Suha Maher Abed1, Mohamed Ghadban Farhan1, Nahidah Kzar Madhloom2 and Batol Imran Dheeb3
1Department of Biology, College of Science, University of Tikrit, Tikrit, Iraq
2Department of Plant Protection, College of Agriculture, University of Tikrit, Iraq
3Department of Pathological Analysis, College of Applaied science, Samaraa university, Iraq
Corresponding Author E-mail: batoolomran@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2550
Abstract
This paper presents the isolation of Acinitobacter baumanii from clinical dources such as wounds, burns and urinary tract infection. A total of 15 isolates of the studied bacteria were collected and identified by using macroscopic features, biochemical tests and the Vitek2 technique. The diagnosis was then confirmed at the species level. Antibiotics susceptibility test was performed following Kirby-Bauer procedure using 12 antibiotics before and after exposing the bacteria to a static magnetic field, to notice changes related to resistance or sensitivity of the antibiotic, in addition to experimenting with the bacterial viable count before and after exposure to the field as well. The results showed that the isolates had a high resistance to antibiotics, so that all the isolates were 100% resistant to both the third generation cephalosporin and ampicillin, while the most effective antibiotic against the isolates was Imipenem giving only 50% susceptibility. When the isolates were exposed to a magnetic field of 0.3 Tesla and for a period of 24 hours incubation at a temperature of 37 ° C, it was observed a decrease in the number of colony forming unit. Concerning with antibiotic testing after exposure, results indicated that Doxycycline was the most variable in the inhibition zone readings, as it increased significantly. We conclude from our study that the magnetic field can change the vital activity of bacteria by reducing its resistance to antibiotics, which is considered a health problem for the life of humans and their animals.
Keywords
Acinitobacter baumanii; Antibiotic Susceptibility; Static Magnetic field
Download this article as:| Copy the following to cite this article: Abed S. M, Farhan M. G, Madhloom N. K, Dheeb B. I. Magnetic Field Exposure to Clinical Isolates of Acinitobacter baumanii. Biomed Pharmacol J 2022;15(4). |
| Copy the following to cite this URL: Abed S. M, Farhan M. G, Madhloom N. K, Dheeb B. I. Magnetic Field Exposure to Clinical Isolates of Acinitobacter baumanii. Biomed Pharmacol J 2022;15(4). Available from: https://bit.ly/3yS8vVa |
Introduction
Acinitobacter baumanii is one of the most widely distributed bacterium in nature, and there are at least 25 different species of it. It covers both Iraq and Afghanistan, which is why it is called the “Bacteria of Iraq”1. This is due to its high ability to acquire resistance against all currently used antibiotics, as well as its ability to stay for a long time in the hospital environment, which led to its rapid spread and outbreak of infection2. Acinitobacter baumanii is also responsible for many hospital-acquired infections, including: Pneumonia, Meningitis, Bacteremia, Soft tissue infection, Peritonitis, Endocarditis, and Urinary tract infection 3. These bacteria possess many virulence factors that contribute to their pathogenicity. The increasing incidence of antibiotics resistance had prompted intensification of research efforts to find new strategies that enhance the role of other antibiotics in therapeutic applications and less likely to develop resistance unlike traditional antibiotics 4. Magnetism sciences have developed and become more complex since the discovery that their properties are related to all physical status: solid, liquid, and even gaseous as well as living things. It deals with transformations, chemical reactions and transport processes between gaseous, liquid, and solid matter. The metabolism of most living organisms can be regarded as multiphase processes involving gases like oxygen and carbon dioxide; liquids like water, blood, lymph, and plant sap; and solid or semisolid substances like bone, tissue, skin, and cellular membranes. Even primitive forms of life and metabolic activity under anaerobic conditions generally involve multiple liquid and solid or semisolid phases structured by cells and membranes. The current diversity of magnetic fields that are generated and used as applications and electronic devices cannot be completely avoided in contrast to the Earth’s magnetic field which is weak, almost uniform, and can be ignored. Therefore, the effect of Magnetic field (MF) exposure depending on field type and strength has become a relevant topic of academic research and appropriate choice of inhibiting or encouraging the growth and reproduction of living cells 5.
Depending on type and intensity of the magnetic field, and the living organism and its nucleic material, the biological effects of electromagnetic fields are explained by two main manners: The first is based on the possible effects on the permeability of ion channels in the membrane. This can affect the transport of ions into cells and can lead to biological changes in organisms due to existence of moving ions and charged groups within the living cells. The second manner is the formation of free radicals due to exposure to the magnetic field. The growth rate depends on the strength of the field and the nature of the magnetic field 6. This work aimed to isolate and identify Acinitobacter baumanii bacteria from different clinical sources and monitoring phenotypic differences before and after exposure to a static magnetic field related to total colony counts and susceptibility to the antibiotic.
Materials and Method
Sample Collection and Isolation
This bacterium were recovered from different sporadic clinical sources in a surveillance, however it was obtained from infected wounds, burns and urine cultures. Samples of wounds and burns injuries were collected by a sterile cotton carrier after sterilization and lifting the bandage under the supervision of the physician. As for urine samples, specimens were taken from patients with urinary tract infection, using sterilized containers designated for this purpose, after directing the patient to sterilize the area and hands with soap and water. Urine was taken from the middle of the stream and neglected the first drops to prevent contamination with the flora present on the skin. Samples were streaked on Maconkey and blood agar at a rate of two replicates per sample and incubated at a temperature of 37°C for 24 hours to obtain single colonies 7.
Identification
Bacterial isolates were diagnosed based on the cultural characteristics of the colonies in terms of shape, size, color and type of growth in liquid media at 37°C, in addition to microscopic examination and biochemical tests. Whereas, it appeared light pink color on the Maconkey agar while on the blood agar in a light gray color and is not hemolytic, due to its inability to produce hemolysin, knowing that all isolates of A. baumannii bacteria have the ability to grow at a temperature of 44 °C which is a physiological characteristic that distinguishes A.baumannii from other species of the genus Acinetobacter 8. Diagnosis was verified using automated Vitek2 technique (Biomeriucs, Marcyl Ettoile, France).
Antibiotics susceptibility testing
The sensitivity of the isolates to the antibiotics used in the study was carried out using the Kirby-Bauer disc diffusion method. Muller Hinton agar medium was inoculated with 0.1 ml of bacterial suspension, antibiotic discs were placed on the surface of the medium, spaced apart and incubated for 18-24 hours at 37°C. The areas of inhibition around each disc were read in mm and the results recorded 9.
Viable count
Total counting of bacterial colonies was performed using Colony Meter by applying the formula: Total number of bacteria (cfu/ ml) = number of colonies × inverted dilution factor. Decimal serial dilution was followed and growing colonies on the plates were visually counted. The viable colony counting was conducted pre and post exposure to the magnetic field. Knowing that dilution 10-6 was used for pre and post colony number measurement. The bacterial suspension prepared by comparing turbidity to McFarland tube that represents 1.5 × 108 cells / ml measured at 600nm spectrophotometry with optical density OD = 0.5 10.
Magnetic Field Exposure testing
A permanent magnet measuring 0.3tesla (Tesla unit) power was provided after measuring the field strength using a Teslometer, where the tubes of bacterial broth were transported were connected between the poles of the magnet and incubated at 37° for 24 hours after which 100µl of bacterial suspension were transferred and cultured on Muller-Hinton medium to test their antibiotics susceptibility as mentioned previously. Considering the results of the sensitivity test for the same isolates before the field exposure as control for the purpose of comparison 11.
Statistical Analysis
The data obtained from susceptibility testing were analyzed using one-way ANOVA test and average means were compared depending on Duncan multiple range with a level of confidence at 0.95 (SPSS 19.0 software program,) 12.
Results and Discussion
Isolates were collected from patients’ wounds, burns and urinary tract infection, fifteen Acinitobacter baumanii were identified out of 100 isolates. Regardless of the total number, the species isolates were obtained more from wounds, followed by urinary tract infection and then burns. The isolates were diagnosed based on routine examinations including microscopic examination, cultural characteristics and biochemical tests, where the genus was diagnosed initially, and then the species was confirmed using Vietek2 technology 13.
Table 1: Results of Biochemical Tests.
| Result | Test |
| Positive | Catalase test |
| Negative | Oxidase test |
| Negative | Indole production |
| Negative | Methyl red |
| Negative | Vogus proskauer |
| Positive | Citrate utilization |
| K/K | Sugars utilization in TSI Agar |
| Negative | H2S gas production |
| Negative (non- flagellated) | Motility test |
| Negative | Urease test |
| Negative | Nitrate reduction |
This study determined the differences in antibiotic resistance. The results indicated high resistance to the used antibiotics at different rates, as they totally (100%) resisted the antibiotics Ampicilin and Cefotaxime, followed by Amikacin and Ciprofloxacin by 83%, then the Gentamicin antibody by 58%, and finally the Imipenem by 50%. These results are in agreement with what was stated by Sohail 14, where their study showed that the isolates of this species gave the least resistance to Imipenem by 5-19%.
Reasons behind the resistance of this bacterium to penicillins may be due to the widespread and indiscriminate use of these antibiotics, as well as the transmission of the resistance through plasmids or transposons. Acinitobacter baumanii strains have the potential to rapidly acquire antimicrobial resistance as newer antimicrobial resistance has been reported worldwide. In general, the resistance to the group of cephalosporins is due to bacterium’s possession of multiple resistance mechanisms and its production of β-lactamase enzymes and that it has the ability to change outer membrane proteins 15. Bacteria resistance to third-generation cephalosporins (Cefotaxime, Cefixime, Cefriaxone) is related to their indiscriminate use, which leads to the development of their resistance. Another cause is their production of broad-spectrum beta-lactamase enzymes as well as production of Ampc-type beta-lactam enzymes that inhibit the action of third and fourth generation cephalosporins. Resistance toward aminoglycosides came up from possession of plasmids that encode of aminoglycosides modifying enzymes represented by the small ribosomal unit 30s, thus preventing them from binding to ribosomes. The antibiotic imipenem was the optimal and effective treatment for this bacterium. It inhibits the activity of the enzyme transpeptidase and stimulates cell disruption. During the past few years from 2010-2018 the resistance of the bacteria under study to this antibiotic has increased, and the reason for this is due to its possession of several mechanisms of resistance, perhaps the most common are Carbapenem-hydrolyzing class D beta lactamase (CHDLs) enzymes that break down carbapenems also break down a protein in the outer membrane known as the core that is related to the resistance against these antibiotics 16.
Magnetic Field Exposure
The growth rate for a culture subjected to static magnetic fields was significantly reduced in comparison with that of the control cultures fig.1 reveals the difference. In an average, number of colonies before exposure was approximately 128 colony forming units while it was 110 after being exposed to same strength of static magnetic field at same incubation’s circumstances. This result was consistent in all fifteen bacterial isolates. This result is in line with Li et al., 17 who stated that the drop number of viable colonies is due to changes in cell physiology, metabolism, and morphology. It was proved that an exposure to the magnetic field was associated with an increase in the permeability of ion channels in the cytoplasmic membrane, formation of free radicals and active oxygen, disintegration of the cell wall, extrusion of cytoplasmic contents, retraction of the cytoplasmic membrane.This result is also consistent with Wenjin 18 who had investigated the morphology of the bacterial strain using scanning and transmission electron microscope and found that the cell surface was damaged. Overall, magnetic fields inhibit the growth of this species.
 |
Figure 1: Viable Count of Colony Isolates Before and After Magnetic Field Exposure. |
There are controversial explanations for the growth of microbes. For example, results from experiments with other bacterial genera suggest that the effects of magnetic fields may be on quorum sensing mediated by the quorum sensing molecule AI-2. The presence of a strong magnetic field in the bacterial medium affects the life of bacteria, as this magnetic field works to create a large physical electrical voltage in the environment of bacteria, so that this effort will overwhelm the natural efforts that exist mainly in the middle of these small cells, which affects the process of ion flow through the cell wall and its loss of control 19. The results of our current study showed clear differences in the sensitivity of isolates to antibiotics compared to the results before exposure to the magnetic field, knowing that the isolates that were originally sensitive to most antibiotics were not counted when treated with the magnetic field, so the total isolates exposed to the field became 12 isolates. Table 2 shows the difference in percentages for each antibiotics before and after exposure to the field. It was found that the inhibition reading of Doxycycline was most variable giving 50 percent susceptibility, unlike the antibiotic Imipenem, whose readings differed only by a small difference.
Table 2: Antibiotic Susceptibility Before and After Exposure to Magnetic Field
| Susceptibility percentage % |
Antibiotic |
|
| Post exposure | Pre exposure | |
| 33.3% | 0% | Ampicilin |
| 11% | 6% | Cefotaxime |
| 20% | 0% | Cefixime |
| 0% | 0% | Cefradine |
| 50% | 25% | Azithromycine |
| 11% | 8% | Cefriaxone |
| 55.5% | 18% | Amikacin |
| 33.3% | 23.7% | Nalidixic acid |
| 55.5% | 25% | Ciprofloxacin |
| 45% | 40% | Gentamicin |
| 88.8% | 33% | Doxycycline |
| 52% | 41.6% | Imipenem |
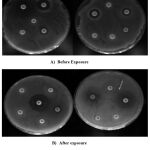 |
Figure 2: Antibiotics Susceptibility Results Before and After Exposure to Static Magnetic Field |
The disc diffusion reading of susceptibility in figure2 shows the phenotypic differences in the diameter of the antibiotic inhibition before and after their exposure to the magnetic field, Table No.3 shows the details of these readings. The result of our research agrees with what was presented by Alkhazan and Saddiq 5, who showed that when Escherichia coli bacteria are exposed to a magnetic field at a frequency of 50Hz and electromagnetic waves 2μT for different periods of time, it caused a significant inhibition in the growth of bacteria after 6 hours and became more sensitive toward antibiotics, with implications for the phenotypic characteristics of reduced bacterial cell length. The magnetic field significantly affects the bacteria wall through the separation of ions and changing the permeability of the cytoplasmic membrane containing porins or protein holes responsible for transporting ions and some other molecules, and one these ions is the proton H+. The flow of this proton through the cell wall is what maintains the pH balance function inside Cell solution. Also, the inhibitory effect may be due to a decrease in the activity of the bacterial cell and its metabolism and a decrease in the rate of replication of its genetic material 20. Li et al.,21 mentioned that static magnetic field had altered bacterial metabolism by decreasing in glycolate oxidase activity and accumulating of long-chain fatty acids (LCFA, with more than 18 carbons) suggesting that free radicals generated by LCFA degradation are the primary target of static magnetic field action, which triggers the bacterial oxidative stress response and ultimately leads to growth inhibition.
From the point of view of statistics using ANOVA test, results depicted as lower-case letters assessing the difference before and after exposure to magnetic field in terms of number isolates (similar lowercase letters horizontally mean that there are no significant differences between them). Capital letters differences refer to antibiotics involved before and after exposure (similar capital letters vertically mean that there are no significant differences between them).
It is clear that isolates number 2,4,7 and 9 had showed significant differences to Azithromycin (AZM) at p< 0.05 where: a>b, while the rest of isolates showed no differences.
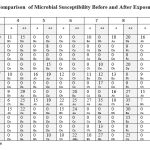 |
Table 3: Statistical Comparison of Microbial Susceptibility Before and After Exposure to the Magnetic Field. |
Ceftriaxone (CTR) showed less differences except for isolate number 1. Same results with Cefradine (CH) since they belong to same group of cefalosporins, approximate interpretation to Woroszyło 22 despite their use of a rotating field test against a specifically different bacterial genus.
Imipenem (IPM) showed differences in isolates number 1,2,4,5,6,8 and10. Amikacin (AK) had statistical differences of the isolates before and after exposure to the magnetic field, as the readings changed in the isolates 1,2,5,6,7,8,9and 12. Anyway Doxycycline (DO) had the largest share in the statistical difference since all isolates gave dramatic changes after exposure according to the alphabetical differences in an ascending order. Differences in inhibition zones diameter around antibiotic discs pre and post exposure illustrated in table3. The current results are compatible with Kamel et al., 23 who posted that moderate intensity static fields induced dramatic changes in the susceptibility to antibiotics and referred to the possibility that magnetic field could interferes with the surface charges of the bacterial membrane or the distribution of the charged antibiotic molecule eventually modifying the rate of antibiotic penetration.
Conclusion
In conclusion the results of this study shed light on the efficiency of the magnetic field in inhibiting the growth of Acinitobacter baumanii and increasing their sensitivity to antibiotics. It can change the life activity of bacteria and may open the way for scientific applications. We propose to monitor the bacterial growth and physiological characteristics of bacteria after exposure to different types of magnetic fields over longer periods of time by measuring optical density or bacterial counting, in addition to highlighting the gene expression of these species.
Conflict of Interest
There is no conflict of interest.
Funding Sources
There is no funding source.
References
- Howard, A., O’Donoghue, M., Feeney, A., & Sleator, R. D. (2012). Acinetobacter baumannii: an emerging opportunistic pathogen. Virulence, 3(3), 243-250.
CrossRef - Aoife H., Michael D., Audery F., and Roy D., Sleator (2012), Acinitobacter baumanii an emerging opportunistic pathogen, Virulence, 3: 243-250.
CrossRef - Ziqing J., Andriana I., Michael L., and Robert S., (2014) Specificity determinants improve therapeutic indices of two antimicrobial peptides pisxidin I and dermaseptin S4 against the gram –negative pathogens Acinitobacter baumanii and Pseudomonas aeruginosa. Pharmaceutical, 7: 366-390.
CrossRef - Peymani A. Farajnia S., Nahaei M., and Azhari F., (2012) Prevalence of class I Integrom Among Multidrug Resistant Acinitobacter baumani in Tabriz, Polish journal of Microbiology, 61: 57-60.
CrossRef - Mousavian-Roshanzamir, S., & Makhdoumi-Kakhki, A. (2017). The inhibitory effects of static magnetic field on Escherichia coli from two different sources at short exposure time. Reports of biochemistry & molecular biology, 5(2), 112.
- Del Re, B., Bersani, F., Agostini, C., Mesirca, P., & Giorgi, G. (2004). Various effects on transposition activity and survival of Escherichia coli cells due to different ELF-MF signals. Radiation and environmental biophysics, 43(4), 265-270.
CrossRef - Mahon, C. R., Lehman, D. C., & Manuselis, G. (2018). Textbook of diagnostic microbiology-e-book. Elsevier Health Sciences.
- Abed SM., Yasin LQ., Mahmood WS., (2021) Inhibitory Effects of Radish Peeled Root Extracts against Selected Pathogenic bacteria, Tropical Journal of Natural Product Research, 5 (8), 1354-1359.
CrossRef - Dijkshoorn, L., Nemec, A. and Seifert, H., An increasing threat in hospitals: multidrug-resistant Acinitobacter baumanii , Nsture reviews Microbiology, 2007, 5: 939-951.
CrossRef - Al-Khaza’leh, K. A., & Al-fawwaz, A. T. (2015). The Effect of Static Magnetic Field on E. coli, S. aureus and B. subtilis Viability. Nat. Sci. Res, 5, 153-157
- Shartooh S M., Abed S M., Hamid N M., Increasing Sterilization Efficiency of Shredder Autoclave on Medical Waste Using Ultraviolet Light Device.
- Abed SM., Mahmood Y SH., Ibrahim I F, Ammar, Antibacterial Activity of Green Synthesized Copper Oxide Nanoparticles,2020, Iraqi Journal of Science, 62(9), 3372–3383.
CrossRef - Poirel, L., & Nordmann, P. (2006). Carbapenem resistance in Acinetobacter baumannii: mechanisms and epidemiology. Clinical Microbiology and Infection, 12(9), 826-836.
CrossRef - Sohail, M., Rashid, A., Aslam, B., Waseem, M., Shahid, M., Akram, M., … & Rasool, M. H. (2016). Antimicrobial susceptibility of Acinetobacter clinical isolates and emerging antibiogram trends for nosocomial infection management. Revista da Sociedade Brasileira de Medicina Tropical, 49, 300-304.
CrossRef - Ghaima, K. K. (2018). Distribution of extended spectrum beta-lactamase (ESBL) genes among Acinetobacter baumannii isolated from burn infections. MOJ Cell Sci Rep, 5(3), 42-46.
CrossRef - Ozdemir S. K., J., He L., Yang L., (2011), Direct estimation of Purcell factor from scatterer- induced mode splitting spectra of an optical microcarity’ Optical society of America, 34 (7): 431.
CrossRef - Li, H., Xie, R., Xu, X., Liao, X., Guo, J., Fang, Y., & Huang, J. 2022). Static Magnetic Field Inhibits Growth of Escherichia coli Colonies via Restriction of Carbon Source Utilization. Cells, 11(5), 827.
CrossRef - Wenjin, J. I., Huimin, H., Aihua, D., & Chunyang, P. (2009). Effects of static magnetic fields on Escherichia col. Micron, 40(4), 894-8.
CrossRef - Brkovic, S., Postic, S., & Ilic, D. (2015). Influence of the magnetic field on microorganisms in the oral cavity. Journal of Applied Oral Science, 23, 179-186.
CrossRef - Al- Barzenji H. A., Al- Jubouri R., Taher Z., (2010) The effect of static magnetic field on some oral microorganisms (an in vitro study), Tikrit Medical Journal, 16(2):34-38.
- Li, H., Xie, R., Xu, X., Liao, X., Guo, J., Fang, Y., & Huang, J. 2022). Static Magnetic Field Inhibits Growth of Escherichia coli Colonies via Restriction of Carbon Source Utilization. Cells, 11(5), 827.
CrossRef - Woroszyło, M., Ciecholewska-Juśko, D., Junka, A., Wardach, M., Chodaczek, G., Dudek, B., & Fijałkowski, K. (2021). The effect of rotating magnetic field on susceptibility profile of methicillin-resistant Staphylococcus aureus strains exposed to activity of different groups of antibiotics. International journal of molecular sciences, 22(21), 11551.
CrossRef - Kamel, F. H., Saeed, C. H., & Qader, S. S. (2014). Magnetic Field Effect on Growth and Antibiotic Susceptibility of Staphylococcus aureus. Al-Nahrain Journal of Science, 17(3), 138-143
CrossRef








