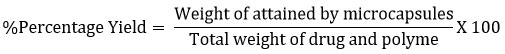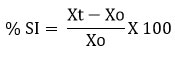Gorantla Naresh Babu 1, 3 , Menaka Muthukarupan1
, Menaka Muthukarupan1 , Hindustan Abdul Ahad2*
, Hindustan Abdul Ahad2* , Veerabomma Sreedhar3
, Veerabomma Sreedhar3
1Department of Pharmacy, Annamalai University, Annamalai Nagar-608002, Tamil Nadu, India.
2Department of Industrial Pharmacy, Raghavendra Institute of Pharmaceutical Education and Research (RIPER) - Autonomous, Ananthapuramu-515721, Andhra Pradesh, India.
3Department of Industrial Pharmacy, Balaji College of Pharmacy, Ananthapuramu-515721, Andhra Pradesh, India.
Corresponding Author E-mail: andulhindustan@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2554
Abstract
In this study, we investigated the mucoadhesive properties of neem fruit mucilage by incorporating it into mucoadhesive microcapsules with Acyclovir (ACR). Methpol-934P and Neem fruit mucilage (NFM) was used to construct 12 different mucoadhesive microcapsules. We assessed FTIR and DSC capabilities for compatibility with ACR and NFM. ACR mucoadhesive microcapsules (ANMM) were characterized for mucoadhesion and ACR release Physico-chemical characteristics. CR was found to be compatible with NFM in the research. The entrapment increased as the levels of NFM in the formulations increased, and mucoadhesion time was longer in formulations with higher levels of NFM. As levels of NFM increase in formulations, the release of drugs is slightly reduced. NFM may be responsible for this due to its release retarding properties. An additive of neem fruit mucilage allowed for the retention of ACR after ingestion when a mucoadhesive polymer (methpol 934P) was used.
Keywords
Acyclovir; anti-viral; Mucoadhesive; Microcapsules; Neem
Download this article as:| Copy the following to cite this article: Babu G. N, Muthukarupan M, Ahad H. A, Sreedhar V. Fabrication and Preliminary Assessment of Neem Fruit Mucilage as Mucoadhesive Abetting Assets with Methpol-934P for Acyclovir Delivery from Mucoadhesive Microcapsules. Biomed Pharmacol J 2022;15(4) |
| Copy the following to cite this URL: Babu G. N, Muthukarupan M, Ahad H. A, Sreedhar V. Fabrication and Preliminary Assessment of Neem Fruit Mucilage as Mucoadhesive Abetting Assets with Methpol-934P for Acyclovir Delivery from Mucoadhesive Microcapsules. Biomed Pharmacol J 2022;15(4). Available from: https://bit.ly/3TqLpw6 |
Introduction
A unique attitude is emerging for drug administration to increase the gastric availability of drugs with patient consent. Among the various dosage forms, gastro retentive microcapsules are of special concern as they can be easily prepared and administered1, 2.
Herpes simplex, herpes zoster, and chicken pox are all contagions treated with acyclovir, a purine nucleoside analogue3, 4. Approximately 15-30% of Acyclovir is bioavailable orally. Acyclovir has a half-life5 of ~2 h. After giving the medication orally, the medication is well absorbed into the stomach.
A mucoadhesive system’s effectiveness is significantly affected by the polymer used. Many patients prefer oral drug administration due to its convenience. Many polymers have been tried for mucoadhesive drives, which are rare and expensive. An ideal polymer would aid mucoadhesion and be derived from nature. The authors plan to examine mucoadhesive microcapsules using neem fruit mucilage (NFM). The antiviral properties of NFM have been demonstrated in studies6, 7. NFM may be helpful in antiviral therapy. In expanded time, ANMM aims to achieve steady-state availability. As they are designed for ease, precision liberation systems are an effective solution for short-acting drugs and those that require incessant medicating.
Materials and Methods
Materials
Acyclovir (ACR) was from Innovative Pharmaceuticals, Hyderabad, Telangana. Methpol 934P, and dichloromethane were from Merck, Hyderabad.
Extraction of mucilage
Depletion of expression was defined8 by Ahad et al., 2021. After washing and removing the outer layer of the neem fruits, they were soaked in water, boiled for an hour, and cooled. The seeds were separated by using ethyl acetate, butanol, and petroleum ether (50%). Using a multilayered muslin cloth bag, the marc was extracted from the mucilage. The mucilage was parted, parched in an oven (ACM-22066) at 40°C, poised, ground, allowed to pass over a # 80 sieve (MSW Labs) and stored in a desiccator (Tarson 402020) at 30°C and 45% RH.
Cleansing of the Mucilage
The NFM was homogenised (APV-1000) with 5% trichloroacetic acid, centrifuged (Systonic S-103), neutralized with NaOH, and dialyzed (SpectraFlo) neutralized with distilled water, as defined by Hindustan et al. 2009. As the last step, ethanol (95%) was treated with the precipitate and scrubbed with acetone9.
Table 1: Composition of the ANMM
| Component | Formulations | |||||||||||
| F-1 | F-2 | F-3 | F-4 | F-5 | F-6 | F-7 | F-8 | F-9 | F-10 | F-11 | F-12 | |
| Acyclovir (mg) | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 |
| Ethyl Cellulose (mg) | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
| NFM (mg) | 25 | 50 | 75 | 100 | 125 | 150 | 175 | 200 | 225 | 250 | 275 | 300 |
| Methpol 934P (mg) | 50 | 75 | 100 | 50 | 75 | 100 | 50 | 75 | 100 | 50 | 75 | 100 |
| Dichloromethane (ml) | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 |
| Span 80 (minims) | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Glutaraldehyde (minims) | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Liquid paraffin (ml) | 300 | 300 | 300 | 300 | 300 | 300 | 300 | 300 | 300 | 300 | 300 | 300 |
Preparation of ANMM
Dichloromethane and acetic acid (2% v/v) were used in the blending of Methpol 934P, ACR, EC, and NFM. Continuous stirring of this mixture into liquid paraffin (containing span 80) was achieved using a three-bladed propeller stirrer (IKA-R1381). We added glutaraldehyde at a rate of 4 drops per minute dropwise to the sample as part of this procedure. Three hours of stirring followed. To remove liquid paraffin, the Acyclovir-Neem mucoadhesive microcapsules (ANMM) were centrifuged and washed with petroleum ether. For the removal of residual glutaraldehyde, an ANMM was suspended in a 5% sodium bisulfite solution for 15 min. After washing with distilled water, the last step was to dry the clothes. Desiccators were used to dry and store the ANMM10-12.
Evaluation parameters
Drug excipient compatibility studies
DSC studies
In a mini pan of DSC, ACR and NFM were mixed in a 1:1 ratio (10 mg) and scanned from 50 to 300°C (Venchal Scientific-412105-USA).
FTIR studies
ACR and NFM were examined using FTIR spectroscopy (Bruker) by scanning at a 4000-400 cm−1 array.
Evaluation of physical properties
Measurement of particle size
ANMM particles were measured using a stage micrometer. Dry ANMM were measured with an eyepiece micrometre on a hygienic glass slide13. At least 100 ANMM were counted per batch.
Production yield
In each of the three trials, the average weight of parched ANMM (W1) recovered from each trial was compared14 to the total of the initial dry weight (W2).
Entrapment Efficiency
100 mg of ANMM were dispersed in 0.1 M HCl overnight with erratic quivering. The mixture was filtered at 254 nm, and the filtrate was analyzed spectrophotometrically (Elico Spectrophotometer, SL-174). An evaluation of entrapment efficiency was based on the ratio between the actual amount of drug in the formulation and the amount initially added15.
Measurement of swelling
An HCl solution of 0.1M was used to swell ANMM. As determined from the following equation16, the weight gain was determined by the difference between the weight gained at time t (Xt) and the beginning time (t = 0 [X0]).
Where Xt-weight of the ANMM after time t; Xo- Initial weight of the ANMM
Mucoadhesion Measurement Study
It was determined that the mucoadhesive time (MT) of a fresh 5 cm piece of sheep stomach gained from a local slaughterhouse within 60 min of the animal’s death and eviscerated by washing with isotonic saline. The mucosal surface was weighed using ANMM and a polyethene plate at a position of 40° relative to the straight line, which was stationary. Infuse HCl (0.1M) at 37±0.5°C at a rate of 5 ml/min, simulating 37±0.5°C. By visual inspection, we measured how long it takes to remove all ANMM from the mucosal surface of the sheep’s stomach17-19.
In Vitro ACR Release Study
As a dissolution medium, 900 ml of HCl (0.1N HCl) was used in the USP-II apparatus at a stirring rate of 50±5 rpm at a temperature of 37±0.5°C. At different breaks, a 5 ml sample was introverted, and the dissolution media were replenished. Spectrophotometric measurements were then performed at 254nm on the samples20-23.
Results and discussion
In addition to evaluating the congeniality of the fresh NFM, its colour was yellowish green, which assists ANMM.
Compatibility studies
Based on the DSC assessment, the pure ACR produced a sharp endothermic peak, indicating its purity. Combining this peak with left-shifting excipients broadens it. DSC observations indicate that ACR does not interact with the excipients.
The ACR pure-form spectrum exhibits FTIR bands for secondary amines, phenyl esters, and carboxylic groups. Blend (F-5) had peaks and stretches like pure drugs. Excipients did not obstruct peaks or stretches of the spectrum of ACR.
Physical properties
Particle size
Optical microscopy was used to fix particle size for all formulations. ANMM array sizes range from 33.92±0.7 to 39.6±0.8 µm (Figure 1), with F-8 having the largest particle size.
Yield of ANMM
ANMM yielded 80.5±2.3 to 94.4±1.7, with F-2 to F-6 showing a maximum (Figure 1).
In vitro drug release
Analyses were conducted to determine whether ANMM dissolves. As NFM shows more binding properties and releases retarding properties, formulations with 50 to 100 mg of NFM show good drug release, whereas formulations with F-5 to F-12 show suppressed drug release.
Swelling Measurement
By analyzing the pattern of ACR release, the ANMM can be used to determine the extent of mucoadhesion. When preparing any mucoadhesive formulation, this study should be considered. Different concentrations of NFM were tested on the swelling indices of the ANMM. The swelling index dropped steadily as the concentration of NFM decreased, and batch F-12 was the most swollen. Several polar compounds are present in NFM, which enable it to absorb and hold water, as well as have swelling assets.
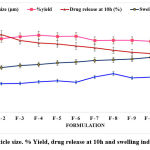 |
Figure 1: Particle size. % Yield, drug release at 10h and swelling index of ANMM |
% Drug entrapment
Entrapment of ANMM ranged from 65.9±1.27 to 79.6±0.98 and formulations with 125mg of NFM gave good entrapment of ACR (Figure 2).
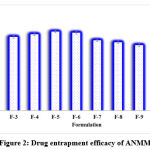 |
Figure 2: Drug entrapment efficacy of ANMM |
In vitro mucoadhesion time
All batches of ANMM had mucoadhesions ranging from 9.2±0.08g (F-1) to 12.4±0.16g (F-12) (Figure 3). The mucoadhesion time was considered to increase with a higher NFM content and higher levels of methpol 934 P.
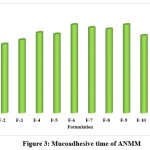 |
Figure 3: Mucoadhesive time of ANMM |
ACR estimation
A UV-VIS spectrometer was used to obtain a calibration curve for ACR estimation in 0.1M HCl solution at 254 nm λmax. According to Beer’s law, the calibration curve ranged from 0-10 µg/ml (repeated three times). Such data can be used to determine the uniformity of content.
Conclusion
Acyclovir (ACR) is released from mucoadhesive polymers at a rate and quantity controlled by the polymers. The ACR is released from the stomach after the microcapsules are digested. To formulate ACR, Neem (Azadirachta indica) fruit mucilage (NFM) combined with methpol 934 P was used. NFM was proven to have antiviral activity in addition to mucoadhesive assets. As earlier studied by Shiraki et al., 2021. Compared to other formulations, F-2 to F-4 with fewer NFM (50 to 100 mg) has more entrapped ACR in addition to the mucoadhesion. An additional increase in NFM retards the ACR release and shows sustained release unlike it was observed by Ghumman et al., 2020. Using the results of this study, it can be concluded that the mucoadhesive microcapsules of ACR with NFM aided by methpol 934 P are an effective way to retain the stomach and support increased bioavailability.
Acknowledgement
The authors feel privileged to thank the department of pharmacy, Annamalai University, Annamalai Nagar, Tamil Nadu, India for their support and encouragement.
Conflict of Interests
The authors declare no conflict of interest.
Funding Sources
There is no funding source.
References
- Chinthaginjala H, Gandla CB, Challa MR, Pradeepkumar B, Ahad HA. Formulation and in vitro evaluation of floating tablets of dicloxacillin sodium using different polymers. Journal of Young Pharmacists 11: 247-251 (2019).
CrossRef - Shravani Y, Ahad HA, Haranath C, gari Poojitha B, Rahamathulla S, Rupasree A. Past Decade Work Done On Cubosomes Using Factorial Design: A Fast Track Information for Researchers. Int J Life Sci Pharma Res. 11: 124-235 (2021).
CrossRef - Kłysik K, Pietraszek A, Karewicz A, Nowakowska M. Acyclovir in the treatment of herpes viruses–a review. Current medicinal chemistry. 27: 4118-4137 (2020).
CrossRef - Shiraki K, Yasumoto S, Toyama N, Fukuda H. Amenamevir, a Helicase-Primase Inhibitor, for the Optimal Treatment of Herpes Zoster. Viruses 2021;13:1547. doi: 10.3390/v13081547
CrossRef - Thomsen AE, Christensen MS, Bagger MA, Steffansen B. Acyclovir prodrug for the intestinal di/tri-peptide transporter PEPT1: comparison of in vivo bioavailability in rats and transport in Caco-2 cells. European journal of pharmaceutical sciences. 23: 319-325 (2004).
CrossRef - Amiri MS, Mohammadzadeh V, Yazdi MET, Barani M, Rahdar A, Kyzas GZ. Plant-Based Gums and Mucilages Applications in Pharmacology and Nanomedicine: A Review. 26: 1770-1778 (2021).
CrossRef - Chandakavathe BN, Deshpande DK, Swamy P, Dhadde SB. Assessment of Toothpaste Formulations Containing Turmeric and Neem Extract for Prevention of Dental Caries and Periodontal Diseases. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences. 88:1523-1539 (2018).
CrossRef - Hindustan AA, Babu UA, Nagesh K, Kiran DS, Madhavi KB. Fabrication of glimepiride Datura stramonium leaves mucilage and poly vinyl pyrrolidone sustained release matrix tablets: in vitro evaluation. Kathmandu university journal of science, engineering and technology. 8:63-72 (2012).
CrossRef - Ahad HA, Kumar BP, Haranath C, Reddy KS. Fabrication and evaluation of glimepiride Ficus benghalensis fruit mucilage matrix transdermal patches. International Journal of Chemical Sciences. 7:2294-2298 (2009).
- Li X, Cui K, Guo Z, Yang T, Cao Y, Xiang Y, Chen H, Xi M. Heterogeneous Fenton-like degradation of tetracyclines using porous magnetic chitosan microspheres as an efficient catalyst compared with two preparation methods. Chemical Engineering Journal. 379:122-124 (2020).
CrossRef - Babu GN, Menaka M, Ahad HA. Neem Fruit Mucilage-Aided Mucoadhesive Microspheres of Acyclovir Using 32 Factorial Design With Design-Expert Software. Applied biological research. 24: 17-27 (2022).
CrossRef - Sailaja K, Ahad HA, Chinthaginjala H, Gudisipalli R, Rajyalakshmi SI, Vagganagari Y. Approaches to Creating and Past Successful Attempts on Microspheres: A Primer for Aspiring Researchers. Research Journal of Pharmaceutical Dosage Forms and Technology. 14: 245-248 (2022).
CrossRef - Ahad HA, Sreeramulu J, Bindu VH, Ramyasree P, Padmaja BS, Sravanthi M. Isolation and physicochemical characterization of Ficus reticulata fruit mucilage. International Journal of Green Pharmacy. 5: 825-828 (2011).
CrossRef - Harsha SS, Ahad HA, Haranath C, Dasari RR, Gowthami M, Varam NJ, et al. Exfoliation Technique of Composing and Depictions of Clopidogrel Bisulphate Afloat Microspheres. Journal of Evolution of Medical and Dental Sciences. 9:1156-1161 (2020).
CrossRef - Hindustan AA, Sreeramulu J, Narasimha RD, Guru PP, Ramyasree P. Fabrication and In vitro Evaluation of High density Gastro retentive Microspheres of Famotidine with Synthetic and Natural Polymers. Indian Journal of Pharmaceutical Education and Research. 46: 48-53 (2012).
- Ghumman SA, Noreen S, tul Muntaha S. Linum usitatissimum seed mucilage-alginate mucoadhesive microspheres of metformin HCl: Fabrication, characterization and evaluation. International journal of biological macromolecules. 155:358-368 (2020).
CrossRef - Chinthaginjala H, Abdul H, Reddy APG, Kodi K, Manchikanti SP, Pasam D. Nanosuspension as Promising and Potential Drug Delivery: A Review. Int J Life Sci Pharma Res. 11: 59-66 (2020).
- da Silva JB, Dos Santos RS, da Silva MB, Braga G, Cook MT, Bruschi ML. Interaction between mucoadhesive cellulose derivatives and Pluronic F127: Investigation on the micelle structure and mucoadhesive performance. Materials Science and Engineering. 119: 111-116 (2020).
CrossRef - Kousar S, Ahad HA, Chinthaginjala H, Babafakruddin P, Lakunde J, Tarun K. Gas Generating Floating Tablets: A Quick Literature Review for the Scholars. Asian Journal of Research in Chemistry. 15:171-175 (2022).
CrossRef - Aher B, Jain N, Jain U, Paithankar A, Bagul T, Gaikwad S. Novel Spectrophotometric Estimation of Acyclovir Using Hydrotropic Solubilizing Agent. Innovational Journal of Chemistry. 1:22-32 (2015).
- Liu H, Pan W, Ke P, Dong Y, Ji L. Preparation and evaluation of a novel gastric mucoadhesive sustained-release acyclovir microsphere. Drug development and industrial pharmacy. 36:1098-1105 (2010).
CrossRef - Gusai T, Dhavalkumar M, Soniwala M, Dudhat K, Vasoya J, Chavda J. Formulation and optimization of microsponge-loaded emulgel to improve the transdermal application of acyclovir—a DOE based approach. Drug Delivery and Translational Research. 11:2009-2029 (2021).
CrossRef