Lakku Sindhura and Md. Nazneen Bobby*
and Md. Nazneen Bobby*
Department of Biotechnology, Vignan's Foundation for Science Technology and Research, Deemed to be University, Vadlamudi, Guntur–522 213, AP. India
Corresponding Author E-mail: drnb_bt@vignan.ac.in
DOI : https://dx.doi.org/10.13005/bpj/2575
Abstract
Passiflora caerulea L. (blue passion flower) is a member of the genus Passiflora, which contains over 500 species and is popular worldwide for its diverse medicinal properties. Qualitative phytochemical screening was carried out for the methanolic extract of the plant leaves and the tests revealed that the plant contains a wide range of medicinally important secondary metabolites such as alkaloids, cyanogenic compounds, phenolic compounds and glycosyl flavonoids. Among a host of organic solvents which were used for extraction, the methanolic extract was particularly found to possess high concentrations of carbohydrates, glycosides, free amino acids, glycosides, fats and alkaloids. Also, flavonoids, phytosterols, saponins, tannins and other phenolic compounds were present at lower levels in the methanolic extract. We tested the antioxidant potential of the P. caerulea methanolic leaf extract and discovered that it possessed nitric oxide scavenging activity of close to 80% w.r.t at 100 μg/mL against ascorbic acid control (100%). In the ABTS and DPPH radical scavenging assays, the extract (100 μg/mL) possessed antioxidant potential of 72.3% and 70%, respectively, w.r.t ascorbic acid control taken at 100 μg/mL. The plant powder was assessed quantitatively for presence of carbohydrates, proteins, lipids, ascorbic acid, tannins and flavonoids and the concentrations of these compounds were found to be 14.26 mg/g, , respectively. MTT assay results showed that the crude methanolic extract of the plant possessed appreciable activity against breast cancer (MCF-7) cells. Although the IC50 values of the extract against MCF-7 cells were much lesser comparatively w.r.t control doxorubicin, the crude extract possessed considerable activity against MCF-7 cells when compared to the standard drug, doxorubicin. These results necessitate deeper investigations into the pharmacological and therapeutic usefulness of purified compounds from P. caerulea. Though P. incarnata is the most widely studied species in the Passiflora genus, P. caerulea is relatively poorly studied. This paper shows the phytochemical composition and biological activities of P. caerulea.
Keywords
Antioxidant/Free Radical Scavenging Activity; Anti-Cancer Activity; Cytotoxicity; Passiflora caerulea; Phytochemical Screening
Download this article as:| Copy the following to cite this article: Sindhura L. Bobby M. N. Phytochemical Profiles, Antioxidant, Antimicrobial and Cytotoxic cell lines activity of Passiflora caerulea L. Biomed Pharmacol J 2022;15(4). |
| Copy the following to cite this URL: Sindhura L. Bobby M. N. Phytochemical Profiles, Antioxidant, Antimicrobial and Cytotoxic cell lines activity of Passiflora caerulea L. Biomed Pharmacol J 2022;15(4). Available from: https://bit.ly/3SLugwN |
Introduction
Phytomedicine
Man has relied on plant-based traditional/ethnic medicines for centuries and we have relied on the secrets of the ancients for treatment and management of various ailments21. Plant secondary metabolites and bioactive principles are produced by various plants in a species-specific manner, depending on the genetic constitution of plants and their ecological environment33. Nearly 70-80% of natural medicines in practices such as Ayurveda, Siddha and Unani medicine come from plant sources 4, 35. Pharmaceutical industries rely heavily on natural compounds for formulation of modern allopathic medicines12. Therefore, the fields of ethnopharmacology and ethnobotany hold the key to unlocking the mechanism of action of these traditional medicinal formulations 15, 18. Also, research into pharmacognosy and phytochemistry provides us with opportunity to identify novel bioactive lead compounds which can be used to treat both communicable and non-communicable human diseases34. Although many plants have been studied in detail for their phytochemical composition and biological activities, there are still many facets to explore. In this vein, we have examined the antioxidant, cytotoxic, anti-inflammatory and antimicrobial activities of the crude methanolic extract of Passiflora caerulea L.
 |
Figure 1: Flower and leaves of P. caerulea L. |
Passiflora caerulea
Passiflora is a genus which was first identified by Spanish explorers in 1529 and this plant is abundantly present in North and South America and in India, West Indies, Italy and Argentina 11. Passiflora caerulea is one of the species, albeit not as popular as Passiflora incarnata (Apricot Vine and Maypop). The flowers of P. species in general have been known to possess a range of compounds possessing anxiolytic, spasmolytic, analgesic, antibacterial, antifungal, aphrodisiac, antidepressant, antispasmatic, anticonvulsant, hypotensive, anticancer, diuretic, vermifuge and nervine sedative (anti-insomnia) actions 19. However, since flowers of this plant are not as abundant as the leaves, we investigated if the leaves also could contain the same phytochemical classes of compounds and also quantified some of the phytochemicals (flavonoids, tannins, phenolics and alkaloids). The plant grows upto 10 m tall vine, which grows well in warm/temperate and tropical climates. Though the flower and fruit of P. incarnata contain harmala alkaloids, the leaves and root of the plant are known to possess much greater quantities of these anti-depressant compounds 14. The compound schaftoside, a glycoside derivative of apigenin, was shown to exhibit antimutagenic effects 38. It is not sure if these same compounds are present in P. caerulea.
Collection of P. caerulea leaves
Passiflora caerulea is comes under angiosperms belongs to Passifloraceae family, upon surveying the area at the foot of the Western Ghats in Nilgiri District of Tamil Nadu, the vines of P. caerulea were found to grow abundantly. 100 g of fresh leaves of the plant were collected and washed. After cleaning the leaves to remove dust and insect eggs, shade drying was done for 2-3 days in a dark room. The dried leaves were weighed and soxhlet extraction was carried out.
Preliminary phytochemical screening
Prior to soxhlet extraction, to identify the best possible solvent that can be used, a range of solvents of various polarities – distilled water, methanol, ethanol, acetone, benzene, chloroform, ethyl acetate and petroleum ether were done by manual extraction of phytochemicals from the powder. Briefly, 1 g of the powder was weighed separately for each of the solvents mentioned above (volume as required, ~5-10 mL), and the powder was ground with a pestle inside a mortar after addition of the solvent. After filtering the solution with a Whatman no. 1 filter paper, the obtained filtrate was used for preliminary phytochemical screening.
Preparation of powder and Soxhlet extraction
The dried leaves were taken and pulverized using a mortar and pestle and then ground to powder using a mixer-blender. A sieve was used to remove debris and the fine powder was loaded into a cellulose thimble purchased from Whatman Co. and the packed thimble kept inside the extraction chamber. The receptacle was packed with analytical grade methanol and the receptacle was placed on a mantle heater and perfused at 65 °C for a few hours until the extraction was completed. The extract was poured into a beaker and then freeze dried. After drying, the powder was weighed and used for quantitative and antioxidant assays. A standard method of extraction was followed 10.
Preliminary phytochemical screening – qualitative assessment
Preliminary qualitative test was performed as per the methods of Harborne 1998 17. The same protocols, as followed by Brinda et al 8 were used for phytochemical screening to detect carbohydrates (specifically- general test, starch test, iodine test, Fehling’s test, Barfoed’s test, Benedict’s test, Molisch’s test, etc.), proteins & free amino acids (Ninhydrin test, biuret test, Millon’s test), fats & oils (spot test, saponification test), alkaloids (Hager’s test, Dragendorff’s test, Mayer’s test, potassium dichromate test and Wagner’s test), flavonoids (ferric chloride test, lead acetate test, test for anthraquinones, test for catachins and test for anthocyanins), phytosterols/steroids (Libermann Burchard test and Salkowski reaction), tannins/phenolics (generic test for tannins/phenolics and phlobatannins test), saponins (hemolysis test and foam test), vitamins and terpenoids. The biochemical tests were performed and the results are shown in Table 1. All of the protocols were followed as given by Brinda and Harbone.
Quantitative test
Dubois method 13 was used for estimating the total sugar content of the plant extract. Total lipids estimation was performed as per gravimetric method 20. Estimation of protein concentration was carried out as per Lowry method 23. The Troll and Cannan method 37 was employed for estimating total free amino acid content of the PC leaf extract. Total phenol content of the PC leaf extract was estimated as per routine Folin-Ciocalteau method of Thangaraj, 2016 36.
Chlorophyll a, b, total chlorophyll and carotenoid content
Chlorophyll and carotenoid content was quantified using the Arnon et al 3 method. After the leaf was shade dried and powdered using a blender, about 0.1g dry powder was taken and ground in a mortar 80% acetone, 5 ml. This solution was subjected to centrifugation at 1,000 rpm (10 minutes) to remove the debris and the supernatant was collected in a fresh tube. After re-homogenization of the pellet with another 5 mL of the solvent (1000 rpm, 10 minutes), the resulting supernatant was combined with the supernatant obtained earlier. The two collected supernatants were pooled and the extract was made up to a final volume of 10 ml 80% acetone. Against an 80% acetone blank, the absorbance of the test solution was measured at 663, 645 and 480 nm against the solvent blank.
DPPH radical scavenging activity
The method of Brand-Williams et al. 8 was used to determine free radical scavenging activity of the PC crude extract, in which, reaction of DPPH radical solution with antioxidants (if present) in the test plant extract sample solution will lead to loss of the intense pink colour of DPPH radical. Antioxidants have the ability to donate electrons to the radical to quench it and the disappearance of pinkish colour is monitored spectrophotometrically at 520 nm. 0.1 mM DPPH radical solution (1.0 mL) prepared in methanol was mixed with 1 ml of P. caerulea leaf extract in the concentration range of 50-500 μg/ml. A blank series comprising of L-ascorbic acid in the same concentration range as test solutions was prepared to estimate the free radical scavenging activity of standard ascorbic acid. Control reaction comprised of 1 ml methanol and 1 ml DPPH control. After incubating the test tubes in the dark, the absorbance differences were tracked at 520 nm and the formula given below was used for calculations:
Inhibition % = (Ac-As/Ac) × 100
Where, Ac corresponds to abs. of the control and as corresponds to the abs. of the sample.
ABTS assay
ABTS decolourization assay of Iverson et al 21 was used for evaluating the antioxidant potential of the MeOH leaf extract of P. caerulea 21. ABTS stock solution was diluted to ~7 mM with pure double distilled water. Around 2.45 mM potassium persulfate was used with ABTS in a 1:1 ratio to generate ABTS radical cation (ABTS•+) by incubating this mixture in a cool dark place and pure double distilled water was used to dilute the generated ABTS•+ to an absorbance of 0.7 at 734 nm. 1 ml of this ABTS radical cation solution was mixed with 1 ml of the methanolic crude extract at various concentrations in a range of 10-100 mg/ml. The ABTS radical cation loses colour and becomes decolorized when it reacts with an antioxidant or reductant. The reaction is followed spectrophotometrically at 734 nm and the radical scavenging ability of the extract was compared to that of standard ascorbic acid reference (also used at 10-100 mM) and the % radical scavenging ability was calculated as follows:
Reducing power assay
Ferric reducing power assay is a method to determine the ability of antioxidants/reductants to convert Fe3+ ions to Fe2+ 32. The plant extract was taken at various concentrations in a range of 10-100 µg/ml. Phosphate buffered saline was used as a diluent (0.2 mM, pH 6.6) and 2.5 ml of 1% potassium ferricyanide, 10% TCA, 1% ferric chloride and L-ascorbic acid were also utilized for this method.
Nitric oxide radical scavenging activity
The protocol of Rao 33 was used for estimation of nitric oxide radical scavenging activity. Although nitric oxide is a vital second messenger gaseous molecule, excess nitric oxide can potentially lead to nitrosative stress. We have done this experiment with the help of Sodium nitroprusside and Greiss reagent. The % nitric oxide scavenging activity was calculated in a manner identical to DPPH assay calculation.
Phosphomolybdenum assay
Phosphomolybdenum method Prieto et al., 31 was used for determination of antioxidant activity. An equal volume (1 ml) of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate) was mixed with plant extract solution. After these solution was reserved in a boiling water bath set at 95 °C for 1.5 hours. After allowing the test tubes to cool, the OD was measured against reagent blank at 765 nm with the aid of a sensitive spectrophotometer. Ascorbic acid was employed as control in the same concentration range as the extract. The results were expressed in terms of ascorbic acid equivalents (AAE)/g plant extract.
Anti-inflammatory activity of P. caerulea leaf extract
The popular HRBC membrane stabilization assay29 was used to determine the anti-inflammatory potential of the P. caerulea leaf extract. Since human RBC membranes have a composition similar to that of lysosomes, RBCs are used for determining the ability of test solutions to prevent lysis owing to inflammation. From healthy volunteers, fresh blood was drawn using standard clinical protocols and to the blood, equal volumes of disinfected Alsever solution were added. Alsever solution was prepared by taking 2% dextrose, 0.8% sodium citrate, 0.9% citric acid and 0.72% sodium chloride, to serve as an an anticoagulant. This blood-Alsever solution mixture was centrifuged at 10,000 rpm for 15 minutes at 37 °C. Post-centrifugation, the supernatant was discarded and the RBCs were washed with 0.9% NaCl repeatedly until the supernatant was clear. The clear RBC suspension was then taken for evaluating the anti-inflammatory activity of the extract. By taking the various concentrations of leaf extract with other reagents like 2 ml of phosphate buffer, 4 ml hyposaline and 0.9 ml of RBC suspension. These reaction mixtures were incubated at room temperature for 30 minutes at 37 °C. The control contained all of the above without sample/plant extract. As positive control, the commercially available drug, aspiring was used at the same concentration range as plant extract. After the incubation, the supernatant was disregarded and discarded. The hemoglobin content of the remaining solution mixture was evaluated spectrophotometrically at 620 nm. Using the formula given below, the hemolysis% was calculated. Higher hemolysis would indicate poor anti-inflammatory activity and vice-versa:
% Hemolysis= T/C x 100
Where, T-Test sample and C-Control sample.
MTT assay
Cytotoxic effect of the plant leaf extract was performed against breast cancer cell line (MCF-7) by in vitro (MTT assay) 5, 7. MCF-7 cell line was procured (NCCS, Pune) and the cells were maintained in DMEM supplemented with FBS (10%), streptomycin (100 μg/mL) and penicillin (100 μg/mL) in a humidified atmosphere of CO2 (50 μg/mL) at room temperature (37 °C). MCF-7cells (1 × 105/well) were seeded in 24-wells plates and then incubated at 37°C with CO2 (5%). After the cells reached confluence, different concentrations of methanolic leaf extract of P. caerulea were mixed and incubated for 24 hrs. The excess plant extract was detached from the well by washing with PBS (pH 7.4) or DMEM lacking serum. Further, 100 μL/well of MTT (0.5%) was mixed and the cells were incubated for 4 hours. After incubation, 1 mL of DMSO was added to all the wells. The absorbance was measured at 570 nm against blank (DMSO) using a UV- spectrophotometer. The required concentration for 50 % inhibition (IC50) was evaluated. Using the following formula, the cell viability (%) was calculated.
% Cell viability = Untreated cells – Treated cells / Untreated cells × 100
Graphs are plotted using the % of Cell Viability on Y-axis and concentration of the sample on X-axis.
Results and Discussion
Preliminary phytochemical analysis
By employing several solvents from non-polar to polar, the qualitative presence of different phytochemical classes was investigated in different extracts which were prepared with using the solvents in decreasing order of polarity- like water, methanol, ethanol, acetone, chloroform and n-hexane. Using various qualitative tests, the presence of most of the phytochemical classes was discovered to be either high or moderate only in the P. caerulea leaf MeoH extract, as opposed to other solvents (Table 1).
Table 1: Phytochemical analysis of Passiflora incarnata leaf extracts.
| S. No | Name of the test | Passiflora incarnata leaf extracts | |||||||||||
| Petroleum ether | Benzene | Chloroform | Methanol | Acetone | Ethyl acetate | Dist. water | |||||||
| 1 | CARBOHYDRATES | ||||||||||||
| a) General test | – | – | + | ++ | + | + | – | ||||||
| b) Sugar test | – | – | + | ++ | + | + | – | ||||||
| c) Barfoed’s test | – | – | + | ++ | + | + | – | ||||||
| d) Molisch’s test | – | – | + | ++ | + | + | – | ||||||
| e) Fehling’s test | – | – | + | ++ | + | + | – | ||||||
| f) Benedict’s test | – | – | + | ++ | + | + | – | ||||||
| g) Iodine test | – | – | + | ++ | + | + | – | ||||||
| 2 | DETECTION OF GLYCOSIDES | ||||||||||||
| a) Legal’ test | – | – | + | + | + | + | – | ||||||
| b) Borntrager’s test | – | – | + | + | + | + | – | ||||||
| c) Ferric chloride test | – | – | + | ++ | + | + | – | ||||||
| 3 | PROTEINS AND AMINO ACIDS | ||||||||||||
| a) Million’s test | – | + | + | ++ | + | – | – | ||||||
| b) Ninhydrin test | – | + | + | ++ | + | – | – | ||||||
| c) Biuret test | – | + | + | ++ | + | – | – | ||||||
| 4 | FIXED OILS AND FATS | ||||||||||||
| a) Spot test | + | + | – | ++ | + | – | – | ||||||
| b) Saponification test | + | + | – | ++ | + | – | – | ||||||
| 5 | DETECTION OF ALKALOIDS | ||||||||||||
| a) Mayer’s test | – | + | – | ++ | + | + | – | ||||||
| b) Dragondroff’s test | – | + | – | ++ | + | + | – | ||||||
| c) Hager’s test | – | + | – | ++ | + | + | – | ||||||
| d) Wagner’s test | – | + | – | ++ | + | + | – | ||||||
| e) Potassium dichromate test | – | + | – | +++ | + | + | – | ||||||
| 6 | DETECTION OF FLAVANOIDS | ||||||||||||
| a) Ferric chloride test | – | – | + | + | + | – | – | ||||||
| b) Lead acetate test | – | – | + | + | + | – | – | ||||||
| c) Test for Anthraquinone | – | – | + | + | + | – | – | ||||||
| d) Test for Catechins | – | – | + | + | + | – | – | ||||||
| e) Test for Anthocyanin | – | – | + | + | + | – | – | ||||||
| 7 | DETECTION OF PHYTOSTEROLS | ||||||||||||
| a) Libermann Burchard test | + | – | – | + | + | – | – | ||||||
| b) Salkowski test | + | – | – | + | + | – | – | ||||||
| 8 | TANNINS AND PHENOLIC COMPOUNDS | ||||||||||||
| a) General test | – | – | + | + | + | – | – | ||||||
| b) Ferric chloride test | – | – | + | + | + | – | – | ||||||
| c) Test for Phlonatannins | – | – | + | + | + | – | – | ||||||
| 9 | DETECTION OF SAPONINS | ||||||||||||
| a) Foam test | – | – | – | + | + | – | – | ||||||
| b) Haemolysis test | – | – | – | + | + | – | – | ||||||
| 10 | Detection of steroids | – | – | – | + | + | – | – | |||||
| 11 | Detection of vitamins | – | – | – | + | + | – | – | |||||
| 12 | Detection of terpenoids | – | – | – | + | + | – | – | |||||
– Not present; + Present; ++ Highly present
From Table 1 shown above, since it was evident that the methanolic extract possessed with widest range of phytochemicals, bulk soxhlet extraction was performed with methanol to obtain the most comprehensive gamut of secondary metabolite classes from P. caerulea. The methanolic extract was found to possess the following phytochemical classes like –carbohydrates, sugars, flavonoids, tannins, saponins, alkaloids and triterpenoids. Sterols may be present in negligible amounts. Acetone was also found to be an excellent solvent, with capacity to extract diverse phytochemical classes, but the relative concentrations of compounds extracted using methanol may be much higher, as indicated by the greater intensity of the colour change in the biochemical tests.
Quantitative assay of constituents in Passiflora caerulea
Passiflora caerulea leaves, much like the other members of the Passiflora genus (eg., Passiflora incarnata) were found to contain chlorophylls a and b, carotenoids, lipid, protein, sugars, tannins, phenolics and free amino acids. Plants of the genus Passiflora contain carbohydrates in abundance and because of the presence of several important metabolites and phytochemicals, this plant is considered to be a nonvitamin and nonmineral nutritional supplement on their own merit 23. Most of the plants in this genus are known to possess flavonoid glycosides, β-carboline alkaloids, phenols, glycosyl flavonoids, cyanogenic flavonoids, vitexin, isovitexin, isoorientin, saponarin, schaftoside, isoschaftoside, isovitexin-2’’-O-beta-glucoside, vicenin-2, lucenin-2, swertisin, Harman, harmol, harmaline, maltol and gynocardin 30.
Table 2: Quantitative estimation of various metabolite classes.
| S. No. | Test | Amount of chemical present (mg/g) |
| 1 | Chlorophyll a | 4.65 ± 0.1 |
| 2 | Chlorophyll b | 7.15 ± 1.2 |
| 3 | Total chlorophyll | 8.12 ± 1.5 |
| 4 | Total carotenoid | 2.05 ± 1.5 |
| 5 | Total lipid | 122.2 ± 6.5 |
| 6 | Total sugar | 43.26 ± 1.0 |
| 7 | Total protein | 0.9 ± 2.1 |
| 8 | Total tannin | 75.25 ± 1.3 |
| 9 | Total phenol | 45.08 ± 1.5 |
| 10 | Total free amino acids | 0.58 ± 0.5 |
| 11 | Total flavonoids | 38.24 ± 0.9 |
Being non-nutritive plant secondary metabolites with diverse biological activities, phytochemicals possess disease preventing and treating attributes and are therefore beneficial to humans by acting as accessory/extrinsic health supplementing agents. Plants produce these secondary metabolites with the help of nature’s blow torch, cytochrome P450. From the phytochemical primary and secondary metabolite quantification assays, it is evident that the leaves of P. caerulea are rich in alkaloids, tannins, flavonoids, saponins and phenols. Alkaloids have a wide range of pharmacological properties including antimalarial, antiasthmatic, anticancer/cytotoxic properties, as reported earlier for other Passiflora genera 6. The primary and secondary metabolites provide nutrition and the antioxidants generated by the plants in such high supra mM concentrations could shield the plants from oxidative stress damage. Moreover, the secondary metabolites have long been considered to confer immunity to plants against invading plant pathogens. For example, steroids and steroidal alkaloids are known to possess good activity and are important anti-inflammatory pharmacological compounds in the drug industry. Moreover, literature survey shows that the flowers and leaves of Passiflora genus possess a wide variety of pharmacological activities such as vasodilatory, antihyperglycemic, cholinomimetic and antiarrythmi 1. Also, some compounds from these species exhibit antibacterial as well as analgesic activities and at much higher concentrations, these molecules can exhibit toxicity 17. Plant of the Passiflora genus possess the ability to act as agents which treat communicable diseases/ailments and many formulations and preparations of Passiflora species have been used in human clinical trials to provide interesting and promising health benefits 25, 27.
Usually, like all green plants, Passiflora sp. contain chlorophyll a, chlorophyll b, carotenoids, lipids, proteins, free amino acids, flavonoids, phenols, tannins and sugars. When compared to the aqueous extract, wider representation of various classes of phytochemicals can be seen in the methanolic extract. Hence, this solvent was chosen for soxhlation and for bulk extraction of metabolites. Methanol is a slightly polar solvent and thus, can be an excellent choice for extraction of a wide variety of both hydrophilic and hydrophobic phytochemicals.
The total chlorophyll content was 8.12 ± 1.5 mg/g DW of P. caerulea (0.8% w/w). Likewise, total carotenoids content was found to be 2.05 ± 1.5 mg/g (Table 2). The plant is rich in photosynthetic pigments and this reveals its high potential to perform photosynthesis. Total carbohydrates (total sugar content) present in P. caerulea extract were found to be 43.26 ± 1.0 mg/g. The total protein present in the leaf extract was found to be 0.9 ± 2.1 mg/g protein (expressed as BSA equivalent). While free amino acids were present, their concentrations were quite low. Phenolic content was found to be 45.08 ± 1.5 mg TAE/g extract. The amount of tannin present was found as 75.25 ± 1.3 mg TAE/g extract. Estimation of total flavonoid content revealed the presence of 38.24 ± 0.9 mg/g flavonoids and total lipid content of the plant extract was about 122.2 ± 6.5 mg/g. These results show that the P. caerulea leaf extracts possessed a broad range of phytochemical classes which could likely possess beneficial pharmacological attributes. Moreover, when Passiflora extracts are administered in recommended doses, these compounds may be well-tolerated and saer to consume than synthetic drugs. This is because when different classes of phytochemicals are present, particular phytochemical moieties can counter-balance the dose-dependent and negative effects of other phytochemicals in the reaction milieu.
Antioxidant activity of P. caerulea methanolic leaf extract
Antioxidants are molecules with donatable electrons (electron rich) and therefore, possess donatable hydrogen atoms as well. When antioxidants react with free radicals, they donate electrons to these molecules to stabilize them electronically and this quells/terminated the free radicals chain oxidation reactions. Natural antioxidants like vitamin C, vitamin E, and many redox-active phytochemicals like polyphenols are known to possess antioxidant activity through which they suppress free-radical mediated chain reactions during oxidative stress. Incomplete reduction of oxygen in the electron transport chain leads through uncoupling leads to formation of ROS, which are deleterious at high concentrations. The chief kinds of ROS are superoxide, hydrogen peroxide and hydroxyl radical. Various ROS have differential reactivity towards different biomolecules. Oxidative stress is characterized by lipid peroxidation of polyunsaturated fatty acids within the membrane, generation of DNA breaks, formation of covalent adducts of DNA/RNA, protein covalent modifications (protein tyrosine nitration, protein carbonylation, formation of Schiff’s base through Maillard reactions etc.). Also, oxidative stress depletes cellular thiol pools and changes the redox potential of glutathione by changing the GSH: GSSG ratio and upregulation of antioxidant genes’ expression (catalase, glutathione peroxidase, thioredoxin reductase, superoxide dismutase etc). These enzymes are expressed during oxidative stress exacerbations and they collectively form the internal cellular antioxidant system. In recent times, there is an increased emphasis on plant-derived natural antioxidants which possess health benefits without exhibiting too much toxicity, like synthetic molecules do. The food industry has picked up the habit of adding antioxidants to foodstuffs to tackle the problem of oxidative stress from consumption of more food calories. Plants are treasure-troves of natural antioxidants and pharmacological agents which can be tested against different ailments. Importantly, using ligand based computer aided drug design and QSAR, the medicinal properties of molecules can be enhanced. These results also contradicted the results with Nejatzadeh Barandozi 28.
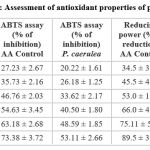 |
Table 3: Assessment of antioxidant properties of phytochemicals. |
In this regard, plant extracts, owing to the rich abundance of many secondary metabolites like phenolics, tannins, flavonoids and carotenoids, can tackle oxidative stress and attempt to restore cellular redox homeostasis by assisting (and acting apart from) the internal antioxidant enzyme system, by virtue of the antioxidant-rich properties of these phytochemicals. Routinely used (and popular) assays to detect and assess the antioxidant properties of phytochemicals include – ABTS assay, DPPH assay, ferric reducing power assay (to detect ability of plant extract and its constituent phytochemical composition) to suppress free radical-mediated cellular damage and cell death. Nitric oxide radical scavenging assay using Griess reagent was also determined to identify the usefulness of the extract in protecting against nitric oxide-mediated damage. The MeOH leaf extract of the study plant was taken at different concentrations, in the range of 10-100 μg/mL. Appreciable activity was detected against free radicals in vitro, in all these assays. The reason for activity of the plant extract against various kinds of radicals is the presence of antioxidant phytochemicals. The values obtained for the reported antioxidant assays are indicated as graphs in Figures 2-5. Table-3
DPPH radical scavenging activity
Antioxidant is well thought out of many health benefits in the field of neutraceutical and medicinal. DPPH assay is very common method for testing on antioxidant molecules. The different types of methods have been followed by the various laboratories on the plant compounds. The standard antioxidant focused mainly on ascorbic acid, BHT and propyl gallate have been followed widely. DPPH is a strong free radical it shows violet in colour mixed with methanol, this solution after adding with antioxidants (like ascorbic acid), changes to yellow in colour indicated antioxidant activity. The P. caerulea extract (100 μg/mL) yielded 63 % antioxidant activity and is low, when matched against L-ascorbic acid (100 μg/mL), as shown in Figure 2.
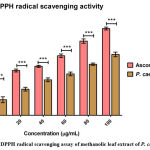 |
Figure 2: DPPH radical scavenging assay of methanolic leaf extract of P. caerulea |
ABTS radical scavenging activity
In the ABTS color reduction assay, the antioxidant activity of P. caerulea extract (100 μg/mL) was shows to be 52.41 ± 1.2 % when compare with ascorbic acid (positive control) activity (which showed low inhibition, as shown in Figure 3). ABTS radical is generated upon reaction with potassium persulphate and this yields the metastable radical cation of ABTS (ABTS+•). Upon accepting an electron from an antioxidant, ABTS radical is neutralized and is converted to ABTS. Using this method, the highest ABTS radical scavenging activity % was found to be 52.12 % at a concentration of 100 μg/mL. Studies on Aloe vera gel also shows antioxidant properties UrmeelaTaukoorah and M.Fawzi Mahomoodally 2016 41. This is comparatively about 72 % of the activity of control ascorbic acid followed same conc. range. A similar ratio of activity of ascorbic acid: plant extract can be seen.
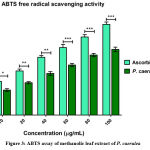 |
Figure 3: ABTS assay of methanolic leaf extract of P. caerulea |
Nitric oxide radical scavenging assay: At normal physiological cellular conditions, nitric oxide is a potent vasodilator and is produced from nitric oxide synthase (NOS). There are various forms of NOS (neuronal/NOS1, inducible/NOS2 and endothelial/NOS3), which metabolize arginine into citrulline, releasing NO radical. Nitric oxide can modulate heme containing proteins by acting as a ligand of heme iron and suppress the activity of these enzymes. Usually, heme containing enzyme systems are known to release lots of ROS. When nitric oxide is in excess, it reacts with other free radicals to form autocollapse products like peroxynitrite (OONO) and is characterized as an RONS. Peroxynitrite is responsible for protein tyrosine nitration, which leads to protein damage. In this assay, nitric oxide free radical was developed by mixing of sodium nitroprusside. The antioxidant activity of P. caerulea methanolic extract (100mg/mL) shows 64.16 ± 1.7 %, which is low down compared with positive control ascorbic acid (Figure 4) activity which was close to 90%.
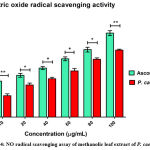 |
Figure 4: NO radical scavenging assay of methanolic leaf extract of P. caerulea |
Ferric reducing power assay
Polyphenols contains may be the maximum free radical scavenging activity amongst phytochemicals. ROS is play a major role on phyto-compounds for the antioxidant activity; so they are respect with singlet oxygen, nitric oxide and superoxide which is chelate with metal ions by the Fenton and Haber-Weiss reactions. Usually plants contains significant amount of bioactive compounds such as phenolics, alkaloids, flavonoids, tannin, saponins and steroids. These capable plant secondary metabolite contains several biological activities and antioxidant activities. Reducing power is suitable assay for antioxidant activity. It is calculated by conversion of Fe3+ to Fe2+ during donating electrons. Reducing power activity of P. caerulea leaf extract was investigated and the data are displayed in Figure 5. The leaf extract possess 45.18 ± 2.2 % at 100 mg/mL, which is very low compared with ascorbic acid (AAE mg/mL).
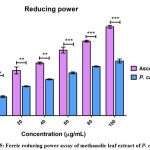 |
Figure 5: Ferric reducing power assay of methanolic leaf extract of P. caerulea |
Anti-inflammatory activity
Inflammation is characterized by phagocyte activation, leading to discharge of chemical mediators such as prostaglandins, TNF-α and interleukins. Leaky gut syndrome is known to be a leading cause of inflammation. Astonishingly, inflammation itself is the underlying cause of several diseases such as cardiovascular disease, cancer and arthritis. Escalation of oxidative stress-mediated tissue damage leads to vascular changes. Plant formulations and extracts such as the “Horchata drink” have potential to improve digestive health, increase memory power and also exhibit hepatic anti-inflammatory activity. The present study demonstrated that the leaf extract of P. caerulea was studied for anti-inflammatory effect through HRBCs membrane stabilization (inhibition of hemolysis), in comparison to the anti-inflammatory effect of aspirin (positive control) (Figure 6). The inhibition (%) was obtained in a dose-dependent manner. The highest inhibition of 61.05 ± 1.5 % was obtained with highest concentration of 800mg/mL extract, while control aspirin exhibited maximum inhibition 90.41 ± 1.3 % at the same concentration. This result indicated that the crude leaf extracts exhibited less toxicity and safe when compared with standard drug aspirin. Similarly studies on antioxidant activity of fermented onions was measured using the 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) radical scavenging activity assay and the hydroxyl radical scavenging activity test. Al-Sahlany & Niamah 2. Studies stated by Ameer A. Mohammed and Alaa Kareem Niamah 26 on antioxidant activity of hyaluronic acid extracted from local isolates of Streptococcus thermophilus.
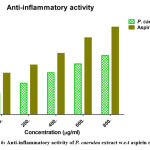 |
Figure 6: Anti-inflammatory activity of P. caerulea extract w.r.t aspirin control |
Antibacterial activity
Phytocompounds that are present in plant extracts are often able to inhibit bacterial and fungal growth. In the case of P. caerulea, its methanolic leaf extract (in concentration range of 100-1000 μg/mL) was tested against two gram negative bacteria – Escherichia coli ATCC25922 (uropathogenic strain) and against Pseudomonas aeruginosa ATCC 27853. (Fig-7). The inhibitory effect of the crude extract was slightly more pronounced against E. coli than against P. aeruginosa. It is not exactly known which of the phytocompounds could have exerted this inhibitory effect. This work requires much in silico (docking, molecular dynamics and DFT studies) and other further in vitro studies (such as binding assays/titration of purified bioactive phytoprinciples with cellular targets). Standard antibiotic solutions of gentamicin 32 (Pulido et al.), Ampicillin10 and ciprofloxacin (CIP) were found to be several folds more potent, even at very low concentrations, as judged by the very large zones of inhibition. Surprisingly, activity of Cefotaxime (CFM) was negligible against both strains selected for this work and AMP did not yield any effect against the E. coli strain for reasons as yet unknown.
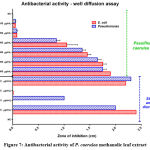 |
Figure 7: Antibacterial activity of P. caerulea methanolic leaf extract |
Cytotoxicity against breast cancer (MCF-7) cells
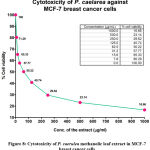 |
Figure 8: Cytotoxicity of P. caerulea methanolic leaf extract in MCF-7 breast cancer cells |
When MTT assay was performed in MCF-7 cell line using the P. caerulea (concentration range of 100-1000 μg/mL), the dose-dependent cellular viability of the given compounds was documented. IC50 value of the extract was found to be 50.22 μg/mL. When the same concentrations of the extract were added to MCF-10A cells (normal breast cell line), the toxicity was much lower, indicating that the plant extract had considerable activity selectively against cancerous cells, as opposed to normal breast cells.
Conclusion
Passiflora caerulea is a relatively poorly studied species of the Passiflora genus. Using qualitative preliminary phytochemical screening methods, the phytochemical classes present in the aqueous, methanolic, benzene, chloroform, ethyl acetate, and petroleum ether extracts of dried leaf sample were studied using established qualitative protocols and the methanolic extract was found to contain possibly higher concentrations of phytocompounds when compared to all other solvents used herein. Using qualitative assays, the content of chlorophyll a and b, total chlorophyll, total carotenoids, total lipids, total sugars, total tannins, total protein, total phenols, total amino acids and total flavonoids was determined using established and routinely used biochemical assays. Antioxidant activity was explored using five different assays and the extract was found to possess appreciable antioxidant activity. Using human RBC membrane stabilization assay, the anti-inflammatory activity of the extract was compared against standard aspirin, a non-steroidal anti-inflammatory drug. The extract possessed modest antibacterial activity against Escherichia coli and Pseudomonas aeruginosa. Anti-breast cancer activity (cytotoxicity) of the plant extract was much higher against breast cancer (MCF-7) cells than against their non-cancerous counterparts (MCF-10A). (Fig-8). These investigations show the remarkable potentials of P. caerulea as a medicinal plant of significant value against inflammation, cancer, infection and oxidative stress. These properties are owing to the presence of various secondary metabolites.
Acknowledgement
We sincerely thank Vignan’s Foundation for Science, Technology and Research (Deemed to be University) for providing the space and lab facilities to carry out this research.
Conflicts of Interest
The authors do not have any conflicts of interest in any form.
References
- Alamgir, A. Secondary metabolites: Secondary metabolic products consisting of C and H; C, H, and O; N, S, and P elements; and O/N heterocycles. In Therapeutic Use of Medicinal Plants and their Extracts; 2: 165-309. (2018).
CrossRef - Al-Sahlany, S. T., & Niamah, A. K. Bacterial viability, antioxidant stability, antimutagenicity and sensory properties of onion types fermentation by using probiotic starter during storage. Nutrition & Food Scienc; (2022).
CrossRef - Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant physiology; 24:1(1949).
CrossRef - Arulmozhi, P., Vijayakumar, S., and Kumar, T. Phytochemical analysis and antimicrobial activity of some medicinal plants against selected pathogenic microorganisms. Microbial pathogenesis; 123:219-226. (2018).
CrossRef - Bahuguna, A., Khan, I., Bajpai, V.K., and Kang, S.C. MTT assay to evaluate the cytotoxic potential of a drug. Bangladesh Journal of Pharmacology; 12:115-118. (2017).
CrossRef - Bezerra, F.W., do N Bezerra, P., de Oliveira, M.S., da Costa, W.A., Ferreira, G.C., and de Carvalho, R.N. Bioactive Compounds and Biological Activity of Croton Species (Euphorbiaceae): An Overview. Current Bioactive Compounds; 16:383-393. (2020).
CrossRef - Borenfreund, E., Babich, H., and Martin-Alguacil, N. Comparisons of two in vitro cytotoxicity assays—the neutral red (NR) and tetrazolium MTT tests. Toxicology in vitro; 2: 1-6. (1988).
CrossRef - Brand-Williams W, CuvelierE, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT – Food Science and Technology; 28 (1):25-30 (1995).
CrossRef - Brinda, P., Sasikala, P., and Purushothaman, K. Pharmacognostic studies on Merugan kizhangu. Bull Med Eth Bot Res; 3: 84-96. (1981).
- Camps, J., and García-Heredia, A. Introduction: oxidation and inflammation, a molecular link between non-communicable diseases. In Oxidative Stress and Inflammation in Non-communicable Diseases-Molecular Mechanisms and Perspectives in Therapeutics. 1-4. (2014).
CrossRef - de Castro, M.L., and Ayuso, L. Soxhlet extraction. Environmental Applications; 2701-2705. (2000).
- Dhawan, K., Dhawan, S., and Sharma, A. Passiflora: a review update. Journal of ethnopharmacology; 94: 1-23. (2004 ).
CrossRef - Di Nardo, G., and Gilardi, G. Natural compounds as pharmaceuticals: the key role of cytochromes P450 reactivity. Trends in biochemical sciences; 45: 511-525. (2020).
CrossRef - Dubois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.t., and Smith, F. Colorimetric method for determination of sugars and related substances. Analytical chemistry; 28: 350-356. (1956).
CrossRef - Feliú-Hemmelmann, K., Monsalve, F., and Rivera, C. Melissa officinalis and Passiflora caerulea infusion as physiological stress decreaser. International journal of clinical and experimental medicine; 6: (2013).
- Ghorbani, A., Naghibi, F., and Mosadegh, M. Ethnobotany, ethnopharmacology and drug discovery. (2006).
- Hameed, I.H., Cotos, M.R.C., and Hadi, M.Y. Antimicrobial, Antioxidant, Hemolytic, Anti-anxiety, and Antihypertensive activity of Passiflora species. Research Journal of Pharmacy and Technology; 10: 4079-4084. (2017).
CrossRef - Harborne, A. Phytochemical methods a guide to modern techniques of plant analysis (springer science & business media). (1998).
- Heinrich, M., Kufer, J., Leonti, M., and Pardo-de-Santayana, M. Ethnobotany and ethnopharmacology—Interdisciplinary links with the historical sciences. Journal of ethnopharmacology; 107: 157-160. (2006).
CrossRef - Ingale, A., and Hivrale, A. Pharmacological studies of Passiflora sp. and their bioactive compounds. African Journal of Plant Science; 4: 417-426. (2010).
- Iverson, S.J., Lang, S.L., and Cooper, M.H. Comparison of the Bligh and Dyer and Folch methods for total lipid determination in a broad range of marine tissue. Lipids; 36: 1283-1287. (2001).
CrossRef - Jayasundar, R. Ayurveda: a distinctive approach to health and disease. Current Science, 908-914. (2010).
- Khan, H., and Nabavi, S.M. Passiflora (Passiflora incarnata). In Nonvitamin and Nonmineral Nutritional Supplements. 361-366. (2019).
CrossRef - Lowry, O.H., Rosebrough, N.J., Farr, A.L., and Randall, R.J. Protein measurement with the Folin phenol reagent. Journal of biological chemistry; 193: 265-275. (1951).
CrossRef - Miroddi, M., Calapai, G., Navarra, M., Minciullo, P., and Gangemi, S. Passiflora incarnata L.: ethnopharmacology, clinical application, safety and evaluation of clinical trials. Journal of ethnopharmacology; 150: 791-804. (2013).
CrossRef - Mohammed, A. A., & Niamah, A. K. Identification and antioxidant activity of hyaluronic acid extracted from local isolates of Streptococcus thermophilus. Materials Today: Proceedings. (2021).
CrossRef - Niamah, A. Determination, identification of bioactive compounds extracts from yellow banana peels and used in vitro as antimicrobial. International Journal of Phytomedicine; 6(4): 625-632. (2015).
- Nejatzadeh-Barandozi, Antibacterial activities and antioxidant capacity of Aloe vera. Organic and Medicinal Chemistry Letter; 3: 1: 5. (2013).
CrossRef - Oyedapo, O., and Famurewa, A.J. Antiprotease and membrane stabilizing activities of extracts of Fagara zanthoxyloides, Olax subscorpioides and Tetrapleura tetraptera. International journal of Pharmacognosy; 33: 65-69. (1995).
CrossRef - Patel, S., Verma, N., and Gauthaman, K. Passiflora incarnata Linn: A review on morphology, phytochemistry and pharmacological aspects. Pharmacognosy Reviews; 3: (2009).
CrossRef - Prieto, P., Pineda, M., and Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Analytical biochemistry; 269: 337-341. (1999).
CrossRef - Pulido, R., Bravo, L., and Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. Journal of agricultural and food chemistry; 48: 3396-3402. (2000).
CrossRef - Rao, M. Nitric oxide scavenging by curcuminoids. Journal of pharmacy and Pharmacology;49: 105-107. (1997).
CrossRef - Saito, K. Phytochemical genomics—a new trend. Current opinion in plant biology, 16: 373-380. (2013).
CrossRef - Saxena, M., Saxena, J., Nema, R., Singh, D., and Gupta, A. Phytochemistry of medicinal plants. Journal of pharmacognosy and phytochemistry 1. (2013).
- Sheldon, J.W., Balick, M.J., Laird, S.A., and Milne, G.M. Medicinal plants: can utilization and conservation coexist? Advances in economic botany; 12: 1-104. (1997).
- Thangaraj, P. Pharmacological assays of plant-based natural products. (2016).
- Troll, W., and Cannan, R.K. A modified photometric ninhydrin method for the analysis of amino and imino acids. Journal of biological chemistry; 200: 803-811. (1953).
CrossRef - Ulubelen, A., Oksuz, S., Mabry, T., Dellamonica, G., and Chopin, J. C-Glycosylflavonoids from Passiflora pittieri, P. alata, P., ambigua and Adenia mannii. Journal of Natural Products; 45:783-783. (1982).
CrossRef - , and M. Fawzi Mahomoodally. Crude Aloe vera Gel Shows Antioxidant Propensities and Inhibits Pancreatic Lipase and Glucose Movement In Vitro. Advances in Pharmacological Sciences; 1-9. (2016).
CrossRef








