Manuscript accepted on :13-10-2022
Published online on: 27-10-2022
Plagiarism Check: Yes
Reviewed by: Dr. Debasis Mitra
Second Review by: Dr. Maysaa Kadhim Al-Malkey
Final Approval by: Dr. Patorn Piromchai
Enas Abd al-Raouf Ammar Semysim
Department of Biology, University of Kufa, Faculty of Science, Najaf, Iraq.
Corresponding Author E-mail: inas.smesim@uokufa.edu.iq
DOI : https://dx.doi.org/10.13005/bpj/2559
Abstract
Curcuma longa L. rhizome extracts have polyphenolic secondary metabolites called curcuminoid and various volatile oils. These compounds exhibit wide spectrum of antibacterial activity. Ethanol and petroleum ether C. longa rhizome extracts were studied for their antibacterial action against two bacteria, Escherichia coli and Staphylococcus aureus. This activity had evaluated by employing Agar Well Diffusion method. Curcuminoid was interpreted by pattern of High Performance Liquid Chromatography (HPLC). The ethanol extract exhibited inhibitory effects against E. coli and S. aureus at concentration 150 mg/ml with diameter of inhibition zone (23.000 ± 0.57735 and 27.000 ± 0.57735mm) respectively. On the contrary, petroleum ether extract had inhibitory effects for E. coli and S. aureus at concentration 150 mg/ml in diameter of inhibition zone (39.000 ± 0.57735 and 41.000 ± 0.57735mm) respectively. Quantitative analysis for the curcuminoid compounds from C. longa rhizome extracts revealed highest curcumin, demethoxycurcumin and bisdemethoxycurcumin (9.12, 5.93 and 23.96 µg /ml) respectively in the extract of petroleum ether. We concluded that the C. longa extracts exhibited inhibitory effects against pathological bacterial growth. The essential oils obtained by petroleum ether extract of C. longa rhizome was more influential inhibition than ethanol extract against E. coli and S. aureus.
Keywords
Curcuma; Curcumin; Diarylheptanoid; Chromatography; Escherichia coli; Staphylococcus aureus
Download this article as:| Copy the following to cite this article: Semysim E. A. R. A. Evaluation of Antibacterial Activity of Extracts of Curcuma Longa L. Rhizome and Estimation of Curcuminoid by HPLC. Biomed Pharmacol J 2022;15(4). |
| Copy the following to cite this URL: Semysim E. A. R. A. Evaluation of Antibacterial Activity of Extracts of Curcuma Longa L. Rhizome and Estimation of Curcuminoid by HPLC. Biomed Pharmacol J 2022;15(4). Available from: https://bit.ly/3TXwuu8 |
Introduction
Curcuma longa L. (C. longa) is an indispensable rhizomatous herb for its ubiquitous usage all across the world as condiment, coloring and cosmetic agent in addition to its medicinal characteristics 1. It is belonged to Family Zingiberaceae. The curcuminoid and volatile oils are the components of C. longa in addition to sugars, proteins and resins 2. The curcuminoid imparts a yellow color and comprises mainly of curcumin (diferuloyl-methane), demethoxycurcumin and bisdemethoxycurcumin while the volatile oils consist of tumerone, atlantone and zingiberone. The curcumin is the major component that responsible for the biological activities of C. longa 3. It is a hydrophobic molecule that dissolved in dimethyl sulfoxide, acetone, ethanol and oils 4. It exhibits wide spectrum activities, such as antibacterial 5, antifungal, antidiabetic 6, anti-inflammatory, antiviral 7, anticancerous, antiallergic 8, antiprotozoal and antioxidant features 9,10. The phenolic compounds including curcuminoid dyes are the most important compounds that responsible for the antioxidant activity 11. Each part of C. longa (such as bulb, leaves, root, barks, peels, etc.) has its own medicinal attributions 12,13. The leaves oil of C. longa exhibits therapeutic, antibacterial, antifungal and cytotoxic properties 14 while the extracts of C. longa roots exhibit an insect repellent and antimicrobial features 15. The powder of the rhizome used to cure gastritis 16 and used externally as an antiseptic 17.
The medicinal plants have been conferred a great attention due to the dilemma of antibiotic resistance that might be emerged during its usage in addition to their fewer potentially harmful effects. Both of these aspects lead to increase the popularity of folk medicine implications in the treatment of various illnesses and searching for newer antibacterial compounds from the plants.
The aim of this study is to assess the antibacterial effectiveness of extracts of C. longa rhizome against example of Gram-positive and Gram-negative bacteria and to estimate the curcuminoid in C. longa rhizome extracts by using High Performance Liquid Chromatography (HPLC).
Materials and Methods
Plant material
longa rhizome (underground stem) was obtained from the local market in Najaf. The plant samples were identified by the taxonomist in department of Botany, Kufa University.
Microbial Strains
Microorganisms: Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) were isolated from patients with infected burn wounds who were admitted to Al-Sader Teaching Hospital in Najaf city.
Preparation of ethanol and petroleum ether extracts
The distilled water used to rinse soil particles out of the rhizomes then left to air dry. By electric grinder powder was made from the dried rhizomes and electric weighing scale used to weighing the powder. Preparation of the extracts accomplished by Soxhlet apparatus with 95% ethanol to obtain ethanol extract and with petroleum ether to obtain essential oil. Then, to remove the solvent, evaporation under reduced pressure by Rotary Evaporator. After that, measuring the weight of the extract 18.
Preparation the extract of phenolic compounds
The extract of phenolic compounds prepared using Reflex Condenser. Fifty grams from C. longa rhizomes powder was taken and put it in a flask volume 1000 ml, 400 ml 2% acetic acid as a solvent was added to it. The extract obtained by Reflex Condenser at 70 0C for 8 hours. Then, filtered out and the obtained solution separated by separator funnel. We added an equal volume of n-propanol. Two layers were resulted, the upper layer contained the phenolic compounds. The Rotary Evaporator accomplished the evaporation of the solvent. The extract was treated with 1% potassium hydroxide alcohol 19.
Antibacterial assay
The extent of antibacterial activity for both C. longa rhizomes extracts, with ethanol and petroleum ether, determined by using Agar Well Diffusion method 20. One of the bacterial suspensions contained Gram-negative bacteria (E. coli) and the other suspension contained Gram-positive bacteria (S. aureus). Each suspension accounted 1x 106CFU/ ml. Pouring Mueller–Hinton Agar (MHA) into Petri dishes (Three Replications for each). Then, spreading the suspension of the bacteria by sterile cotton swabs in the media plates after solidifying. The plates were allowed to dry for 15 minutes. Four wells were made using sterile borer (8 mm) on the MHA plates.
The extract was dissolved in Dimethyl Sulphoxide (DMSO) to prepare different extracts concentrations at (50, 100, 150 mg /ml). We took a volume 100µl for each extract concentration and added it to 3 wells while the 4th well was left as a control for DMSO. To allow diffusion of the extract into the agar we left the plates for 10 minutes at room temperature then incubated for 24 hours at 37 0C. At last, we determined bacterial growth by measuring the diameter of zone of inhibition and expressed it in millimeters (mm) around each well in the plates.
Determination of curcuminoid by HPLC
HPLC operating conditions:The curcuminoid were estimated by HPLC according to method that mentioned by Wichitnithad et al 21. HPLC analysis was performed using a Shimadzu system that consist of CBA-20A system controller, an LC-6AD, VP pump, DGU-20A3R degasser, SPD-M20A Photo diode array detector, Lab solution software and C-18 column (250 × 4.6 mm).
Reverse-phase HPLC: It comprises isocratic system with 2.0 ml/min flow rate, 35°C column temperature, acetonitrile mobile phase and 2% acetic acid 40:60 (v /v). The injected volume was 20 µl. The qualitative identification of the compounds was obtained by matching retention time and UV spectrum (190-900 nm) for each compound, while the quantitative measurement was obtained by calculating the peak area for each compound at a wavelength of 425 nm.
Statistical analysis
The results were interpreted by T-test according to (GraphPad Prism, version 9) software. The mean and standard error (SE) for each value was determined. P value less than 0.05 was considered as statistically significant.
Results
Antibacterial activity: The ethanol extract of C. longa rhizomes exhibited growth inhibition for both E. coli and S. aureus bacteria at high concentration (150 mg/ml) with inhibition zone diameter (23.000 ± 0.57735 and 27.000 ± 0.57735 mm) respectively, while at low concentration (50 mg/ml) the inhibition zone diameters were (18.333 ± 0.33333 mm) against E. coli and (19.000 ± 0.57735 mm) against S. aureus, Table 1 and Figure 1.
Table 1: Antibacterial activity of C. longa extracted with ethanol against two types of aerobic pathogenic bacteria.
| Bacteria | Diameter of inhibition zone (mm) | |||||
| At 50 mg/ml
(Mean±SE) |
P value | At 100 mg/ml
(Mean±SE) |
P value | At 150 mg/ml
(Mean±SE) |
P value | |
| E. coli | 18.333 ± 0.33333 | 0.3739
|
21.000 ± 0.57735 | 0.0053**
|
23.000 ± 0.57735 | 0.0080**
|
| S. aureus | 19.000 ± 0.57735 | 24.667 ± 0.33333 | 27.000 ± 0.57735 | |||
Note: * Statistically significant p value ie. p ≤ 0.05.
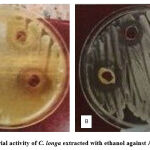 |
Figure 1: Antibacterial activity of C. longa extracted with ethanol against A. E. coli B. S. aureus. |
The petroleum ether extract of C. longa rhizome gave more achievable inhibition for growth of both E. coli and S. aureus in this study. Their inhibition zones diameters were (39.000 ± 0.57735 and 41.000 ± 0.57735 mm) respectively at concentration 150 mg/ml. On the contrary, the concentration (50 mg/ml) of petroleum ether extract attained inhibition zone diameter (20.333 ± 0.33333 mm) for E. coli and (29.000 ± 0.57735 mm) for S. aureus, Table 2 and Figure 2.
Table 2: Antibacterial activity of C. longa extracted with petroleum ether against two types of aerobic pathogenic bacteria.
| Bacteria | Diameter of inhibition zone (mm) | |||||
| At 50 mg/ml
(Mean±SE) |
P value | At 100 mg/ml
(Mean±SE) |
P value | At 150 mg/ml
(Mean±SE) |
P value | |
| E. coli | 20.333 ± 0.33333 | 0.0002***
|
37.000 ± 0.57735 | 0.0668
|
39.000 ± 0.57735 | 0.0705
|
| S. aureus | 29.000 ± 0.57735 | 35.333 ± 0.33333 | 41.000 ± 0.57735 | |||
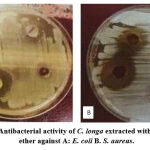 |
Figure 2: Antibacterial activity of C. longa extracted with petroleum ether against A: E. coli B. S. aureus. |
In comparison between ethanol and petroleum ether as antibacterial solvents of C. longa rhizome against E. coli and S. aureus the petroleum ether solvent showed more effective antibacterial activity than an ethanol solvent. The diameter of the inhibition zone for E. coli at the petroleum ether solvent was (39.000 ± 0.57735 mm) in (150 mg/ml) concentration, while the diameter of the inhibition zone of the ethanol solvent was (23.000 ± 0.57735 mm) at the same concentration. In the (50 mg/ml) concentration, the diameter of the inhibition zone for E. coli was (20.333 ± 0.33333mm) for petroleum ether solvent, while in ethanol solvent was (18.333 ± 0.33333 mm). The maximum value of the diameter of the inhibition zone for S. aureus was (41.000 ± 0.57735 mm) at (150 mg/ ml) concentration for petroleum ether solvent, while the minimum value was (19.000 ± 0.57735 mm) at (50 mg/ml) concentration for ethanol solvent, Table 3.
Table 3: Comparison between ethanol and petroleum ether as a solvents of C. longa in antibacterial activity against two types of aerobic pathogenic bacteria.
| Bacteria | Solvent | Diameter of inhibition zone (mm) | |||||
| At 50 mg/ml
(Mean±SE) |
P value | At 100 mg/ml
(Mean±SE) |
P value | At 150 mg/ml
(Mean±SE) |
P value | ||
| E. coli | Ethanol | 18.333 ± 0.33333 | 0.0132*
|
21.000 ± 0.57735 | < 0.0001***
|
23.000 ± 0.57735 | < 0.0001***
|
| Petroleum ether | 20.333 ± 0.33333 | 37.000 ± 0.57735 | 39.000 ± 0.57735 | ||||
| S. aureus | Ethanol | 19.000 ± 0.57735 | 0.0003*** | 24.667 ± 0.33333 | < 0.0001*** | 27.000 ± 0.57735 | < 0.0001*** |
| Petroleum ether | 29.000 ± 0.57735 | 35.333 ± 0.33333 | 41.000 ± 0.57735 | ||||
Estimation of curcuminoid by HPLC: The presence of curcuminoid and phenolic compounds in C. longa rhizome extracts of ethanol and petroleum ether were estimated by HPLC, a C-18 column at flow rate of 2.0 ml/min and detection at a wavelength of 425 nm, a mobile phase of acetonitrile and 2% acetic acid (40:60 v/v).
Curcuminoid compounds identified by retention time and UV spectrum (190-900 nm) for each compound. Figures 3,4,5 and Tables 4, 5, 6 demonstrate determination of each peak in extract samples and retention time for each compound in comparison with the retention time of the standard, Figure 6 and Table 7. The HPLC analysis revealed three major peaks, the curcumin showed the highest retention time followed by demethoxycurcumin and bisdemethoxycurcumin. For each compound, the peak area was determined to calculate the concentration of compounds in extracted samples using linear regression, Figures 7,8,9 and Tables 8, 10, 12. The curcumin, demethoxycurcumin and bisdemethoxycurcumin yielded the higher concentrations (9.12, 5.93 and 23.96 µg/ml) respectively in petroleum ether extract. The lower concentrations for curcumin, demethoxycurcumin and bisdemethoxycurcumin were (0.21, 0.56 and 3.07 µg/ml) respectively in ethanol extract, Tables 9, 11, 13.
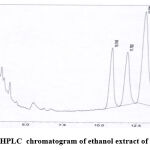 |
Figure 3: HPLC chromatogram of ethanol extract of C. longa. |
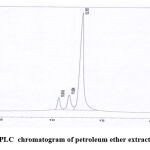 |
Figure 4: HPLC chromatogram of petroleum ether extract of C. longa. |
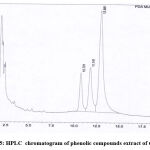 |
Figure 5: HPLC chromatogram of phenolic compounds extract of C. longa. |
Table 4: HPLC analysis of ethanol extract.
| Peak | Retention time (min) | Area | Height | Concentration | Unit | Name |
| 1 | 10.745 | 18792 | 1002 | 0.000 | Bisdesmethoxycurcumin | |
| 2 | 11.782 | 20097 | 886 | 0.000 | Desmethoxcurcumin | |
| 3 | 12.939 | 46978 | 1541 | 0.000 | Curcumin |
Table 5: HPLC analysis of petroleum ether extract.
| Peak | Retention time (min) | Area | Height | Concentration | Unit | Name |
| 1 | 10.618 | 18343 | 9279 | 0.000 | Bisdesmethoxycurcumin | |
| 2 | 11.654 | 30682 | 11468 | 0.000 | Desmethoxcurcumin | |
| 3 | 12.767 | 20860 | 74041 | 0.000 | Curcumin |
Table 6: HPLC analysis of phenolic compounds.
| Peak | Retention time (min) | Area | Height | Concentration | Unit | Name |
| 1 | 10.701 | 34205 | 1666 | 0.000 | Bisdesmethoxycurcumin | |
| 2 | 11.741 | 51951 | 1913 | 0.000 | Desmethoxcurcumin | |
| 3 | 12.881 | 14872 | 4390 | 0.000 | Curcumin |
Table 7: HPLC analysis of the standard.
| Peak | Retention time (min) | Area | Height | Concentration | Unit | Name |
| 1 | 10.638 | 2413 | 234 | 0.000 | Bisdesmethoxycurcumin | |
| 2 | 11.702 | 17215 | 782 | 0.000 | Desmethoxcurcumin | |
| 3 | 12.868 | 78307 | 2560 | 0.000 | Curcumin |
Table 8: Linearity and range of the standard.
| The standard | Curcumin concentration
(mg/ml) |
Peak area |
| 1 | 0.33 | 78307 |
| 2 | 1.56 | 349147 |
| 3 | 8.33 | 1721531 |
| 4 | 15.7 | 3590597 |
| 5 | 156.7 | 33863875 |
Table 9: Linearity and range of samples.
| Sample | Peak area | Curcumin concentration (µg/ml) |
| S1 (ethanol extract) | 46978 | 0.21 |
| S2 (petroleum ether extract) | 2086011 | 9.12 |
| S3 (phenol compounds extract) | 148724 | 0.66 |
Table 10: Linearity and range of standard.
| The standard | Desmethoxycurcumin concentration (mg/ml) | Peak area |
| 1 | 0.33 | 17215 |
| 2 | 1.96 | 63203 |
| 3 | 7.891 | 412312 |
| 4 | 15.7 | 829201 |
| 5 | 156.7 | 9051622 |
Table 11: Linearity and range of sample.
| Sample | Peak area | Desmethoxycurcumin concentration (µg/ml) |
| S1(ethanol extract) | 20097 | 0.56 |
| S2(petroleum ether extract) | 306821 | 5.93 |
| S3(phenol compounds extract) | 51951 | 1.15 |
Table 12: Linearity and range of the standard.
| The standard | Bisdesmethoxycurcumin concentration
(mg/ml) |
Peak area |
| 1 | 0.33 | 4213 |
| 2 | 1.56 | 6841 |
| 3 | 8.7 | 61124 |
| 4 | 15.7 | 118304 |
| 5 | 156.7 | 2181574 |
Table 13: Linearity and range of sample.
| Sample | Peak area | Desmethoxycurcumin concentration (µg/ml) |
| S1(ethanol extract) | 18792 | 3.07 |
| S2(petroleum ether extract) | 183437 | 23.96 |
| S3(phenol compounds extract) | 34205 | 5.03 |
 |
Figure 6: Reference standard analysis HLPC Chromatogram. |
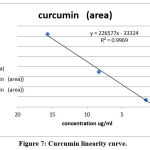 |
Figure 7: Curcumin linearity curve. |
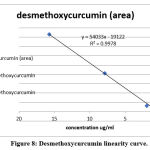 |
Figure 8: Desmethoxycurcumin linearity curve. |
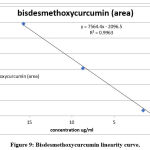 |
Figure 9: Bisdesmethoxycurcumin linearity curve. |
Discussion
In this review, the antibacterial activity of C. longa rhizomes ethanol and petroleum ether extracts were investigated. For that purpose, Agar Well Diffusion method was used and zone of bacterial growth inhibition was measured with different concentrations of ethanol and petroleum ether extracts.
Apparently, increasing ethanol extract active components concentration of C. longa leads to more antibacterial activity. The underling explanation for this effect of ethanol extract is attributed to phenolic compounds content, curcuminoid (diarylheptanoid). There are three main curcuminoids isolated from C. longa, curcumin is one of them 22 which exhibits numerous pharmacological activities and antibacterial properties 23,24,25. The phytochemical screening of ethanol extract of C. longa confirms presence of saponins, tannins, flavonoids and alkaloids 2. The phenolic compounds confers protein denaturing property, may change cell permeability leading to swelling and rupture of the bacterial cells 26 while antibacterial activities ascribe to the presence of the alkaloids 27. The essential oils of C. longa are rich in terpene (monoterpene, oxygenated monoterpene and sesquiterpene) 28. These comprising oily compounds play an important role in its antibacterial activity 29. In addition, it inhibits the bacterial growth by influencing certain metabolic pathways of microbial cells 28.
These results confirm that the ethanol and petroleum ether extracts have antibacterial effect against both Gram-negative and Gram-positive bacteria, though it was more prominent against Gram-positive. The presence of lipopolysaccharides layer in their outer cell membrane confers Gram-negative bacteria marginal protection to the extracts 30. On the contrary, the presence of large amount of peptidoglycan in the Gram-positive bacterial cell walls with relatively small amount of lipid contributes to its wall sensitivity to ethanol and petroleum ether extracts 31.
The petroleum ether evidently could be a good solvent for extraction of C.longa rhizome and to isolate essential oils that contained active components. Some active components may affect the metabolic functions of bacterial cells 32. The bacterial inhibitory effect of the essential oils attributes to its role on various enzymes metabolic reactions that related to structural synthesis and energy production of the bacterial cells 33.
HPLC method proves to be sensitive, precise and accurate for quantifying of curcuminoid in the extracts 34. It used for both qualitative and quantitative measurements. The difference that obtained in concentrations of curcuminoid might be due to the solvents difference and the extraction conditions.
Conclusion
From this study, we can conclude that the C. longa extract exhibits inhibitory action against pathological bacterial growth. The essential oils of C. longa rhizome that obtained from petroleum ether have a capability to inhibit bacterial growth more effectively than ethanol extract. The petroleum ether can be considered as a good solvent for extraction of C. longa rhizome components and isolation of bioactive constituents in a higher concentration.
Conflict of interest
The author declares no conflict of interest.
Funding Source
This article receives no specific funding grants from any agency in public.
References
- Jayaprakasha G, Jagan L, Rao M, Sakariah K. Chemistry and biological activity of Curcuma longa. Trend Food Sci Tech.n 2005, 16:533-548.
CrossRef - Chairman K, Jayamala M, Vijila R, Ranjit A. Phytochemical screening and antimicrobial activity of Curcuma longa natural dye. General Med. 2015, 3(2): 2327-5146.
- Singh R, Jain D. Evaluation of antimicrobial activity of curcuminoids isolated from turmeric. Int. J. of Pharm. and Life Sci. 2012, 3(1):1368-1376.
- Abas F, Lajis N, Shaari K, Israf M, Stanslas J, Yusuf U, Raof S. Diterpene glucoside from the rhizomes of Curcuma mangga. J. Nat Prod 2005, 68:1090-1093.
CrossRef - Rai D, Singh J, Roy N, Panda D. Curcumin inhibits FtsZ assembly: an attractive mechanism for its antibacterial activity. Biochem. J. 2008, 410:147-155.
CrossRef - Mohamed A, Abdel-Aziz A, El-Sherbiny E, Mors R. Anti-diabetic effect of Aloe vera juice and evaluation of thyroid function female diabetic rate. Bioscience Research 2009, 6:28-34.
- Siddiqui A, Cui X, Wu R, Dong W, Zhou M, Hu M, Simms H, Wang P. The anti-inflammatory effect of curcumin in an experimental model of sepsis is mediated by up-regulation of peroxisome proliferators-activated receptor-gamma. Crit. Carit. Care. Med. 2006, 34:1874-1884.
CrossRef - Suzuki M, Nakamura T, Iyoki S, Fujiwara A, Watanabe Y, Mohri K, Isobe K, Ono K, Yano S. Elucidation of anti-allergic activities of curcumin related compounds with a special reference to their anti-oxidative activities. Biol. Pharm. Bull. 2005, 28: 1438-1443.
CrossRef - Menon V, Sudheer A. Antioxidant and anti-inflammatory properties of curcumin, Adv. Exp. Med. Biol. 2007, 595:105-125.
CrossRef - Reddy R, Vatsala P, Keshamouni V, Padmanaban G, Rangarajan P. Curcumin for malaria therapy. Biochem. Biophys. Res. Commun. 2005, 326: 472-474.
CrossRef - Antunes A, Weber S, Schittler L, Gomes G. Synergistic and antimicrobial properties of commercial turmeric (Curcuma longa) essential oil against pathogenic bacteria. Science Technology Aliment Campinas, 2012 32:525-530.
CrossRef - Anne-Catherine O. Medicinal plants. A Botanic Garden for the Nation. Bot. Garden. 2007, p. 121.
- Chattopadhyay I, Biswas K, Bandyopadhyay U, Banerjee R. Turmeric and curcumin biological actions and medicinal applications. Curr. Sci. 2004, 87 (1): 44-53.
- Essien E, Newby J, Walker T, Setzer W, Ekundayo O. Chemotaxonomic characterization and in-vitro antimicrobial and cytotoxic activities of the leaf essential oil of Curcuma longa grown in southern Nigeria. Medicines J. 2015, 2: 340-349.
CrossRef - Rudrappa T, Bais H. Curcumin, a known phenolic from Curcuma longa, attenuates the virulence of Pseudomonas aeruginosa PAO1 in whole plant and animal pathogenicity models. Journal of Agriculture and Food Chemistry 2008, 56:55-62.
CrossRef - Thapa S. Medico-ethnobotany of Magar community in Salija VDC of Parbat district, central Nepal. Our Nat. 2012, 10: 176-190.
CrossRef - Mehra A, Bajpai O and Joshi H. Diversity, utilization and sacred values of ethno-medicinal plants of Kumaun Himalaya. Trop. Plant Res. 2014, 1(3):80-86.
- Harborne JB. Physiological Methods: a guide to modern techniques of plant analysis. London. Chapman and Hall, 1984, p. 288.
- Riberean-Gayon P. Plant phenoles. Oliver and Boyd. USA. 1972, p. 254.
- Mukherjee K, Balasubramanian P, Saha K, Saha P, Pal M. Antibacterial efficiency of Nelumbo Nucifera (Nymphaeceae) rhizome extract. Indian Drugs 1995, 32:274-276.
- Wichitnithad W, Jongaroonngamsang N, Pummangura S, Rojsitthisak P. A simple isocratic HPLC method for the simultaneous determination of curcuminoids in commercial turmeric extracts. Photochemical Analysis 2009, 20 (4): 314-319.
- Wichitnithad W, Jongaroonngamsang N, Pummangura S, Rojsitthisak P. A simple isocratic HPLC method for the simultaneous determination of curcuminoids in commercial turmeric extracts. Photochemical Analysis 2009, 20 (4): 314-319.
CrossRef - Mun S, Joung D, Kim Y, Kang O, Kim S, Seo Y, Kim y et al. Synergistic antibacterial effect of curcumin against methicillin-resistant Staphylococcus aureus. Phytomedicine 2013, 20(8-9): 714-718.
CrossRef - Negi S, Jayaprakash G, Jagan L, Rao M, Sakariah K. Antibacterial activity of turmeric oil: by product from curcumin manufacture. J. Agric. Food Chem. 1999, 47: 4297-4300.
CrossRef - Nisar T, Iqbal M, Raza A, Safdar M, Iftikhar F, Waheed M. Turmeric: A promising spice for phytochemical and antimicrobial activities. Am-Euras. J. Agric. And Environ Sci. 2015, 15(7):1278-1288.
- Ho C, Lee C, Huang M (Eds.). Phenolic compounds in food and their effects on health. ACS symposium Series 506. 1992.
CrossRef - Pundir R, Jain P. Comparative studies on the antimicrobial activity of black pepper (Piper nigrum) and turmeric (Curcuma longa) extracts. Int. J. Appl. Biol. Pharmac. Technol. 2010, 1(2): 492-501.
- Parveen Z, Nawaz S, Siddique S, Shahzad K. Composition and antimicrobial activity of the essential oil from leaves of Curcuma longa L. Kasur variety. Indian J.Pharm. Sci. 2013, 75(1): 117-122.
CrossRef - Singh P, Jain A. Evolution of antimicrobial activity of volatile oil and total curcuminoids extracted from turmeric. Int. J. of Chem Tech Res. 2011, 3(3):1172-1178.
- Alzoreky NS, Nakahara K. Antibacterial activity of extracts from edible plants commonly consumed in Asia. Int. J. Food Microbial. 2003, 80:223-230.
CrossRef - Park M, Bae J, Lee D. Antibacterial activity of (10) gingerol and (12) – gingerol isolated from ginger rhizome against periodontal bacteria. Phytother. Res. 2008, 22(11): 9-1446.
CrossRef - Saifur M, Hasan B, Muslima J, Biswas K, Haque A, Islam R, Haque E, Tama S, Nirupam B. Ethanol extract of Curcuma longa leaf, a potential drug candidate against Bacillus species mediated infections. International Journal of Biosciences 2014, 4 (7): 9-14.
CrossRef - Wilkins KM, Board RG. Natural antimicrobial systems. In: Gould GW, editor. Mechanisms of Action of Food Preservation Procedures. London. Elsevier 1989, p. 285.
- Hastati S, Hadju V, Alam G. Determination of the curcumin pigment in extract curcuma Domestica Val from South Sulawesi, Indonesia, by High Performance Liquid Chromatography. International Journal of Scientific and Technology Research 2015, 4(4):95-98.







