Syahrida Dian Ardhany1* , Susi Novaryatiin1
, Susi Novaryatiin1 and Guntur Satrio Pratomo2
and Guntur Satrio Pratomo2
1Department of Pharmacy Faculty of Health Sciences, Muhammadiyah University of Palangkaraya, Palangka Raya, 73111.
2Bawi Bakena Tuntung Bahalap Industry, Palangka Raya, 73111.
Corresponding Author E-mail: chass501@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2558
Abstract
Several studies revealed that the ethanolic extract, cream, and loose powder of Bawang Dayak bulbs (Eleutherine bulbosa (Mill.) Urb.) are effective in inhibiting the growth of different acne-causing bacteria, including S. aureus, S. epidermidis, and P. acne. Several product series have also been developed using the plant for the treatment of acne-prone skin. Therefore, this study aims to carry out a primary irritation test of E. bulbosa loose powder using the patch test method on rabbits and humans to determine its safety before it is marketed. The results showed that the primary irritation index of E.bulbosa loose powder on rabbits was 0.125, which was classified in the negligible category, and there were no signs of erythema or edema in humans. This indicates that it does not cause irritation and has the potential to be developed into anti-acne products.
Keywords
Acne; Bawang Dayak; Eleutherine bulbosa; Patch Test
Download this article as:| Copy the following to cite this article: Ardhany S. D, Novaryatiin S, Pratomo G. S. Irritation Test of Bawang Dayak (Eleutherine Bulbosa (Mill.) Urb.) Loose Powder for Acne Vulgaris. Biomed Pharmacol J 2022;15(4). |
| Copy the following to cite this URL: Ardhany S. D, Novaryatiin S, Pratomo G. S. Irritation Test of Bawang Dayak (Eleutherine Bulbosa (Mill.) Urb.) Loose Powder for Acne Vulgaris. Biomed Pharmacol J 2022;15(4). Available from: https://bit.ly/3ibpw7m |
Introduction
Acne is a chronic skin condition, which often requires long-term management. 1-2 It also has several effects depending on its severity, including psychological damage, such as depression, anguish, as well as removal from social life. 3 The use of cosmetics and personal care products is increasing due to the need to improve skin texture, promote beauty, and overcome emotional and self-esteem issues. However, there has also been a rapid increase in side effects of these products, such as mild to severe skin irritation responses.4
Bawang dayak (Eleutherine bulbosa (Mill.) Urb.) is a species of medicinal plant that is extensively distributed in Central Kalimantan, Indonesia, with various health benefits.5 Previous studies reported that it has several bioactivities including anticancer, antibacterial, and antioxidant6-9. E. bulbosa has the potential to be used as a cosmetic raw material, especially as an anti-acne. In previous studies, its ethanolic extract inhibited several acne-causing bacteria, such as S. aureus, S. epidermidis, and P. acnes. 10 Therefore, this study aims to produce an E.bulbosa product series, including cream, clay mask, and sheet mask for acne-prone skin. The process was then continued with the development of loose powder products. Based on the results of the study, loose powder of E.bulbosa can prevent the growth of acne-causing bacteria, especially P.acnes.11 Several clinical studies need to be carried out to determine the product’s safety before it is introduced to the market. In this study, an irritation test was performed for the loose powder of E.bulbosa using test animals and people.
Materials and Methods
Plant material and preparation of extract
The part of Bawang Dayak used was fresh bulbs, which were taken from the harvest of self-planting, while the seedlings were collected from Sei Gohong Bukit Batu, Palangka Raya, Central Kalimantan, Indonesia. This study was authenticated by the Indonesian Institute of Sciences Research Center for Biology, Bogor Indonesia with reference number 2119/IPH.1.01/If.07/VIII/2018. Bulbs of Bawang Dayak were processed into powder and then extracted with 96% ethanol using the percolation method.
Loose powder of Bawang Dayak preparation
All materials in Table 1 were weighed, and ZnO was sieved using a 40-mesh sieve. The mortar and stamper were then heated using hot water. The ethanol extract of E. bulbosa bulbs was prepared by adding a little 95% ethanol. It was then ground until it was homogeneous and then dried with corn starch. In another mortar, menthol and peppermint oil were added with a little ethanol and then dried with ZnO and talcum. The two mixtures were then combined and ground until homogeneous by adding the remaining talcum. When the mixing was complete, the powder was sieved using 40-mesh and 100-mesh with a sieve shaker, followed by packing. Loose powder base using as a control.
Table 1: Formulation of Bawang Dayak loose powder.
| Materials | Loose powder base (mg)
as a control |
Bawang Dayak loose powder (mg) |
| E. bulbosa ethanol extract | – | 1500 |
| Peppermint oil | 10 drops | 10 drops |
| ZnO | 300 | 300 |
| Menthol | 100 | 100 |
| Corn starch | 4000 | 4000 |
| Sterile Talc ad | 10000 | 10000 |
 |
Figure 1: E. Bulbosa loose powder |
Rabbit Skin Irritation test
This study was approved by the Health Research Ethics Committee of Aisyiyah University of Yogyakarta with ethical approval No. 2221/KEP-UNISA/VII/2022. Rabbit skin irritation test is an early detection irritation before testing on humans, if the results of the test on rabbits show a moderate or severe irritation this study is not continued to human trials.
An evaluation was carried out using a total of 4 rabbits, and their backs were clipped free of fur with an electric clipper. The clipped areas were divided into four sites for each animal with a surface area of 49 cm (7×7 cm). The loose powder was then applied immediately to one site with different doses of 250 mg, 500 mg, and 1000 mg. Another site served as a control, and a loose powder base was administered. The gauze was used to cover the treated and controlled areas, and a non-occlusive bandage was applied to the rabbit’s back. Subsequently, the animals were brought back to their cages. The bandage was removed 24 hours after application and checked for skin irritation. The locations were observed for 24 and 72 hours.12 The reactions, defined as erythema and oedema were presented as scores from observation based on the classification score for skin reaction, as shown in Table 2. The PII was then calculated, and the results are presented in Table 3. The primary irritation index was calculated using the formula given below12-16:
Table 2: Classification score for skin reaction (erythema and oedema) 13-16
| Reaction | Score |
| No erythema/oedema | 0 |
| Very slight erythema/oedema (barely perceptible) | 1 |
| Well-defined erythema/oedema | 2 |
| Moderate to severe erythema/oedema (raising approximately 1 mm) | 3 |
| Severe erythema (beet redness) to eschar formation/ oedema (raised more than 1 mm and extending beyond the area of exposure) | 4 |
| Total possible score for primary irritation (erythema and oedema) | 8 |
Table 3: Response categories of irritation in rabbit12,15-16
| Category | Primary Irritation Index (PII) |
| Negligible | 0-0.4 |
| Slight irritation | 0.5-1.9 |
| Moderate irritation | 2-4.9 |
| Severe irritation | 5-8 |
Human skin irritation
This is a pre-post-test-controlled study with the human patch test method. The inclusion criteria include healthy participants, between the ages of 18 and 30, without a history of an allergy-related disease, cooperative, willing to participate, and informed consent must be obtained from the volunteers.17
An unhealthy individual with excessive sweating, a wet wound or abnormal skin were excluded. This study was approved by the Health Research Ethics Committee of Aisyiyah University of Yogyakarta with ethical approval No. 2221/KEP-UNISA/VII/2022. The loose powder was applied to 20 healthy volunteers using two hands for each volunteer, one for the treated group given loose powder of Bawang Dayak, and one for the control administered with a loose powder base. The volunteers were informed not to carry out extra work or activities causing increased sweating, scratching, as well as avoid water.18 The test was carried out with loose powder of Bawang Dayak and loose powder base on the right and left forearm, respectively, which were previously marked with a size of 4 x 2.5 cm. After applying both treatments, the test location was covered with a non-occlusive opsite post-op bandage of 6.5 x 5 cm. The patch test was removed after 24 hours and observed using the grading criteria of skin reactions, as shown in Table 213-16.
Results and Discussion
E. bulbosa (Bawang Dayak) is a medicinal plant with several health benefits. Previous studies revealed that the ethanolic extract of its bulbs has the potential to be used to treat acne. Another study stated that it can inhibit acne-causing bacteria, including S.aureus, S.epidermidis and P.acne. Consequently, it was developed into products, such as creams, clay masks, and loose powder, which were processed into a product, namely the E.bulbosa series for acne-prone skin19. The Formulation of Bawang Dayak loose powder contains ZnO, which acts as an adjuvant with synergy anti-acne effects20-21. The cream has been subjected to several phases of irritant testing on both people and animals. The same test was carried out for the loose powder before further development study was performed, specifically a clinical trial on human faces with acne.
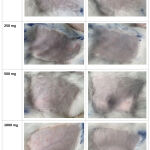 |
Figure 2: Appearance of rabbit skin irritation test |
In acne, sebum produced by sebaceous glands, content changes and reactive oxygen species (ROS), namely hydroxyl, superoxide, and nitrous oxide can be released from the damaged follicular walls.22-23 The occurrence of irritation during the acne infection is caused by these free radicals. Previous studies revealed that medications used to treat the condition work by reducing ROS because oxidative stress as well as inflammation plays a critical role in acne pathogenesis.24 Alternative antioxidants from natural sources are advantageous in facilitating the healing of damage caused by these free radicals. The use of natural products has become a substitute for topical treatment of several diseases, such as acne bacterial, due to resistance to antibiotics.25 Based on a previous study, E. bulbosa contains flavonoids with antibacterial and antioxidant abilities, and they were more dominant in the bulbs compared to other plant parts, such as leaves and flowers.26-28
Table 4: Irritation test on rabbits
| Rabbits | Treatment | 24h | 72h | Primary Irritation Index (PII) | ||
| Erythema | Oedema | Erythema | Oedema | |||
| 1 | Control | 0 | 0 | 0 | 0 | 0.125 |
| 250mg Loose powder of E. bulbosa | 0 | 0 | 0 | 0 | ||
| 500mg Loose powder of E. bulbosa | 0 | 0 | 0 | 0 | ||
| 1000mg Loose powder of E. bulbosa | 1 | 0 | 0 | 0 | ||
| 2 | Control | 0 | 0 | 0 | 0 | |
| 250mg Loose powder of E. bulbosa | 0 | 0 | 0 | 0 | ||
| 500mg Loose powder of E. bulbosa | 0 | 0 | 0 | 0 | ||
| 1000mg Loose powder of E. bulbosa | 1 | 0 | 0 | 0 | ||
| 3 | Control | 0 | 0 | 0 | 0 | |
| 250mg Loose powder of E. bulbosa | 0 | 0 | 0 | 0 | ||
| 500mg Loose powder of E. bulbosa | 0 | 0 | 0 | 0 | ||
| 1000mg Loose powder of E. bulbosa | 0 | 0 | 0 | 0 | ||
| 4 | Control | 0 | 0 | 0 | 0 | |
| 250mg Loose powder of E. bulbosa | 0 | 0 | 0 | 0 | ||
| 500mg Loose powder of E. bulbosa | 0 | 0 | 0 | 0 | ||
| 1000mg Loose powder of E. bulbosa | 0 | 0 | 0 | 0 | ||
| Total | 2 | 0 | 0 | 0 | ||
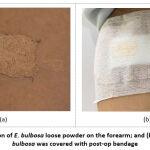 |
Figure 3: (a) Application of E. bulbosa loose powder on the forearm; and (b) application of the E. bulbosa was covered with post-op bandage. |
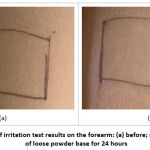 |
Figure 4: Appearance of irritation test results on the forearm: (a) before; and (b) after application of loose powder base for 24 hours. |
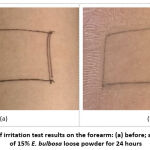 |
Figure 5: Appearance of irritation test results on the forearm: (a) before; and (b) after application of 15% E. bulbosa loose powder for 24 hours |
Pharmaceutical and herbal skin care products must undergo in vitro and in vivo testing for biological evaluation. Assessment of their irritation and sensitization potential with natural compounds is a significant step in the evaluation of their biocompatibility. An irritation test was carried out to determine the irritating effect of a product after use on the skin to know the level of product safety before selling it to the public. Irritated skin often appear as erythema, edema, and desquamation due to the direct contact of substances with the dermal layer of the skin.29
The primary irritation test of E.bulbosa loose powder in this study was carried out on rabbits and humans using the patch test method, as shown in Figure 2. The in vivo rabbit test is the benchmark against which new approach methodologies for skin irritation are usually compared. No alternative method offers a complete replacement of animal use for this endpoint for all regulatory applications.30-31
Based on the results, the sample was in a negligible category (PII= 0.125), which indicates that it was harmless, as shown in Table 4. There was also no sign of erythema or edema among the 20 volunteers, as shown in Table 5. Each volunteer was interviewed to find out whether there was itching or burning on the skin after the application of E. bulbosa loose powder, as shown in Figures 3 to 5. Furthermore, only 4 of them had a little itchy feeling. Itching occurs when they were sweating excessively during activities, but it was minimal and didn’t disrupt their functions. For the loose powder base (control), all volunteers had no itchy or hot sensation on the skin. The results of these two studies revealed that E.bulbosa loose powder did not cause significant irritation. Therefore, this study can proceed to the clinical trial stage using volunteers who have acne-prone faces to determine its effectiveness.
Table 5: Irritation test on volunteers.
| Volunteers | Loose powder base | Bawang Dayak loose powder |
| 1 | 0 | 0 |
| 2 | 0 | 0 |
| 3 | 0 | 0 |
| 4 | 0 | 0 |
| 5 | 0 | 0 |
| 6 | 0 | 0 |
| 7 | 0 | 0 |
| 8 | 0 | 0 |
| 9 | 0 | 0 |
| 10 | 0 | 0 |
| 11 | 0 | 0 |
| 12 | 0 | 0 |
| 13 | 0 | 0 |
| 14 | 0 | 0 |
| 15 | 0 | 0 |
| 16 | 0 | 0 |
| 17 | 0 | 0 |
| 18 | 0 | 0 |
| 19 | 0 | 0 |
| 20 | 0 | 0 |
Conclusion
Loose powder of E. bulbosa was in the negligible category (PII= 0.125) on rabbit skin and does not irritate human skin. Further studies are needed for the clinical stage using volunteers who have acne-prone faces to determine its effectiveness. The suggest of this study is apply ZnO and the extract of E.bulbosa on two separate groups to compare the effect on acne.
Acknowledgement
This study was funded by The Ministry of Education and Culture Republic of Indonesia, through the grant of Penelitian dan Produk Vokasi No: 133/SPK/D4/PPK.01.APTV/VI/2022.
Conflict of Interest
There is no conflict of interest
References
- Tan J, Alexis A, Baldwin H, Beissert S, Bettoli V, Rosso JD, Dreno B, Gold LS, Harper J, Lynde C, Thiboutot D, Weiss J, Layton AM. The personalized acne care pathway-recommedations to guide longitudinal management from the personalising acne: consensus of experts. JADD Int., 2021;5: 101-111
CrossRef - Leung AKC, Barankin B, Lam JM, Leong KF, Hon KL. Dermatology: how to manage acne vulgaris. Drugs Context.,2021;10:1-18
CrossRef - Diogo MLG, Campos TM, Fonseca ESR, Pavani C, Horliana ACRT, Fernandes KPS, Bussadori SK, Fantin FGMM, Leite DPV, Yamamoto ATA, Navarro RS, Motta LJ. Effect of blue light on acne vulgaris: a systematic review. Sensors., 2021; 21 (6943):1-13
CrossRef - Kose O, Erkekoglu P, Sabuncuoglo S, Gumusel BK. Evalution of skin irritation potentials of different cosmetic products in Turkish market by reconstructed human epidermis model. Regul Toxicol Pharmacol., 2018; 98: 268-273
CrossRef - Atikah TA, Wardiyati T, Nihayati E, Saputera, Nendissa DR. Cultivation technology innovation of dayak onion (Eleutherine palmifolia Merr) to increase productivity and economic feasibility analysis. Agromix., 2021; 12(1):39-46
CrossRef - Muti’ah R, Listiyana A, Nafisa BB, Suryadinata A. Kajian efek ekstrak umbi bawang dayak (Eleutherine palmifolia (L.) Merr) sebagai antikanker. J. Islamic Pharm., 2020; 5(2):14-26
CrossRef - Shi P, Du W, Wang Y, Teng X, Chen X, Ye L. Total phenolic, flavonoid content, and antioxidant activity of bulbs, leaves, and flowers made from Eleutherine bulbosa (Mill.) Urb. Food Sci Nutr., 2019;7:148-154
CrossRef - Putra AMP, Sari RP, Musiam S. Combination of bawang dayak extract and acarbose against male white rat glucose levels. Borneo J Pharm., 2021;4(2):84-90
CrossRef - Kamarudin AA, Sayuti NH, Saad N, Razak NAA, Esa NM. Eleutherine bulbosa (Mill.) Urb. Bulb: Review of the pharmacological activities and Its prospects for application. Int J Mol Sci., 2021;22(13) 6747:1-20
CrossRef - Novaryatiin S, Ardhany SD. Potential anti-acne: bawang dayak (Eleutherine bulbosa (Mill.) Urb.) from Central Kalimantan-Indonesia. Pharmacogn J.,2020;12(1):52-57
CrossRef - Novaryatiin S, Amalia NR, Ardhany SD. Formulation of anti acne loose powder of bawang dayak (Eleutherine bulbosa (Mill.) Urb.) ethanol extract. Borneo J Pharm., 2022;5(2):153-160
CrossRef - Kamkaen N, Phuntuwate W, Samee W, Boonrod A, Treesak C. The investigation of the rabbit and human skin irritation of herbal anti-wrinkle cream. Thai Pharmaceut. Sci. J.,2017;2(1):20-25
- Nyigo VA, Mdegela R, Mabiki F, Malebo HM. Assesment of dermal irritation and acute toxicity potential of extracts from Synadenium glaucescens on healthy rabbits, wistar albino rats and albino mice.European J Med Plants.,2015;10(4):1-11
CrossRef - Gatne MM, Tambe K, Adarsh, Ravikanth K. Acute dermal irritation study of polyherbal gel mastilep in rabbits. Int J Pharm Sci Res., 2015;6(8):3473-3476
CrossRef - Sohail T, Yasmeen S, Imran H, Ferheen S, Rehman A, Khan RA. Standardadization and skin irritation potential of herbal analgesic cream containing Nigella sativa seed oil.Bangladesh J Med Sci., 2020;19(1):163-168
CrossRef - Lebang JS, Siampa JP, Nurmiati. Studies of wound healing activity and irritation test of chili (Capsicum frutescens) leaves gel on rats (Rattus novergicus). Galenika J Pharm., 2021; 7(1): 54-65
CrossRef - Mekjaruskul C, Kumkarnjana S, Fangkrathok N, Kengkittipat W, Sirichat N. Potential cosmeceutical applications and evaluation of human skin irritation of Tagetes erecta flower extract. Pharmacogn Res., 2021; 13(4):199-207
CrossRef - Srichaipor N, Pongcharoen P, Kanokkangsadal P, Itharat A. Skin irritation and allergic testing of thai herbal extracts (Ha-Rak with turmeric) in healthy volunteers. Thammasat Med J., 2020;20(2):165-174
- Ardhany SD, Putra CD, Novaryatiin S. Modification of anti-acne bawang dayak (Eleutherine bulbosa (Mill.) Urb.) cream to Propionibacterium acnes. J Adv Pharm Technol Res., 2021; 12(1):94-98
CrossRef - Bae JY, Park SN. Evaluation of anti-microbial activities of ZnO, citric acid and a mixture of both against Propionibacterium acnes. Int J Cosmet Sci., 2016; 38(6):550-557
CrossRef - Abendrot M, Kalinowska-Lis U. Zinc-containing compounds for personal care applications. Int J Cosmet Sci., 2018; 40(4): 319-327
CrossRef - Arican O, Kurutas EB, Sasmaz S. Oxidative stress in patients with acne vulgaris. Mediators Inflamm., 2005; 2005(6): 380-384
CrossRef - Vora J, Srivastava A, Modi H. Antibacterial and antioxidant strategies for acne treatment through plant extracts. Inform Med Unlocked., 2017; 2017: 1-5
- Sahib AS, Al-Anbari HH, Raghif ARA. Oxidative stress in acne vulgaris: an important therapeutic target. J Mol Pathophysiol., 2013; 2: 27-31
CrossRef - Silva MV, Moura NG, Motoyama AB, Ferreira VM. A review of the potential therapeutic and cosmetic use of propolis in topical formulations. J App Pharm Sci., 2020; 10 (1):131-141
CrossRef - Shi P, Du W, Wang Y, Teng X, Chen X, Ye L. Total phenolic, flavonoid content, and antioxidant activity of bulbs, leaves, and flowers made from Eleutherine bulbosa (Mill.) Urb.. Food Sci Nutr., 2019; 7(1): 148-154
CrossRef - Shamsudin NF, Ahmed QU, Mahmood S, Shah SAA, Khatib A, Mukhtar S, Alsharif MA, Parveen H, Zakaria ZA. Antibacterial effects of flavonoids and their structure-activity relationship study: a comparative interpretation. Molecules., 2022; 27(1149): 1-43
CrossRef - Adamczak A, Ozarowski M, Karpinski TM. Antibacterial activity of some flavonoids and organic acids widely distributed in plants. J Clin Med., 2020; 9(109): 1-17
CrossRef - Kojima H, Nakada T, Yagami A, Todo H, Nishimura J, Yagi M, Sugiyama M, Yamamoto K, Ikarashi Y, Sakaguchi H, Yamaguchi M, Hirota M, Ikeda H, Imai N, Hatao M. A step-by-step approach for assessing human skin irritation without animal testing for quasi-drugs and cosmetic products. Appl Vitro Toxicol., 2021;7(3):144-154
CrossRef - Rooney JP, Choksi NY, Ceger P, Daniel AB, Truax J, Allen D, Kleinstreuer N. Analysis of variability in the rabbit skin irritation assay. Regul Toxicol Pharmacol., 2021;122:104920
CrossRef - Jung H, Seo W, Jeong T, Kang HW, Kim S. A study on the skin irritation toxicity test of processed sulfur in new Zealand white rabbit. J Pharmacopuncture., 2022;25(1):46-51
CrossRef









