Fidia Rizkiah Inayatilah* , Lailatul Mas’udah1, Ria Ramadhani Dwi Atmaja1, Mayu Rahmayanti
, Lailatul Mas’udah1, Ria Ramadhani Dwi Atmaja1, Mayu Rahmayanti , Burhan Ma’arif
, Burhan Ma’arif and Hajar Sugihantoro
and Hajar Sugihantoro
Department of Pharmacy, Faculty of Medical and Health Science, Maulana Malik Ibrahim State Islamic University, Malang,
Corresponding Author E-mail: fidiarizkiah9@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2574
Abstract
Red fruit ( Lam.) contains high levels of fatty acids, α-tocopherol, and β-carotene which can accelerate wound healing. Red fruit oil (RFO) is formulated as an emulgel because it is comfortable to use, has high penetration, and has a low risk of irritation. Emulgel RFO must meet the standards for physical properties and stability of topical preparations.The purpose of this study was to determine the effect of variations in RFO concentration on the physical properties and physical stability of the RFO emulgel. Emulgel was made with various RFO concentrations of 5% w/v (F1), 10% w/v (F2), and 15% w/v (F3). Then there was a physical evaluation and tested for physical stability after storage for 28 days at room temperature (30ºC±2ºC). Variations in RFO concentration decreased dispersion and adhesion values in the evaluation of physical qualities. In the 28-day storage stability test, variations in RFO concentration decreased the pH value, dispersion and increased adhesion. Emulgel RFO before and after the cycling test did not change. After performing the mechanical test, all the RFO emulgels did not occur in phase separation. It can be concluded that the variation of RFO concentration affects the physical properties, namely, dispersion and adhesion. However, it did not affect the organoleptic, homogeneity, and pH of the emulgel. After 28-day storage, variations in RFO concentration significantly affected pH, spreadability, and adhesion, while homogeneity and organoleptic had no significant effect. Variations in RFO concentration did not affect the stability of the cycling test and mechanical test.
Keywords
Emulgel; Formulation; Physical Properties; Red Fruit Oil (Pandanus conoideus Lam.); Stability
Download this article as:| Copy the following to cite this article: Inayatilah F. R, Mas’udah L, Atmaja R. R. D, Rahmayanti M, Ma’arif B, Sugihantoro H. Formulation and Physical Stability Test of Red Fruit Oil (Pandanus conoideus Lam.) Emulgel Using Carbopol 940 Base as Wound Treatment. Biomed Pharmacol J 2022;15(4). |
| Copy the following to cite this URL: Inayatilah F. R, Mas’udah L, Atmaja R. R. D, Rahmayanti M, Ma’arif B, Sugihantoro H. Formulation and Physical Stability Test of Red Fruit Oil (Pandanus conoideus Lam.) Emulgel Using Carbopol 940 Base as Wound Treatment. Biomed Pharmacol J 2022;15(4). Available from: https://bit.ly/3BHxLyJ |
Introduction
When exposed to sharp objects or heat, the skin, which protects the body from the outside world, can be damaged or injured. Following an injury, the body employs a sophisticated mechanism to regenerate and replace the damaged tissue 1. Both internal and external factors, like oxygenation, infection, age, sex hormones, stress, medications, smoking, and nutrition, can affect how quickly a wound heals 2.
Topical medications are an external component that can hasten wound healing. Common wound topical medications on the market, such as 10% povidone-iodine, have side effects such as irritation, the risk of bacterial resistance, allergies, inner ear damage, and excessive administration that limit wound granulation and fibroblast cell proliferation 3-5. As a result, little side-effect alternative treatments are required. Herbal medicines have fewer side effects than synthetic drugs and are cheaper. They also have more active ingredients that work together to make them more effective 6.
As a topical medication to accelerate wound healing, Red fruit (Pandanus conoideus Lam) is a special and typical plant (endemic) which grows at Papua Island. This plant belongs to the genus Pandanus and the family Pandanaceae 7. Red fruit oil contains linoleic acid, oleic acid, palmitic acid, tocopherol, and beta-carotene, which can speed up wound healing by reducing inflammation and promoting the formation of new blood vessels, collagen growth, and re-epithelialization 8–10. Emulgel is one of the dose forms designed for RFO. Emulgel is created by dissolving an O/W emulsion in a gel base. RFO is used in the production of emulgels because it can hold hydrophobic medications in a water-soluble gel base, does not feel sticky or oily, and is easily washed 11. Because it is non-toxic and non-irritating to the skin, carbopol 940 is used as a gel base 12.
Physical properties of topical preparations that affect medication transport into the skin include organoleptic, pH, dispersibility, adhesion, and homogeneity. The choice of an appropriate formulation has a significant impact on the achievement of therapeutic goals 13. Other factors, such as the stability of pharmaceutical preparations, are also important. Because the product’s viscosity, homogeneity, scent, physical appearance, and attributes are the same as when a new sample was generated, stability is a parameter indicating that the product will survive within a specified range during storage and use. Because pharmaceutical preparations are mass-produced and subjected to temperature, shock, and storage for extended periods before reaching customers. The presence of active chemicals can affect the physical stability of pharmacological formulations 14. The purpose of this study is to see if differences in red fruit oil concentrations of 5% w/v, 10% w/v, and 15% w/v have an influence on the physical properties and physical stability of the red RFO emulgel produced.
Materials
Red fruit oil (Pandanus conoideus lam.) was obtained from CV Made Mulya under the trademark “Planta Sehat Sari Buah Merah”, aquadest, carbopol 940, triethanolamine (TEA), propylene glycol, propyl paraben, methyl paraben, butylatedhydroxytoluene (BHT), and tween, were purchased from Merck (Darmstadt, Germany).
Methods
Production of RFO Emulgel
In the first step, a gel basis was created by dissolving carbopol 940 in distilled water and homogenizing it at 6000 rpm for two minutes. Allow 15 minutes for the complete expansion of carbopol 940. After 15 minutes, the gel base was added TEA and stirred with a stirring rod until a clear and thick gel base formed. The aqueous phase is then combined, which consists of distilled water, propylene glycol, propylparaben, methylparaben, and tween 80. In the oil phase, RFO, BHT, and Span 80 are mixed together. Both phases are heated to 70-75 degrees Celsius. After reaching the point of melting, the oil phase was injected into the aqueous phase and homogenized for 15 minutes at 6000 rpm. The mortar and stamper were then heated using heated aquadest. Using a tissue, dry the mortar and stamper after heating them. The gel base was briefly ground in the mortar, and then the emulsion was slowly added while the mixture was stirred very hard. A thick and homogenous emulsion is generated once the gel basis and emulsion have been homogeneously spread. After that, the final emulgel is kept in a firmly sealed container. The composition of the ingredients can be seen in Table 1 15.
Table 1: Formula of RFO Emulgel
| Ingredient | Function | Formula (% w/v) | Range Concentration (%)13 | ||
| F1 | F2 | F3 | |||
| RFO | Active ingredients | 5 | 10 | 15 | |
| Carbopol 940 | Gelling agent | 1 | 1 | 1 | 0.5–2.0 |
| TEA | Alkalizer | 1 | 1 | 1 | |
| Tween 80 | Emulsifier | 4,53 | 4,53 | 4,53 | 1–15 |
| Span 80 | Emulsifier | 0,46 | 0,467 | 0,46 | 1–15 |
| Propylene glycol | Enhancer | 10 | 10 | 10 | 5–80 |
| Methyl paraben | Preservative | 0,18 | 0,18 | 0,18 | 0.02–0.3 |
| Propyl paraben | Preservative | 0,02 | 0,02 | 0,02 | 0.01–0.6 |
| BHT | Antioxidant | 0,02 | 0,02 | 0,02 | 0,0075–0,1 |
| Aquadest | Gel base | 40 | 40 | 40 | |
| Emulsion | 37,78 | 32,78 | 27,78 | ||
Organoleptic test
Observed color, odor (rancid or not), and the physical appearance of the emulgel 16.
Homogeneity test
0.10 g of emulgel were placed between two slides and observed. It was observed that the emulgel preparation contained coarse particles and was homogeneous. Emulgel must look the same on the outside, with no lumps of fillers or poorly mixed active ingredients 16.
pH test
Using a pH meter, by mixing 10 mL of distilled water with 1 g of the sample 16.
Spreadability test
The middle position of the glass was filled with 0.50 g of emulgel, which was then covered with another glass and left for 1 minute. After one minute, 50 g of weight were added. Every minute, 50 to 200 g were added 16.
Adhesion test
Within 5 minutes, 1 g of emulgel is loaded with 500 g of water. The weight is then raised, and the load on the adhesion test equipment (60 g) is released, and the time when the two slides are released 16.
Physical Stability Test
Storage test
The emulgel was stored for 28 days at room temperature (30ºC±2ºC). The evaluations carried out were organoleptic tests, homogeneity, pH, dispersion, and adhesion 16.
Cycling test
After 24 hours at 4ºC in the refrigerator, the emulgel was transferred to a 40ºC oven for 24 hours. These two storages were considered one cycle. After six cycles, the smell of the emulgel was checked to see if there was phase separation or crystallization 17.
Mechanical test
Fill a centrifuge tube with 10 mL of emulgel. The emulgel was then centrifuged at 3800 rpm for 5 hours. The phase separation is expressed in the value of F, i.e., (initial emulgel height-upper phase separation height)/initial eulgel height. The closer the F value to 1, the more stable the emulgel is 18.
Data analysis
The results of the descriptive analysis included homogeneity and organoleptic tests after manufacturing F1, F2, and F3 on physical properties tests and after 28 days of storage at room temperature (30ºC±2ºC) and day 28 of storage at room temperature (30ºC±2ºC) and mechanical test results. After 28 days of storage at room temperature (30ºC±2ºC), SPSS analysis was conducted on pH, dispersibility, and adhesion physical property tests. utilized Pearson’s product moment correlation analysis. The analysis of Simple Linear Regression will be used to keep going with data that has been checked for correlation and shows that the independent and dependent variables are related in a significant or significant way.
Because RFO active ingredient contains a high proportion of unsaturated fat, it is easily oxidized. is therefore added as an antioxidant. In addition, mixing emulsions and gels is a crucial step in the production of emulgels, so vigorous and constant stirring is required. The optimal stirring speed and time will yield a homogenous, high-quality emulgel preparation 19. When the gel base and emulsion are mixed together well, they make a thick, even emulsion (Figure 1).
 |
Figure 1: (F1 with 5% RFO; F2 with 10% RFO; F3 with 15% RFO) |
Evaluation of the Physical Properties
Organoleptic test
The color of F1, F2, and F3 orange-red. However, the addition of RFO concentration to the emulgel had no discernible effect on its color. In theory, increasing the RFO content will impart more color to the emulgel. This is because RFO is an extremely dark red color. This result is likely due to the fact that carbopol 940, when used as a gel, produces a transparent gel. Thus, the addition of emulgel concentration did not significantly alter the color.
Homogeneity test
All of the F1, F2, and F3 emulgels were homogeneous according to the homogeneity test results. This demonstrates that all of the emulgels have been thoroughly blended.When applied to the skin, homogeneous topical medicines will feel comfortable.
pH test
According to Pearson’s analysis, the strength of the correlation between concentration and pH was moderate (0.46) and not significant (p-value = 0.21), with a negative correlation direction. This indicates that increasing the emulgel concentration does not affect the pH of the finished emulgel. The linear regression test is not performed since the correlation test has no effect. Because RFO has an acidic pH of 5.24, adding RFO concentration to the emulgel can theoretically lower the pH of the emulgel 20. The pH values of all three formulas are still between 4.2 and 6.5, which is a good range for a topical treatment 14.
Spreadability test
The Pearson test revealed a strong (0.95) and statistically significant (p<0.00) inverse relationship between RFO concentration and dispersion, indicating that increasing concentration decreases the dispersion of the resulting emulgel. Figure 2’s linear regression equation indicates that adding 1 percent RFO can reduce emulgel dispersion by 0.07 centimeters. The addition of RFO concentration, which can inhibit spreadability, is motivated by the viscosity factor. There is a direct relationship between dispersion and viscosity; the greater the viscosity, the smaller the dispersion 21. This is because the higher the viscosity of a substance, the greater its ability to prevent its spread across a surface. All RFO emulgel formulations met the criteria for effective topical preparation, namely a thickness of between 5 and 7 centimeters 22.
Adhesion test
The positive correlation between RFO concentration and adherence was strong (0.76) and significant (p-value = 0.03), indicating that adding RFO concentration boosted RFO emulgel adhesion significantly. Figure 2 shows the equations derived in the linear regression test, which reveals that adding 1% RFO concentration increases adhesive power by 0.06 seconds. This is because the viscosity and cohesive forces increase as the concentration rises. As a result, the more viscous a liquid is, the more difficult it is for the liquid’s molecules to separate. Still, all of the RFO emulgel formulas (F1, F2, and F3) met the requirements for correct topical dosing, which was more than 1 second.
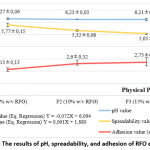 |
Figure 2: The results of pH, spreadability, and adhesion of RFO emulgel |
Physical Stability Test
Storage test
The three emulgel formulations (F1, F2, and F3) were uniform, reddish-orange, smelled of RFO but were not rancid, and did not separate. The results of the organoleptic and homogeneity tests revealed that the concentration variation of RFO did not change during storage. After performing the correlation test, the pH test revealed that the concentration of RFO and pH had a very strong (0.92) and statistically significant (p<0.00) negative correlation. This means that, statistically, increasing the concentration will result in a significant decrease in the pH of the emulgel after storage. Using the regression equation, Figure 3 demonstrates that a 1 percent increase in RFO concentration decreases the pH value by 0.009 after the storage test. RFO contains a multitude of different unsaturated fatty acids with double bonds. The presence of double bonds in fatty acids weakens the C-H bonds between adjacent C atoms. Consequently, it is easier to remove the H atom from the C-H bond 23. The liberated H+ ions raise the pH of the solution after oxidation, making it more acidic. Due to its high RFO, Emulgel F3 has the lowest pH and is the most oxidized. The pH of the three RFO emulgel formulations was measured after they had been stored, and it was found to be within the range for a good topical preparation 14.
The correlation test revealed that the fluctuations in RFO concentration and dispersion were highly correlated (0.92), significant (p<0.00), and negative. A strong and significant correlation exists between an increase in RFO concentration and a decrease in emulgelspreadability. The addition of 1% RFO reduced emulgel dispersion by 0.062 cm after storage, according to the dispersion regression equation (Figure 3). Consistent with the results of a study that investigated the effect of temperature and concentration on viscosity, these findings support the hypothesis. Increasing concentration has a greater impact on viscosity than increasing temperature 24. This is due to the fact that the addition of concentration increases particle contact regardless of whether the temperature is altered. Following storage, Emulgel F1, F2, and F3 still met the 5-7 cm dispersion standards.
The Pearson test indicates that the effect of RFO concentration on adhesion is positively correlated (0.85) and statistically significant (p<0.001) in a positive direction. This suggests that a statistically significant and significant correlation exists between an increase in RFO concentration and an increase in emulgel stickiness after storage. According to the adhesion regression test equation, adding 1% RFO to the emulgel can increase its adhesiveness by 0.081 seconds after storage (Figure 3). Adhesion is inversely proportional to spreadability, and the adhesion result supports the spreadability result. Emulgel F1, F2, and F3 met the requirements for topical preparations, which were that they stick to the skin for more than one second.
The F1, F2, and F3 emulgels were reddish-orange in color, homogeneous, and separation-free prior to the cycling test. Theoretically, the cycle test treatment on a preparation should affect the rate of oxidation in the preparation. This is as a result of the influence of high temperatures (40 ºC), one of the factors that contribute to oxidation. After the cycling test of emulgels F1, F2, and F3 revealed the presence of dew on the tube wall, the emulgel in the bottle remained reddish-orange, possessed a flavor characteristic of RFO, was homogeneous, and did not separate. This demonstrates that the three emulgel formulations are stable and have not undergone oxidation, making them suitable for topical treatment. Dangerous to the body, substances have negative effects on the user 25. The instability of pharmacological preparations can diminish the efficacy of drugs.
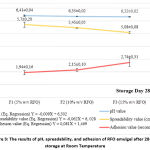 |
Figure 3: The results of pH, spreadability, and adhesion of RFO emulgel after 28-day storage at Room Temperature |
Mechanical test
The mechanical tests showed that the RFO F1, F2, and F3 emulgels did not separate into phases. Calculations to get F emulgel RFO values of F1, F2, and F3 were then performed. The F value of the RFO emulgel F1, F2, and F3 was 1 since there was no phase separation in the RFO emulgel following the mechanical test. Centrifugation for 5 hours at 3800 rpm was equivalent to the gravitational force created by a year of storage. Emulsion stability is a situation in which the dispersing phase’s molecular size is uniform and the dispersed phase has a suitable conformation. When the forces of attraction and repulsion between the phases in an emulgel system are equal and the density difference between the two phases is high, the particles in the emulgel system won’t stick together. This makes the system more stable 26.
Conclusion
There is an effect of variations in red fruit oil with concentrations of 5% w/v (F1), 10% w/v (F2), and 15% w/v (F3) on the physical properties of the red fruit oil emulgel. The greater the concentration of RFO, the lower the spreadability and the greater the adhesion. Meanwhile, the organoleptic results, homogeneity, and pH showed no significant effect on variations in the concentration of RFO emulgel. The concentration of RFO also affects the physical stability of the emulgel. The increase in the concentration of RFO increased the pH, decreased the dispersion and increased the adhesion significantly after the stability test was carried out at room temperature (30ºC±2ºC) for 28 days. Emulgel RFO F1, F2 and F3 did not show any organoleptic differences before and after the cycling test. After performing the mechanical test, all emulgel formulas did not undergo phase separation, so that F = 1.
Acknowledgement
Because of his cooperation in providing red fruit oil, the authors would like to express their gratitude to Drs. I Made Budi, M.Si from Cenderawasih University in Papua particular work.
Conflict of Interest
All authors declare there is no conflict of interest in this manuscript.
References
- Handayani LT. Studi meta analisis perawatan luka kaki diabetes dengan modern dressing. Theindonesian journal of health science, 2016;6(2).
- Guo SA, DiPietro LA. Factors affecting wound healing. Journal of dental research, 2010;89(3):219-229.
CrossRef - Avisha VBN, Zakiyah R, Andriana D. Perbandingan Efek Perasan Lidah Buaya (Aloe vera) dengan Povidone Iodine 10% terhadap Jumlah Sel Neutrofil Darah dan Jaringan Kulit Luka Sayat Punggung Tikus Wistar Jantan. Jurnal Kedokteran Komunitas, 2019;7(1).
- Ali IR, Lestari RD, Andriana D. Perbandingan Efek Perasan Lidah Buaya (Aloe vera) dengan Povidone Iodine Terhadap Kadar Superoxid Dismutase dan Malon dialdehid Serum Tikus Wistar dengan Luka Sayat. Jurnal Kedokteran Komunitas, 2020;8(1).
- Piromchai P. Ototoxicity of povidone-iodine – A case report. J Otol, 2019; 14(1):30-32.
CrossRef - Ningsih IY. Studi Etno farmasi Penggunaan Tumbuhan Obat oleh Suku Tengger di Kabupaten Lumajang dan Malang, JawaTimur. Pharmacy: Jurnal Farmasi Indonesia (Pharmaceutical Journal of Indonesia), 2016;13(1).
- Rohman A, Windarsih A. Characterization, biological activities, and authentication of red fruit (Pandanus conoideus Lam) oil. Food Research, 2017;2(2)
CrossRef - Lin TS, AbdLatiff A. Abd Hamid NA, and Mazlan Evaluation of Topical Tocopherol Cream in Cutaneous Wound Healing in Streptozotocin-Induced Diabetic Rats. Evidence-Based Complementary and Alternative Medicine, 2012;49(1).
CrossRef - Rodrigues HG, VinoloMA, Sato FT, Magdalon J, Kuhl CM, Yamagata AS, et al. Oral Administration of Linoleic Acid Induces New Vessel Formation and Improves Skin Wound Healing in Diabetic Rats. PloS one, 2016;11(10).
CrossRef - Polcz ME, Barbul A. The Role of Vitamin A in Wound Healing. Nutrition in Clinical Practice, 2019;34(5).
CrossRef - Yadav SK, Mishra MK, Tiwari A, Shukla A. Emulgel: a New Approach for Enhanced Topical Drug Delivery. Int J Curr Pharm Res, 2016;9(1).
CrossRef - Safitri FI, Nawangsari D, Febrina D. Overview: Application of Carbopol 940 in Gel. International Conference on Health and Medical Sciences (AHMS 2020), 2021: 80-84
CrossRef - Ali NW, Yamlean PVY. Pengaruh Perbedaan Tipe Basis Terhadap Sifat Fisik Sediaan Salep Ekstrak Etanol Daun Tapak Kuda (Ipomoea Pescaprae (L) Sweet). Pharmacon, 2015;4(3).
- Dewi DRN, Zakkia LU, Khoiruddin W,Harismah K. Pengaruh pH Terhadap Lamanya Penyimpanan Sediaan Esktrak Daun Seligi dan Eugenol dari Minyak Daun Cengkeh Sebagai Obat Anti In: Prosiding SNST Fakultas Teknik Universitas Wahid Hasyim, 2018: 97-100.
- Rowe RC, Sheske P, Quinn M. Handbook of Pharmaceutical Excipients. New York: Libros Digitales- Pharmaceutical Press,
- Chavda V, Rupapara V. Formulation and Evaluation of Naproxen Emulgel for topical delivery by a modified method. Int J Compr Pharm, 2013;4(07):1-4.
- Arianto A, Lie DYL, Bangun HB. Preparation and evaluation of nanoemulgels containing a combination of grape seed oil and anisotriazine as sunscreen. Open Access Macedonian Journal of Medical Sciences, 2020;8(B):994-999.
CrossRef - Baskara IBB, Suhendra L, Wrasiati LP. Pengaruh Suhu Pencampuran dan Lama Pengadukan terhadap Karakteristik Sediaan Krim. Jurnal Rekayasa dan Manajemen Agroindustri,
CrossRef
- Lestari ABS. The Optimization of Mixing Rate and Mixing Time in the Formulation of Green Tea Extract Jurnal Ilmu Kefarmasian Indonesia, 2012;10(2).
- Febrina E, Gozali D, Rusdiana T. Formulasi Sediaan Emulsi Buah Merah (Pandanus conoideus ) sebagai Produk Antioksidan Alami. Penelitian Peneliti Muda-UNPAD dalam Lembaga Penelitian Muda-UNPAD. Bandung. 2007.
- Nailufa Y. Formulasidan Evaluasi Gel Hand Sanitizer dengan Moisturizer Alga Hijau (Spirulina platensis) dan Vitamin E. Jurnal Syntax Idea, 2020;2(6).
- Dominica D,Handayani D. Formulasi dan Evaluasi Sediaan Lotion dari Ekstrak Daun Lengkeng (Dimocarpus Longan) sebagai Jurnal Farmasi dan Ilmu Kefarmasian Indonesia, 2019;6(1).
CrossRef - Simamora A. Efek Tokoferol pada Peroksida Lipid. Meditek, 2003;11(28).
- Ghanavati G, Shojaei MJ, Ramazani ASA. Effects of asphaltene content and temperature on viscosity of Iranian heavy crude oil: Experimental and modeling study. Energy Fuels, 2013;27(12).
CrossRef - Putri MA. Stabilitas Fisika dan pH Mikroemulsi dan Gel Mikroemulsi Magnesium Ascorbyl Phospate dan Tocopherol Acetate dan Rice Bran Oil menggunakan Konsentrasi Surfaktan Non Ionik dan Kosurfaktan Sebesar 27% dengan Perbandingan 12: 1. Jurnal Ilmiah Mahasiswa Universitas Surabaya, 2016;5(1).
- Sarungallo ZL, Hariyadi P, Andarwulan N,Purnomo EH. Characterization of Chemical Properties, Lipid Profile, Total Phenol A and Tocopherol Content of Oils Extracted from Nine Clones of Red Fruit (Pandanusconoideus). Agriculture and Natural Resources, 2021;49(2)








