from normotensive mothers weighed more than 3500 gr., 22.58% among those born to women with gestational hypertension had a high birth weight, and 17.70% born to women with preeclampsia also had a high birth weight.
The Institute of Public Health from Guanajuato State (IPHGS) is the public health system in Guanajuato State; it has 20 Community Hospitals, 15 General Hospitals, and 4 Maternal Hospitals that receive pregnant mothers to obstetric resolution.
The objective of this study was to corroborate whether there is a relationship between gestational hypertension and high weight birth (≥3500 g) in newborns. For this purpose, the study considers women admitted for obstetric resolution and their products treated in hospitals in Guanajuato State, Mexico, dependent on the IPHSG with hypertension developing after 20 weeks of pregnancy.
Patients and methods
Study design
It is a retrospective quantitative study, on the database of deliveries from hospitals from IPHSG, in the Mexican state of Guanajuato. The study considered all registries of deliveries in hospitals from IPHSG with the data of the mother and newborn during 2020 with hypertension (140 systolic or 90 diastolic, mm Hg) after 20 weeks of pregnancy.
The selection criteria were pregnant mothers admitted to IPHSG hospitals for obstetric resolution, with complete data in the registry from mothers and outcomes, with hypertension after the 20 weeks of pregnancy (with systolic pressure 140 or higher and diastolic pressure 90 or higher, mm Hg).
The exclusion criteria were mothers with twin pregnancies. The elimination criteria were incomplete registries.
There was no sampling; the study included all the complete records.
The following sociodemographic variables were included: age, marriage status, number of pregnancies, and gestational age upon admission to the hospital.
The independent variables included the following:
Systolic pressure. It is a continuous quantitative variable; it is the pressure of the blood to circulate in the circulatory system during the heart systole. It is measured in mm Hg and is presented as mean and standard deviation.
Diastolic pressure. It is a continuous quantitative variable; it is the pressure of the blood to circulate in the circulatory system during the diastole of the heart. It is measured in mm Hg and is presented as mean and standard deviation.
Gestational hypertension. It is a binary categorical variable; it is defined as systolic pressure 140 mm Hg or diastolic pressure 90 mm Hg or higher, from 20 weeks of pregnancy, without proteinuria; it is measured as yes or no, and it is presented with frequencies and percentages.
Preeclampsia/eclampsia. It is a binary categorical variable; it is defined as systolic pressure 140 mm Hg or diastolic pressure 90 mm Hg or higher, from 20 weeks of pregnancy, with proteinuria; it was measured as yes or no, and it is presented with frequencies and percentages.
Mode of delivery. It is a binary categorical variable. It is how the newborn was born; measured as vaginal delivery or cesarean section; presented with frequencies and percentages.
The outcome variables were:
Birthweight. It is a continuous quantitative variable. It is the bodyweight after the delivery. It is measured in grams and is presented as mean ± standard deviation.
Classification of birthweight. It is an ordinal categorical variable. It is the body mass expressed by grams: it is measured as low (less or equal to 2499 gr), adequate (2500-3499 gr), and high (≥ 3500 gr) and presented as frequencies and percentages.
Dichotomic birthweight. It is a dichotomic variable. It is the bodyweight after the delivery. It is the body mass expressed by grams: it is measured as low-normal (less or equal to 3499 gr) and high (≥ 3500 gr). It is presented as frequencies and percentages.
Procedures and sample size calculation
After the approval of the research and research ethics committees of the General Hospital of Penjamo, the files of pregnant women admitted for obstetric resolution from the IPHSG hospitals were reviewed. The study included the book of deliveries and the registries from mothers admitted to the hospitals for review. Data privacy is guaranteed as no personnel data for identification was used.
Assuming that women with preeclampsia-eclampsia have 7.8% of newborns weighing more than 3500 g and those with gestational hypertension have 3.8%11, the minimum sample size is 535 in the gestational hypertension group and 535 in the preeclampsia-eclampsia group. This computation was performed with a confidence level of 95% and 80% of power (Epidat 4.2, 2016. Xunta de Galicia, OPS, and CES University).
Statistical analysis
Descriptive statistics are shown for all the variables. The Chi-squared test and its P-value were computed to study a relationship between gestational hypertension and preeclampsia or eclampsia with the classification of birthweight from newborns. Computation of Odds Ratio (OR) and 95% Confidence Intervals (95% CI) were used to show the effect of pregnancy disease hypertension on birthweight. We also fitted a multivariate logistic regression model including age group, marital status, number of previous pregnancies, and gestational age as covariates.
To study the relationship between systolic and diastolic pressure with birthweight, it has been computed the Pearson correlation, adjusted a linear regression model, and made scatterplots.
The alpha value was fixed at .05 as a threshold for statistical significance. The statistical analysis was performed in STATA 13.0 ® (Stata Corp., College Station, TX, USA).
Results
The number of reviewed registries was 1,675. They were collected in eight Community Hospitals, eight General Hospitals, and two Maternal Hospitals. All included a diagnosis of hypertension induced by pregnancy.
Table 1 shows the characteristics of mothers with pregnancy-induced hypertension, divided into those with gestational hypertension or preeclampsia-eclampsia.
Only for the number of pregnancies, there were no statistically significant differences between mothers with gestational hypertension and preeclampsia-eclampsia. For the variables age, gestational age at hospital admission, systolic and diastolic blood pressure, and birth weight of the products, there are differences between the groups of mothers (P<.05).
Table 1: Quantitative characteristics from the mothers by type of hypertension.
| Variable | Gestational hypertension (n=968) | Preeclampsia/ eclampsia (n=707) | t-Student test, df, P-value |
| Age (years)
Range Mean ± SD |
14 to 43 25.25 ± 6.66 |
14 to 46 25.99 ± 7.24 |
-2.16, 1673, .03 |
| Number of pregnancies
Range Median, IQR |
0 to 8 1, 1 |
0 to 9 1, 1 |
0.92, .34 (Medians) |
| Gestational age (weeks)
Range Mean ± SD |
25 to 42 38.0 ± 2.24 |
21.5 to 44.3 37.24 ± 2.63 |
6.37, 1673, .0000 |
| Systolic pressure (mmHg)
Range Mean ± SD |
110 to 192 142.09 ± 11.53 |
120 to 243 149.81 ± 15.64 |
-11.63, 1673, .0000 |
| Diastolic pressure (mmHg)
Range Mean ± SD |
40 to 130 92.24 ± 8.68 |
68 to 150 96.67 ± 10.02 |
-9.66, 1673, .0000 |
| Birthweight (grs)
Range Mean ± SD |
680 to 4685 2968.28 ± 624.58 |
330 to 5300 2847.55 ± 662.96 |
3.81, 1673, .0001
|
Source: Database for analysis (15)
Table 2 shows the categorical variables by the groups of hypertensive mothers. The chief characteristics of the women with gestational hypertension were cohabitation (56%), childbirth by cesarean (69.11%), and full-term pregnancy (68.80%). Regarding birth weight, 21.18 % had products with high weight (greater or equal to 3500 gr.). Meanwhile, the chief characteristics of women with preeclampsia-eclampsia were cohabitation (47.38%), childbirth by cesarean (80.20%), and full-term pregnancy (52.90%). For this group, 29.56% had products with low birthweight (less than 2500 gr.).
Table 2: Distribution of categorical variables by mothers with gestational hypertension or preeclampsia/eclampsia
| Variable | Gestational hypertension
(n=968) n % |
Preeclampsia/ eclampsia
(n=707) n % |
|
| Civil Status
Single Married Separated Divorced Widowed Cohabitation |
114 11.78 300 30.99 5 0.52 6 0.62 0 0.00 543 56.10 |
119 16.83 238 33.80 11 1.56 2 0.28 1 0.14 335 47.38 |
Z for two proportions
-2.97, P=.003 – 1.22, P=.22 – 2.16, P=.03 0.99, P=0.32 N/A 3.53, P<0.01 |
| Childbirth
Vaginal Cesarean |
299 30.89 669 69.11 |
140 19.80 567 80.20 |
X2= 25.97 P=.000 |
| Gestational age (weeks)
Preterm (37 or less) Full-term (38-42) |
302 31.20 666 68.80 |
333 47.10 374 52.90 |
X2=43.89 P=.000 |
| Birthweight (gr)
Low (≤ 2499) Adequate (2500-3499) High (≥3500) |
203 20.97 560 57.85 205 21.18 |
209 29.56 375 53.04 123 17.40 |
X2= 16.93 P=.000 |
Source: Database for analysis (15)
The study fitted several logistic regression models for the dichotomic birthweight variable. For the model just including gestational hypertension as covariable, the OR was 1.28 (95% CI=1.0 to 1.63). We considered gestational age a potential confounder variable, obtaining an OR for gestational hypertension of 1.05 (95% CI= 0.81 to 1.36). The likelihood ratio test for the model’s comparison was 128.43 and a p-value lesser than 0.001. Hence, gestational age plays a confounding role.
Regarding the linear relationship between systolic and diastolic blood pressure with birthweight, systolic blood pressure showed a non-statistically significant (P=0.2) low negative correlation (r=-0.07) among women with gestational hypertension. For the women with preeclampsia-eclampsia, the correlation was not statistically significant (Figure 1).
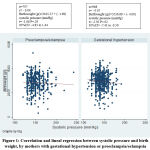 |
Figure 1: Correlation and lineal regression between systolic pressure and birthweight, by mothers with gestational hypertension or preeclampsia/eclampsia |
Source: Registries from hospitals
Figure 2 shows a statistically significant (P=0.048) low negative correlation (r=-0.06) between diastolic blood pressure and birth weight among women with gestational hypertension. For women with preeclampsia-eclampsia, the correlation was non-statistically significant.
 |
Figure 2: Correlation and lineal regression between diastolic pressure and birthweight, by mothers with gestational hypertension or preeclampsia/eclampsia |
Source: Registries from hospitals
Discussion
From the sample of 1675 records, it has been shown that gestational hypertension is related to high birth weight (≥3500 gr) (X2 = 16.93, P<.05, and the effect is OR =1.28 (1.0 to 1.63). Gestational age confounds this effect (OR=1.05, 95%CI 0.81 to 1.36). Meanwhile, childbirth, marital status, and the number of pregnancies do not improve the model (P>.05).
Liu et al.12, reported 3.58% of newborns with low birth weight from mothers with gestational hypertension and 6.02% from mothers with preeclampsia-eclampsia. In Guanajuato, 20.97% of newborns born to mothers with gestational hypertension weighed less than 2,500 grams, and 29.56% of mothers with preeclampsia-eclampsia (Table 2).
Women with preeclampsia-eclampsia show higher systolic and diastolic blood pressure13. It was observed too among women treated in Guanajuato State, with statistically significant differences in mean systolic and diastolic blood pressure among women with gestational hypertension or preeclampsia-eclampsia (P<.05) (Table 1).
In Taiwanese women, it was reported that those with preeclampsia-eclampsia had higher systolic (Mean 179.5 ± 19.7 mmHg) and diastolic (108.7 ± 16.5 mmHg) blood pressure than women with gestational hypertension (147.9 ± 8.1 systolic and 87.8 ± 10.2 diastolic)11. This difference in diastolic and diastolic blood pressure levels is reported among pregnant women in Guanajuato State, Mexico, with preeclampsia-eclampsia or gestational hypertension (Table 1).
Another similarity between Taiwanese and Mexican women is that there were more cesarean sections among women with preeclampsia-eclampsia (87.3%, and 80.20%, respectively) than those with gestational hypertension (77.4% and 69.11%, respectively) (12) (Table 2). The results are like those in Shen et al.14, where 35.4% of newborns were born by cesarean section among mothers with gestational hypertension and 53.6% among mothers with preeclampsia-eclampsia.
The average birth weight in children of mothers with preeclampsia-eclampsia in Taiwan was 1957.0 ± 89.61 g, and for those with gestational hypertension was 2459.7 ± 794.6 g12. In products from mothers in this study, an average birth weight of 2968.28 ± 624.58 is reported among mothers with gestational hypertension and 2847.55 ± 662.96 gr among mothers with preeclampsia/eclampsia (Table 1).
Magalhäes et al.15, analyzed leptin, adiponectin, and ghrelin levels in cord blood of products with less than 37 weeks of gestation from mothers with or without hypertension. They found elevated levels in children of hypertensive mothers compared to non-hypertensives. It could be a possible reason for high or low birth weight in newborns from mothers with pregnancy-induced hypertension.
Limitations
We could not obtain all registries of women admitted to IPHSG hospitals because they were incomplete. It can introduce selection bias and did not include normotensive pregnant women since this study aimed to compare the birthweight between gestational hypertension and preeclampsia among pregnant Mexican women
Conclusion
There is a mild association and effect between gestational hypertension and high birth weight (≥3500 g). Nevertheless, it is confounded by gestational age at birth. The association is lost, and the effect decreases while adjusting by this covariable. Linear regression between systolic-diastolic pressure and birthweight shows little support for this association. The evidence is low and further studies are advisable.
Acknowledgments
The authors thank the Nursing interns from all the hospitals, who collaborated in obtaining data from the files: Alexa Loreley Cuevas- García, Alejandra Vázquez-Medina, María Luisa Cruz-Ayala, Jaime Martínez-Baltazar, Gloria del Carmen López-Martínez, Jorge Daniel Arriaga-Becerra, Rosa Emilia Bustos-Gutiérrez, Laura Berenice Carpio-Verdín, Blanca Vanessa González-Zermeño, Selene Shunaxi Alvarado-Matus, Luz Fernanda Torres-Preciado, Mónica Salinas-Candelas, Emmanuel Antonio Manríquez-Saucedo, Blanca Sarahy Irazú Angulo-Pérez, Jimena Lisett Cardial-González, Claudia Gabriela Gutiérrez-Santamaría, Brenda Araceli Crespo-Peña, América Fernanda Rocha-Morales, Melissa Sánchez Rocha, María del Rosario Tapia -Torres, Adriana Patricia Hernández-Domínguez, Emanuel Galván Tapia.
Author Contributions
Nicolás Padilla-Raygoza, designed the protocol and submitted to Research and Ethics Committees. Analyzed the data and wrote the manuscript.
Edna Griselda Lara-Mares, trained to Nurses to collect the data from registries of the hospitals, participated in writing the manuscript.
Efraín Navarro-Olivos, checked and clean the data, participated in analysis pd the data and writing the manuscript.
Jorge Lira – Gómez, obtained the permissions from directors from hospitals to contact nurses and obtained the data; participated in writing the manuscript.
Gilberto Flores-Vargas, participated in analysis of data and writing the manuscript.
María de Jesús Gallardo-Luna, participated in writing the protocol and the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Sources
No funding for this research.
References
- Say L, Chou D, Gemmill A, Tunҫalp O, Moller AB, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014; 2:e323–33. Doi: https://doi.org/10.1016/S2214-109X(14)70227-X
CrossRef - Steegers EAP, Dadelszen P, Duvekot RP, Johnnes J. Pre-eclampsia. Lancet. 2010; 376 (9741): 631–44. Doi: https://doi.org/101016/ S0140-6736(10)60279-6
CrossRef - WHO, UNICEF, UNFPA. The World Bank and the United Nations Population Division. Trends in maternal mortality: 1990 to 2013. Estimates by WHO, UNICEF, UNFPA, The World bank and the United Nations Population Division. 2014. Available from: www.who.int
- Tranquilli A, Dekker G, Magee L, Roberts J, Sibai B, Steyn W, et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: A revised statement from the ISSHP. Pregnancy Hypertens. 2014; 4:97–104. Doi: https://doi.org/ 10.1016/j.preghy.2014.02.001
CrossRef - Instituto Mexicano del Seguro Social. Guía de Práctica Clínica. Prevención, diagnóstico, y tratamiento de preeclampsia en el Segundo y tercer nivel de atención. 2017. Accessed July 27, 2020. Available at: http://www.imss.gob.mx/sites/all/ statics/guiasclinicas/ 020GER.pdf
- Lindheimer MD Taler SJ, Cunningham FG. ASH position paper: hypertension in pregnancy. J Clin Hypertens (Greenwich) . 2009; 1(4):214-25. Doi: https://dx.doi.org/10.1111/j.1751-7176.2009.00085.x
CrossRef - Gutiérrez Santana D, Balderas Pedrero M. Preeclampsia y eclampsia. [Thesis]. Morelia, Michoacán: UNAM; 2017. pp.19-38. Accessed August 23, 2022. Available in: https://repositorio.unam.mx/contenidos/preeclampsia-y-eclampsia-433334?c=4MEeZ4&d=false&q=*:*&i=1&v=1&t=search_0&as=2
- Xiong X, Mayes D, Demianczuk N, Olson DM, Davidge ST, Newburn-Cook C, et al. Impact of pregnancy-induced hypertension on fetal growth. Am J Obstet Gynecol. 1999; 180(1): 207-213.
CrossRef - Xiong X, Demianczuk N, Buekens P, Saunders LD. Association of pre-eclampsia with high birth weight for age. Am J Obstet Gynecol. 2000; 183(1): 148-55.
CrossRef - Padilla-Raygoza N, Díaz-Guerrero R, Ruiz-Paloalto ML, Canfield CM, Avecilla-Hernández AA. Hipertensión inducida por el embarazo y peso de los productos al nacer. Acta Universitaria. 2013; 23(1): 3–8. Doi: https://doi.org/10.15174/au.2013.384
CrossRef - Xiong X. Impact of preeclampsia on birth outcomes. Supercourse of Epidemiology, Internet and Global Health. Available in: https://sites.pitt.edu/~super1/lecture/jec567/001.htm
- Liu CM, Cheng PJ, Chang SD. Maternal complications and perinatal outcomes associated with gestational hypertension and severe preeclampsia in Taiwanese women. J Formos Med Assoc. 2008; 107(2):129-138 Doi. https://doi.org/10.1016/S0929-6646(08)60126-6
CrossRef - Franco-Martinez N, Campos de Oliveira Filgueira G, De Souza Rangel Machado J, Tanus Dos Santos JE, Cristina Sandrim V, Duarte G, et al. Clinical and laboratory characteristics of pregnant women with preeclampsia versus gestational hypertension. Rev Bras Ginecol Obstet.2014;36(10):461-466. Doi: https://doi.org/10.1590/so100-720320140005029
CrossRef - Shen M, Smith GN, Rodger M, White RR, Walker MC, Wen SW. Comparison of risk factors and outcomes of gestational hypertension and pre.eclampsia. Plos One.2017;12(4):e0175914. Doi: https://doi.org/10.1371/journal.pone.0175914
CrossRef - Magalhäes ESDS, Méio MDBB, Peixoto-Filho FM, Gonzalez S, da Costa ACC, Moreira MEL. Pregnancy-induced hypertension, preterm birth, and cord blood adipokiner levels. Eur J Pediatr.2020; 179(8):1239-1246. Doi: https://doi.org/10.1007/s00431-020-03586-8 ,
CrossRef - Padilla-Raygoza N. Relationship between gestational hypertension and high birthweight.2022. Retrieved from: https://osf.io/xg26d








