Duraivel M1 , Arunkumar R2*
, Arunkumar R2* and Ruckmani A2
and Ruckmani A2
1Department of Pharmacology, Madha Medical College and Research Institute, Thandalam, Kovur, Chennai 600128, Kanchipuram district, Tamilnadu, India.
2Department of Pharmacology, Chettinad Hospital and Research Institute, Chettinad Academy of Research and Education, Kelambakkam, 603103, Chengalpet District, Tamilnadu, India.
Corresponding Author E-mail: drarunvp@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2547
Abstract
Introduction: Cardiac patients are generally treated with cardiac medications but, when they develop other common conditions, they may have to be given the necessary non-cardiac medications. There are few such medications which, when given to the cardiac patients produces a potentially lethal drug interaction. Aim: The main aim of the study was to evaluate the prescription of non-cardiac medications that could cause QT interval prolongation among cardiac patients. Materials and methods: The medical records of 100 cardiac patients were collected from both the outpatients and inpatients of cardiology department. The list of medications prescribed to each subject was recorded and classified as cardiac and non-cardiac medications. The ECG changes reported in the literature for both cardiac & non-cardiac medications were collected. Frequency analysis of these medications having effect on QT interval was analyzed. Results: Among the 100 cardiac patients, there were 70 males and 30 females. 86 of them were inpatients and 14 were outpatients. Majority of the patients (63%) were in the age group between 51-70 years. Aspirin (80%) and paracetamol (20%) were found to be the most commonly prescribed cardiac and non-cardiac medications respectively. Conclusion: Many cardiac patients received non-cardiac medications which are known to cause changes in ECG. Hence, wherever possible these medications should be replaced by an appropriate alternative drug which does not cause ECG changes. In situations where prescription of these medications becomes unavoidable, they should be used with caution in recommended doses and for the optimal period to prevent adverse cardiac effects.
Keywords
Cardiac Medications; Cardiac Patients; Drug Interaction; ECG; Non-cardiac medications; QT interval
Download this article as:| Copy the following to cite this article: Duraivel M, Arunkumar R, Ruckmani A. Non-Cardiac Medications Induced QT Prolongation in Cardiac patients: A Retrospective Analysis. Biomed Pharmacol J 2022;15(4). |
| Copy the following to cite this URL: Duraivel M, Arunkumar R, Ruckmani A. Non-Cardiac Medications Induced QT Prolongation in Cardiac patients: A Retrospective Analysis. Biomed Pharmacol J 2022;15(4). Available from: https://bit.ly/3g7YRY8 |
Introduction
Cardiac patients are usually treated with medications such as ACE inhibitors, beta blockers, calcium channel blockers, diuretics, nitrates, cardiac glycosides, antiarrhythmic drugs and other groups of drugs. These patients may develop other common conditions such as respiratory infections, gastrointestinal problems or genitourinary disorders and they may have to be given the necessary non-cardiac medications. There are few non-cardiac medications which, when given to the cardiac patients could affect the cardiac function, and thereby produces additive drug interactions between non-cardiac and cardiac medications which can be potentially fatal.1,2 Around 40-50% of all the deaths due to cardiovascular causes are because of sudden cardiac deaths (SCDs)3-5 and about 80-85% of these deaths are caused by ventricular arrhythmias.6-8 Prolongation of ventricular repolarization is considered to be an important etiological factor responsible for causing ventricular arrhythmia9,10 which may provoke a condition called as torsades de pointes (TdP), a specific type of abnormal heart rhythm that can lead to SCDs.11-13
One of the important risk factor thought to be responsible for TdP is the use of QT prolonging drugs.14 QT prolongation can either be congenital or acquired.15 While congenital QT prolongation is an inherited condition16, acquired QT prolongation is most often drug-related.17 There are certain factors that predispose the patients to QT prolongation/TdP and that include: age more than 65 years, female gender, bradycardia, diseases like hypertension, heart failure, myocardial ischemia, diabetes mellitus, hyperthyroidism, electrolyte abnormalities such as hypokalemia, hypocalcemia and hypomagnesaemia and use of certain cardiac and non-cardiac medications.18-24
QT prolongation is a well known side effect of the cardiac medications, mainly anti-arrhythmic medications,25 but it can also occur with many non-cardiac medications.26-30 The list of medications that are associated with QT prolongation/TdP are given below in table 1.
Table 1: Examples of medications that have association with QT prolongation/TdP
| Cardiac medications | Non-cardiac medications |
| Antiarrhythmic drugs
Quinidine Amiodarone Procainamide Disopyramide Dofetilide Ibutilide Sotalol |
Antipsychotic drugs
Haloperidol Thioridazine* Chlorpromazine Queitapine Risperidone Ziprasidone Clozapine |
| Cardiovascular drugs
Bepridil Dopamine Dobutamine Adrenaline Indapamide Isradipine Nicardipine Moexipril |
Antidepressant drugs
Amitriptyline Desipramine Citalopram Escitalopram Doxepin Fluoxetine Sertraline Venlafaxine |
| Antihistaminic drugs
Terfenadine* Astemizole* |
|
| Antimotility drugs
Domperidone Cisapride* |
|
| Antiemetic drugs
Ondansetron Dolasetron |
|
| Antimicrobial drugs
Levofloxacin Moxifloxacin Grepafloxacin* Sparfloxacin* Erythromycin Azithromycin Metronidazole |
|
| Antimalarial drugs
Quinine Chloroquine |
|
| Antifungal drugs
Ketoconazole Fluconazole |
|
| Antiprotozoal drugs
Pentamidine |
|
| Antiviral drugs
Amantadine |
|
| Anticancer drugs
Tamoxifen Nilotinib Lapatinib |
Drugs withdrawn or restricted from its use in the market
In the recent years, several non-cardiac medications have been withdrawn or restricted from its use in the market because of its potential to cause QT prolongation/TdP and sudden cardiac death. 10, 11, 31-33
When these non-cardiac medications are given to cardiac patients, it becomes evident that the pro-arrhythmogenic potential of those medications can pose a significant public health problem34 and there is a possibility for lethal drug-drug interactions.35-39 So such medications need to be identified and a suitable alternative should be prescribed.
Hence, this retrospective study has been planned to evaluate the prescription of non-cardiac medications that could cause QT interval prolongation among cardiac patients.
Materials and methods
Before initiating the study, Institutional ethics committee clearance (IHEC/05/2013/Desp.No.265/Dt:27.11.2013) was obtained.
This is a type of observational study which was conducted retrospectively by collecting the data from the medical records of 100 cardiac patients who have attended the outpatient department of cardiology and also admitted in the cardiology ward as inpatients. The personal identity of the patients was not revealed in any part of the manuscript. All the data collected from the patients was analyzed for the lists of medications prescribed to them and were recorded. It was then classified into cardiac and non-cardiac medications. The influence of these cardiac and non-cardiac medications on ECG was gathered through literature search. The effect of non-cardiac medications on the ECG, particularly QT interval was recorded and evaluated.
Inclusion criteria
Medical records of patients who were taken the cardiac or non-cardiac medications that are known to cause QT prolongation were included in the study
Both sexes were included in the study and the upper age limit was set at 70 years
Exclusion criteria
Medical records of patients with other causes for QT prolongation such as heart failure, myocardial ischemia, hypertension, diabetes mellitus, hyperthyroidism, and electrolyte abnormalities such as hypokalemia, hypocalcemia and hypomagnesemia were excluded from the study
The data generated from this study was entered in Microsoft excel sheet. Descriptive analysis was done for all the categorical variables such as age, gender, category of medications and data were expressed as frequencies (percentages).
Results and Discussion
The analysis of the medical records of 100 cardiac patients showed that 86 of them were inpatients and 14 were outpatients.
The frequency analysis of the different characteristics of the study participants such as age, sex and the category of medications is listed below in table 2.
Table 2: Frequency analysis of the different characteristics of the study participants
| Age (in years) n=100 | |
| 30-50 | 37 |
| 51-70 | 63 |
| Sex n=100 | |
| Male | 70 |
| Female | 30 |
| Medications | |
| Cardiac | 36% |
| Non-cardiac | 64% |
The above frequency analysis table represents that among study participants, 70% were males 30% are females. Most of the participants (63%) were in the age group between 51-70 years. The analysis also showed that the study participants had higher incidence of taking non-cardiac medications (64%) than cardiac medications (36%).
Eroglu TE et al in their study observed that the users of non-cardiac QT prolonging medications have a higher risk of SCDs and death than users of cardiac QT prolonging medications. They also showed that women are more vulnerable to QT prolongation and confer a higher SCDs risk than men and that their risk was more elevated with non-cardiac QT prolonging medications.8
In a population-based study by Straus SM et al, even with more modest QT prolongation, nearly three-fold risk of SCDs were observed in approximately 8000 elderly men and women over the age of 55 years.40
Cardiac medications and QT interval
The cardiac medications which were given to the cardiac patients include aspirin, clopidogrel, enalapril, metoprolol, carvedilol, amlodipine, nitroglycerine and digoxin. Among these medications, aspirin (80%) was the most commonly prescribed cardiac medication in cardiac patients. The list of commonly prescribed non-cardiac medications to the cardiac patients is given below in figure 1.
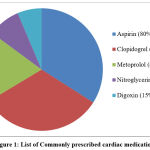 |
Figure 1: List of Commonly prescribed cardiac medications |
From the list of cardiac medications prescribed to the cardiac patients, it was found that none of the patients were found to be taking cardiac medications that have significant effect on QT interval in ECG. The most commonly prescribed cardiac medications in our study are aspirin, clopidogrel and metoprolol. However, there are no any reports or evidences to prove the fact that these cardiac medications have an effect on QT interval prolongation.
Non-cardiac medications and QT interval
The non-cardiac medications that were given to the cardiac patients include paracetamol, domperidone, azithromycin, ciprofloxacin, levofloxacin, cotrimoxazole, fluoxetine, amitriptyline fluconazole and ketoconazole. Among these medications, paracetamol (20%) was the most commonly prescribed non-cardiac medication in cardiac patients. The list of commonly prescribed non-cardiac medications to the cardiac patients is given below in figure 2.
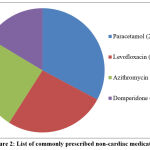 |
Figure 2: List of commonly prescribed non-cardiac medications |
From the list of non-cardiac medications prescribed to the cardiac patients, it was found through literature search that 60% of the non-cardiac medications have significant effect on QT interval in ECG. 26,28-31 Among the prescribed non-cardiac medications, the drugs that were found to significantly affect the QT interval in ECG include levofloxacin, azithromycin, domperidone, amitriptyline and fluconazole. There are many literature evidences available to prove the fact that these commonly prescribed non-cardiac medications have a significant effect on QT interval. However, there are no any reports of QT interval prolongation documented with the most commonly prescribed non-cardiac medication in our study, paracetamol.
An observational study conducted by Li K et al to determine the effect of intravenous ondansetron on QT interval showed that administration of 4 mg intravenous ondansetron showed a significant increase in QT interval (p-value < 0.05).41
In a study done by Field J et al, it was observed that domperidone at the conventionally used doses causes QT prolongation in 6% of patients.27
Catelya LG et al studied about the effect of levofloxacin on QT interval in 24 patients who received 500 mg of the drug once daily. Of all the patients who received levofloxacin, six patients (25%) had developed QT prolongation and two of them showed significant QT prolongation (p-value < 0.05).42
In a study done by Choi Y et al, to evaluate the risk of exposure of the antibiotic azithromycin on QT prolongation, it was showed that the risk of QT prolongation was increased in the elderly people aged between 65-79 years.43
In a retrospective, observational study conducted by Funai Y and colleagues on 87 patients receiving tricyclic antidepressants (TCAs) it was found that TCAs even at lower than normal doses significantly prolonged the QT interval (p value < 0.01).44
In one case study report by Tholakanahalli VN et al, a 68-year-old female patient was given oral fluconazole for her Candida infection with otherwise no any risk factors for TDP. The patient was normal for 7 days after starting fluconazole and on day 8, she suddenly developed TDP which gets resolved when fluconazole was discontinued.45
Assumptions and limitations
As the data this study was collected retrospectively from the case sheets of the cardiac patients, only limited information available in the case sheets could be considered. Inclusion of more number of subjects and prospective collection of data would have added more value to the present study.
Future extension of the work
The authors are planning to collect the same data prospectively in cardiac patients and to assess the ECG changes with various non-cardiac medications.
Conclusion
Cardiac patients frequently receive medications for other associated ailments. These non-cardiac medications may affect the cardiac functions and influence the effect of cardiac medications. From this study, it was found that many cardiac patients received non-cardiac medications that significantly affect the QT interval. Hence, wherever possible these medications should be replaced by any other suitable one that does not affect the heart. If it is not possible, these medications should be used with caution as per recommended dose for very short period to avoid any cardiac effects such as myocardial infarction, heart failure and life threatening ventricular arrhythmias including TdP. Since the development of TdP is rare and multifactorial, the knowledge on drug that causes QT prolongation/TdP is essential while treating patients.
Acknowledgement
We owe our heartfelt thanks to the technical staffs in the medical records department for their assistance in this work. We express our sincere gratitude to the almighty for helping us in completing this work.
Conflict of Interest
There is no conflict of interest
Funding Source
There is no funding source.
References
- Straus SM, Sturkenboom MC, Bleumink GS, Dieleman JP, van der Lei J, de Graeff PA, Kingma JH, Stricker BH. Non-cardiac QTc-prolonging drugs and the risk of sudden cardiac death. Eur Heart J. 2005 Oct;26(19):2007-12. doi: 10.1093/eurheartj/ehi312. Epub 2005 May 11. PMID: 15888497.
- Viskin S, Justo D, Halkin A, Zeltser D. Long QT syndrome caused by noncardiac drugs. Prog Cardiovasc Dis. 2003 Mar-Apr;45(5):415-27. doi: 10.1053/pcad.2003.00101. PMID: 12704598.
- Myerburg RJ, Kessler KM, Castellanos A. Sudden cardiac death: epidemiology, transient risk, and intervention assessment. Ann Intern Med 1993; 119: 1187–97.
- Myerburg RJ, Interian A Jr,Mitrani RM, Kessler KM, Castellanos A. Frequency of sudden cardiac death and profiles of risk. Am J Cardiol 1997; 80: 10F–19F.
- Gorgels AP, Gijsbers C, de Vreede-Swagemakers J, Lousberg A,Wellens HJ. Out-of-hospital cardiac arrest – the relevance of heart failure. The Maastricht Circulatory Arrest Registry. Eur Heart J 2003; 24: 1204–9.
- Myerburg RJ, Castellanos A. Emerging paradigms of the epidemiology and demographics of sudden cardiac arrest. Heart Rhythm 2006; 3: 235–9.
- Josephson M,Wellens HJ. Implantable defibrillators and sudden cardiac death. Circulation 2004; 109: 2685–91.
- Eroglu TE, Barcella CA, Blom MT, et al. Out-of-hospital cardiac arrest and differential risk of cardiac and non-cardiac QT-prolonging drugs in 37 000 cases. Br J Clin Pharmacol. 2022;88(2):820-829. doi: 10.1111/bcp.15030
- Straus SM, Kors JA, De Bruin ML, van der Hooft CS, Hofman A, Heeringa J, Deckers JW, Kingma JH, Sturkenboom MC, Stricker BH, Witteman JC. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol 2006; 47: 362–7.
- Lasser KE, Allen PD,Woolhandler SJ, Himmelstein DU, Wolfe SM, Bor DH. Timing of new black box warnings and withdrawals for prescription medications. JAMA 2002; 287: 2215–20.
- Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med 2004; 350: 1013–22.
- Al-Khatib SM, LaPointe NM, Kramer JM, Califf RM. What clinicians should know about the QT interval. JAMA 2003; 289: 2120–7.
- Yap YG, Camm AJ. Drug induced QT prolongation and torsades de pointes. Heart 2003; 89: 1363–72.
- Myerburg RJ. Sudden cardiac death: exploring the limits of our knowledge. J Cardiovasc Electrophysiol., 2001 Mar; 12(3): 369-81.
- Cohagan B, Brandis D. Torsade de Pointes. [Updated 2021 Aug 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459388/
- Nakano, Y., Shimizu, W. Genetics of long-QT syndrome. J Hum Genet61, 51–55 (2016). https://doi.org/10.1038/jhg.2015.74
- Li M, Ramos LG. Drug-Induced QT Prolongation And Torsades de Pointes. P T. 2017 Jul;42(7):473-477. PMID: 28674475; PMCID: PMC5481298.
- Keating MT, Sanguinetti MC. Molecular and cellular mechanisms of cardiac arrhythmias. Cell 2001; 104: 569–80.
- Al-Khatib SM, LaPointe NM, Kramer JM, Califf RM. What clinicians should know about the QT interval. JAMA 2003; 289: 2120–7.
- Brown DW, Giles WH, Greenlund KJ, Valdez R, Croft JB. Impaired fasting glucose, diabetes mellitus, and cardiovascular disease risk factors are associated with prolonged QTc duration. Results from the Third National Health and Nutrition Examination Survey. J Cardiovasc Risk 2001; 8: 227–33.
- Passino C, Franzoni F, Gabutti A, Poletti R, Galetta F, Emdin M. Abnormal ventricular repolarization in hypertensive patients: role of sympatho-vagal imbalance and left ventricular hypertrophy. Int J Cardiol 2004; 97: 57–62.
- Gaudron P, Kugler I, Hu K, Bauer W, Eilles C, Ertl G. Time course of cardiac structural, functional and electrical changes in asymptomatic patients after myocardial infarction: their inter-relation and prognostic impact. J Am Coll Cardiol 2001; 38: 33–40.
- Davey PP, Barlow C, Hart G. Prolongation of the QT interval in heart failure occurs at low but not at high heart rates. Clin Sci (Lond) 2000; 98: 603–10.
- van Noord C, van der Deure W, Sturkenboom M, Straus S, Hofman A, Visser T, Kors J, Witteman J, Stricker B. High free T4 levels are associated with QTc prolongation in males. J Endocrinol 2008; 198: 253–60.
- Taira CA, Opezzo JA, Mayer MA, Höcht C. Cardiovascular drugs inducing QT prolongation: facts and evidence. Curr Drug Saf. 2010 Jan;5(1):65-72. doi: 10.2174/157488610789869229. PMID: 20210721.
- Tabrizi S, Heidari S, Rafiei H. Investigation role of ondansetron on long QT interval among non-cardiac patients. Ann Med Surg (Lond). 2021 Oct 19;71:102971. doi: 10.1016/j.amsu.2021.102971. PMID: 34712483; PMCID: PMC8531560.
- Field J, Wasilewski M, Bhuta R, Malik Z, Cooper J, Parkman HP, Schey R. Effect of Chronic Domperidone Use on QT Interval: A Large Single Center Study. J Clin Gastroenterol. 2019 Oct;53(9):648-652. doi: 10.1097/MCG.0000000000001183. PMID: 30720577.
- Kervezee L, Gotta V, Stevens J, Birkhoff W, Kamerling I, Danhof M, Meijer JH, Burggraaf J. Levofloxacin-Induced QTc Prolongation Depends on the Time of Drug Administration. CPT Pharmacometrics Syst Pharmacol. 2016 Sep;5(9):466-74. doi: 10.1002/psp4.12085. Epub 2016 Aug 1. PMID: 27479699; PMCID: PMC5036421.
- Rochester MP, Kane AM, Linnebur SA, Fixen DR. Evaluating the risk of QTc prolongation associated with antidepressant use in older adults: a review of the evidence. Ther Adv Drug Saf. 2018 Jun;9(6):297-308. doi: 10.1177/2042098618772979. Epub 2018 May 4. PMID: 29854391; PMCID: PMC5971403.
- Mok NS, Lo YK, Tsui PT, Lam CW. Ketoconazole induced torsades de pointes without concomitant use of QT interval-prolonging drug. J Cardiovasc Electrophysiol. 2005 Dec;16(12):1375-7. doi: 10.1111/j.1540-8167.2005.00299.x. PMID: 16403073.
- Woosley RL. Drugs that prolong QTc interval and/ induce Torsade de Pointes [online]. Available at http://www.qtdrugs.org/medical-pros/drug-lists/drug-lists.cfm
- De Ponti F, Poluzzi E, Cavalli A, Recanatini M, Montanaro N. Safety of non-antiarrhythmic drugs that prolong the QT interval or induce torsade de pointes: an overview. Drug Saf 2002; 25: 263–86.
- Roden DM. Cellular basis of drug-induced torsades de pointes. Br J Pharmacol 2008; 154: 1502–7.
- Viskin S. Long QT syndromes and torsade de pointes. Lancet 1999 Nov 6; 354(9190): 1625-33.
- Yap YG, Camm AJ. Drug induced QT prolongation and torsades de pointes. Heart 2003; 89: 1363–72.
- Moss AJ. The QT interval and torsade de pointes. Drug Saf 1999; 21 (Suppl. 1): 5–10; discussion 81–7.
- Dresser GK, Spence JD, Bailey DG. Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition. Clin Pharmacokinet 2000; 38: 41–57.
- Cholerton S, Daly AK, Idle JR. The role of individual human cytochromes P450 in drug metabolism and clinical response. Trends Pharmacol Sci 1992; 13: 434–9.
- Aerssens J, Paulussen AD. Pharmacogenomics and acquired long QT syndrome. Pharmacogenomics 2005; 6: 259–70.
- Straus SM, Bleumink GS, Dieleman JP, van der Lei J, Stricker BH, Sturkenboom MC. The incidence of sudden cardiac death in the general population. J Clin Epidemiol. 2004 Jan;57(1):98-102. doi: 10.1016/S0895-4356(03)00210-5. PMID: 15019016.
- Li K, Vo K, Lee BK, Addo N, Coralic Z. Effect of a single dose of i.v. ondansetron on QTc interval in emergency department patients. Am J Health Syst Pharm. 2018 Mar 1;75(5):276-282. doi: 10.2146/ajhp161070. Epub 2018 Jan 9. PMID: 29317399.
- Catelya, L & Tjahjono, Cholid & Hanafi, A. (2020). Levofloxacin and Drug-Induced Long QT Syndrome (diLQTS): The Incidence and How to Prevent It. IOP Conference Series: Earth and Environmental Science. 441. 012190. 10.1088/1755-1315/441/1/012190.
- Choi Y, Lim HS, Chung D, Choi JG, Yoon D. Risk Evaluation of Azithromycin-Induced QT Prolongation in Real-World Practice. Biomed Res Int. 2018 Oct 14;2018:1574806. doi: 10.1155/2018/1574806. PMID: 30406128; PMCID: PMC6204160.
- Funai Y, Funao T, Ikenaga K, Takahashi R, Hase I, Nishikawa K. Use of tricyclic antidepressants as analgesic adjuvants results in nonhazardous prolongation of the QTc interval. Osaka City Med J. 2014 Jun;60(1):11-9. PMID: 25272563.
- Tholakanahalli VN, Potti A, Hanley JF, Merliss AD. Fluconazole-induced torsade de pointes. Ann Pharmacother. 2001 Apr;35(4):432-4. doi: 10.1345/aph.10210. PMID: 11302406.
List of abbreviations
ECG – Electrocardiogram
SCDs – Sudden cardiac deaths
ACE – Angiotensin converting enzyme
TdP – Torsades de Pointes
TCAs – Tricyclic antidepressants








