Fayez K. Fouda1 , Ahmed Saad Montaser2, Gehan T. El-Bassyouni3
, Ahmed Saad Montaser2, Gehan T. El-Bassyouni3 , Esmat M.A. Hamzawy4
, Esmat M.A. Hamzawy4 , Eman Refaat Youness5
, Eman Refaat Youness5 * and Mohamed S. Abd El-Aziz6
* and Mohamed S. Abd El-Aziz6
1Medical Biochemistry Department, Medical Research and clinical studies Institute,National Research Centre, Doki, Giza, Egypt
2Textile Research Institute, National Research Centre, Cairo, Giza, Egypt.
3Refractories, Ceramics and Building Materials Dept., National Research Centre, Dokki, Cairo, Egypt.
4Glass Research Department., National Research Centre, Dokki, Cairo, Egypt.
5Medical Biochemistry Department,Medical Research and clinical studies Institute, National Research Centre, Doki, Giza, Egypt
6Genetic Engineering and Biotechnology Institute, Microbial Chemistry Department, National Research Centre (NRC), Dokki, Egypt
Corresponding Author E-mail: hoctober2000@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2548
Abstract
Aspergillosis is a fungal infection cause reduction or suppression of innate immune system. Occasionally silver nanoparticles showed antibacterial activity rather than anti-fungal activity. In the current paper three different shapes and concentrations of silver nanoparticles (AgNPs) were synthesized using dextrin and starch, then applied to cotton for acquiring antifungal properties. TEM illustrates the size, shape and homogeneity of the AgNPs. Atomic absorption spectroscopy (AAS) quantified the AgNPs in the colloidal solution. Attenuated total reflection ATR/FT-IR spectroscopy and scanning electron microscope (SEM) coupled with Energy Dispersive X-Ray Analysis (EDX) to screen the homogeneity and dispersion of the AgNPs. Different shapes (rode and triangular) of AgNPs (10-17nm) were produced using 10~5 ppm of the AgNPs. Antifungal activity towards Aspergillus Niger fungus was documented using the disc diffusion method. Cotton treated with AgNPs showed significant effective antifungal properties at lower concentrations (~10 ppm) preserving its white color compared to the higher concentrations (>100 ppm)of the AgNPs. Manufactured cotton fabrics treated with low concentrations of AgNPs were examined towards the recognition of the antifungal properties with minimal industrial footprint which may expose a novelpossibility for antifungal applications.
Keywords
Anti-Fungal; Aspergillosis; Cotton, Textile; Silver Nanoparticles; Tissue Resist Fungus
Download this article as:| Copy the following to cite this article: Fouda F. K, Montaser A. S, El-Bassyouni G. T, Hamzawy E. M. A, Youness E. R, El-Aziz M. S. A. Eco-Friendly Low Concentration Silver Nanoparticles Acquiring Cotton Textile Antifungal Activities for Pulmonary Aspergillosis Protection. Biomed Pharmacol J 2022;15(4). |
| Copy the following to cite this URL: Fouda F. K, Montaser A. S, El-Bassyouni G. T, Hamzawy E. M. A, Youness E. R, El-Aziz M. S. A. Eco-Friendly Low Concentration Silver Nanoparticles Acquiring Cotton Textile Antifungal Activities for Pulmonary Aspergillosis Protection. Biomed Pharmacol J 2022;15(4). Available from: https://bit.ly/3X07a99 |
Introduction
Acute aspergillosis is a fungal disease with an ominous prognosis and a high mortality rate if not cured rapidly1. The fungal aspergillus spores are ubiquitous, and every day numerous persons respire in fungal spores. However, in individuals having the reduced or suppressed innate immune system, the fungal spores could overwhelm the respiratory tract barriers causing a pulmonary infection, identified as acute pulmonary aspergillosis (APA). The main etiological factors for the development of APA are systemic treatment with glucocorticoids or another immunosuppressive drugs, hematological diseases, sepsis, stem cell transplants, infection with influenza, liver cirrhosis and HIV, which all lead to diminished cell-mediated immune system 2.
Aspergillus Niger is a moldthat is hardly recognized as an etiological cause of pneumonia 3. Although uncommon when a patient is affected by this mold due to being immunosuppressed probably due to a long-term steroidal treatment; it became evident that the conventional drugs used for fungal infections such as voriconazole showed negative results 4.
Nanotechnology is becoming increasingly efficient in allowing researchers to inspect new characteristics of elements in a diameter range that was not possible before. The minute size of nanoparticles provides more surface area of action as compared to non-nanomaterials to the same given volume thus improving the collision frequency between particles and attaining a higher degree of agglomeration 5. Silver was long used as an antibacterial agent and now it is being utilized by nanotechnology to develop AgNPs which plays a vital role in numerous biomedical applications. It has great potential owing to the non-toxicity of the active Ag+ to human cells and their innovation being a long durable biocide with low volatility and high-temperature stability6,7. The nano-sizeof the AgNPs exhibited more effective antibacterial activity at low concentrations. To fulfill the necessity of AgNPs, several procedures have been approved for their synthesis8,9.
Chemical reduction methods for producing AgNPs included consuming synthetic or natural reducing agents. Carbohydrates are an eco-friendly method for producing nanoparticles. At the alkaline medium, carbohydrate chains degrade along with the reduction of the metal cations producing acetyl groups and temporarily reduced nanoparticles. At low concentrations, AgNPs presented antibacterial activity nevertheless there is a lot of disputes about its cell infiltration and toxicity10. Preparation conditions for instance time, temperature, and source of metal cation all regulate nanoparticles growth, shape and efficiency. Among the numerous synthesis methods for AgNPs, biological procedures are the simplest, fastest, safest, reliable, and the eco-friendliest that could produce well-defined size and morphology in enhanced conditions11,12.
After synthesis accurate particle characterization was essential due to the physicochemical properties of particles could have a significant influence on their biological properties. Occasionally silver nanoparticles showed antibacterial activity rather than anti-fungal activity13, 14. Currently, textile fabrics treated with different nano-metals such as silver, zinc and titanium showed efficient antibacterial activity. Many routes were used for stabilizing nanomaterials either in-or ex-situ. One of the drawbacks of silver nanoparticles is the yellow coloration that becomes more prominent with higher concentrations 15.In the current work, two concentrations were produced through chemical reduction methods using two natural products, producing two different particle shapes showing antifungal activity at low concentrations. Produced nanoparticles applied on textile fabrics showed antifungal activity which to our knowledge was not once demonstrated before. Modern investigating tools were utilized to examine the AgNPs size and distribution16. The antifungal activity of the produced textile fabrics was also studied.
Materials and Methods
Materials
Silver nitrate (AgNO3), dextrin & sodium hydroxide (NaOH) were obtained from Sigma-Aldrich Co. USA. Starch was provided by Glucose and Yeast Co. Egypt. Pure cotton fabrics (100%) were provided from Golden Tex Company, Egypt; whose weight was 118 g/m2 and thickness was 0.251 mm. All chemical reagents are of analytical grade and were altogether utilized without any additional purification.
Preparation of Silver Nanoparticles
Dried starch and dextrin were solubilized under alkaline conditions. 0.1N AgNO3 solution was dropped wisely added to the alkali solution during magnetic stirring. The AgNO3 alkali-treated polymer (starch or dextrin) water system was allowed to react at 70°C in an oven for 30 min. At the end of the reaction, the high concentrated color (50-100 ppm) mixture turns into pale yellow; confirming the formation of AgNPs.
Textile Surface Modification with (AgNO3)
Two different solutions of silver nanoparticles were applied to cotton fabric using the exhaustion method. Firstly, cotton fabric was immersed in the mentioned solutions for 24 hours under continuous shaking at 100 rpm. Secondly, exhausted fabrics were dried at 100°C for 30 min. Treated fabrics were washed with water/ethanol several times to remove extra salts.
Characterization of the prepared Nanomaterial and obtained fabrics
Attenuated total reflection (ATR)-FTIR spectroscopy (ATR/FTIR):
ATR/FTIR spectroscopy is more advanced than FTIR that are employing by governing the chemical properties on the polymer surface. ATR/FTIR spectroscope (JASCO Infrared System, Japan) recorded and analyzed the blank fabrics and treated samples either with copolymer and/or the polymer nanocomposites17.
X-Ray Diffraction (XRD)
XRD a standard analytical technique used for the analysis of both crystal and molecular structures. XRD measurements were carried out utilizing Philips Expert powder diffraction system (Philips Analytical, Netherlands) equipped with a vertical goniometry in the Bragg-Brentano focusing geometry. The X-ray generator operated at 40 kV and 50 mA, using the CuKα line at λ = 1.54056 Å as the radiation source.
Scanning Electron Microscopy (SEM)
SEM microscopy was carried out to characterize the morphology and dispersion of AgNPs in the polymer matrix. SEM is a surface imaging routine, used to determine different size distributions, particle sizes, surface morphology and nanomaterial shapes of the produced particles at the nanoscales. Energy dispersive X-ray (EDX) spectroscopy was used to attain the composition and the elemental analysis of the nanomaterial. The internal structure of the textile samples was analyzed using a scanning electron microscope. The fabric and treated fabrics were dehydrated and scanned. Scanning electron microscopy was achieved by JEOL (JXA-840A); electron probe micro analyzer Edwards, England.
Transmission Electron Microscope (TEM)
TEM is an important procedure for the characterization of nanomaterials, to get quantitative measures of particle and/or grain size, morphology and size distribution. The crystallinity and nature of AgNPs were verified using a high-resolution transmission electron microscope (HR-TEM), Joel model JEM-2100, Japan. The aqueous dispersion of the particles was prepared then drop-casted on a carbon-coated copper grid, air-dried at room temperature in advance of being deliberated microscopically. Particle sizes of the sample were characterized by the particle size analyzer technique for measuring the size distribution by number.
Atomic Absorption
For measuring the AgNPs concentration, a definite amount of silver nanoparticles solution was removed by a syringe and the aqueous phase was delivered to the nebulizer of a flame atomic absorption spectrometer (FAAS). Atomic absorption calibrated with different concentrations of silver was exploited to measure the unknown silver concentration.
Antimicrobial Activities
Disc agar plate method has been recognized to estimate the antifungal activities of diverse textile fabrics treated with different AgNPs. Aspergillus Niger was selected to evaluate the antifungal activities; the fungal test microbe was cultivated on a potato dextrose agar (PDA) medium. The culture was diluted with sterilized distilled water to 107– 108 colony forming units (CFUs)/mL, and then 1mL of each was used to inoculate 1LErlenmeyer flask containing 250 mL of solidified agar media. These media were put onto previously sterilized Petri dishes (10cm diameter having 25ml of solidified media). Textile discs loaded with nanosilver. The discs were dried at room temperature under sterilized conditions and then placed on the agar plates seeded with test microbe and incubated for 24h, at the suitable temperature of the test organism18. Antifungal activities were recorded as the diameter of the clear zones (comprising the disc itself) that appeared around the discs. Cycloheximide (100µg/disc) was used as antifungal standards19.
Results and Discussion
Treatment of starch with NaOH at high concentration results in swelling of starch particles and create more Starch-O-Na. A large amount of starch hydroxyls might organise with Ag+ preventing their accumulation after being converted to Ag0 throughout the reduction process via capping and/or encapsulation. The ultimate reduction of Ag+ to AgNPs is mostly because of the interaction of Ag+ with reducing groups of starch-O-Na at C6. In the present work, the well-known factors controlling conditions as temperature (70oC) and duration for 30 min were employed throughout AgNPs the synthesis. At the end of the reaction, the powder of alkali-treated starch turns yellowish-brown gradually after adding AgNO3; a point that obviously determines the AgNPs formation.
UV-Vis Spectroscopy
Silver nanoparticles as a powerful antibacterial agent successfully prepared through reduction method using starch and dextrin. Carbohydrates (starch and dextrin) at basic conditions oxidized to form acetyl structure combined with metal cation reduction. Nano-metals especially silver forming a specific absorption peak at UV-Vis spectrophotometer between 400-450 nm at UV-Vis spectrophotometer. The produced AgNPs spectrum confirmed the formation of AgNPs under previous reduction conditions. The two UV spectra werelying between 400 and 450 nm. Not only wavelengths give information about metal synthesis, but also the broadness and/or sharpness of the peak.It is clear from Fig.1 that the alkaline solutions of carbohydrates successfully turned Ag+into AgNPs, for both solutions of St-AgNPsand Dx-AgNPs.Peaks spectra at 400 nm reflect the presence of AgNPs20. Additionally, the same coincident peaks represent the same concentration of silver nanoparticles.
 |
Figure 1: UV spectra of prepared St-AgNPs and Dx-AgNPs solution, b) UV spectra of cotton, cotton/St-AgNPs and cotton/Dx-AgNPs. |
X-ray Diffraction (XRD)
Produced nanomaterial exposed to X-ray diffraction to confirm nanostructure through diffraction patterns. Fig. 2 showed the X-ray diffraction patterns of the cotton fabrics at the2θ position of 18, 23 and 37. For Fig. 2 a, b, c and d, there is a comparison between the cotton fabric and AgNPs produced through chemical reduction methods. X-ray patterns showed the same peaks with little change in sharpness in addition to small peaks represents AgNPs at 2θ position 34, 43, 46 and 48. It is assumed that there is a low concentration of silver on examined fabrics. XRD observations also gave information about the ignored changes in cotton crystallinity after being exposedto the AgNO3 treatment21.
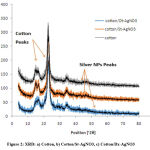 |
Figure 2: XRD: a) Cotton, b) Cotton/St-AgNO3, c) Cotton/Dx-AgNO3 |
ATR/FTIR
ATR/FTIR wasused to discover the electrostatic binding between the cotton fabric and AgNPs. Fig. 3 illustrated the ATR resonance between 400-4000. ATR exhibited peaks 3500, 2900, 1050 nm which attributed to the hydroxyl, ethylene and ether linkage of glucose chains of cotton fabrics. There are no significant changes with cotton treated with AgNPs compared to pristine cotton. Moreover, binding between AgNPs and cellulose chains appears clearly at declined hydroxyl peaks, such decline perhaps credited to the binding of silver cations into the active negative (anionic) charge on cellulose (hydroxyl groups) 22.
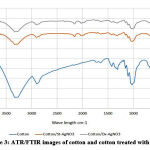 |
Figure 3: ATR/FTIR images of cotton and cotton treated with AgNPs. |
Scanning Electron Microscope (SEM)
Fig. 4 demonstrates the consistency of the treated fabrics with starch/AgNPs and dextrin/AgNPs. The illustrated smooth elongated compressed fibre with interspace belonged to blank cotton fabric. Treated cotton/St-AgNO3and cotton/Dx-AgNO3 composites showed significant changes Fig. 4b, reveals samples treated with dextrin, it shows coated fibre with white precipitation on the surface. Coating probably attributed to dextrin used for the AgNPs process, while white precipitate could be accredited to silver nanoparticles on the fiber surface. Fig. 4c showed the morphology of textile fabrics treated with starch/AgNPs. The polymer was coated with highly arranged and dispersed needle-like silver on the fibre surface. The different morphology of the AgNPs with different shapes expected a superior result at microbiological evaluations. Dispersion of silver nanoparticles on the surface of textile fabrics appeared as presented inFig. (4). Elemental distribution elucidated the content of carbon, oxygen and silver as accessible in Table (1).
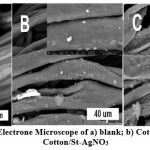 |
Figure 4: Scanning Electrone Microscope of a) blank; b) Cotton/Dx-AgNO3and c) Cotton/St-AgNO3 |
Table 1: Elemental distribution of Cotton/St-AgNO3andCotton/Dx-AgNO3
| Cotton/Sr-AgNO3 | Cotton/Dx-AgNO3 | ||||
| Element | Atomic weight | Molecular weight | Element | Atomic weight | Molecular weight |
| C | 48.29 | 55.61 | C | 47.12 | 54.64 |
| O | 51.26 | 44.54 | O | 51.97 | 45.25 |
| Ag | 0.43 | 0.06 | Ag | 0.91 | 0.12 |
Transmission Electron Microscope (TEM)
The influence of the preparation procedure on AgNPs morphology demonstrated at TEM images. Two different shapes, spherical and triangular, attributed to dextrin and starch polymers, respectively were successively determined. Fig. 5 presents the two shapes with aggregations owing to the utilization of polymer as a reducing and capping agent. Furthermore, TEM showed that the two shapes are homogeneously distributed with particles size in the range of 10-30 nm. Besides, it was obvious from the scanned image that clear the agglomeration of the starch molecules were (Fig. 5b) more than that of dextrin (Fig. 5a), which may be attributed to the chain length on the two polymers23. As known, dextrin derived from starch through hydrolysis. From this point, we can interpret the agglomerated silver in the case of St-AgNO3 compared with Dx-AgNO3. The same reason could cause more chance for the growth and nucleation of silver on the cotton fabric forming needle structure on them while forming spherical structure in the case of Dx-AgNO324.
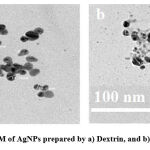 |
Figure 5: TEM of AgNPs prepared by a) Dextrin, and b) Starch. |
Atomic Absorption Spectroscopy (AAS)
Based on AAS results there were no significant differences between concentrations used in the current study. The two concentrations of AgNPs were approximately 10~5 ppm/ml.
Microbiological Evaluations
The fungal activities of cotton fabrics treated with St-AgNO3 and Dx-AgNO3 against Aspergillus Niger clearly elucidated (Fig.6). However, AgNPs was addressed as a powerful antibacterial agent, meanwhile, there was a shortage of the antifungal activity of AgNPs. Herein, a low concentration of two different shapes showed a powerful antifungal activity towards Aspergillus Niger with inhibition zones of 25 and 29 mm for sphere and triangle AgNPs subsequently. These results were assumed to be attributed to the cell membrane interaction with the two shapes of AgNPs. As known AgNPs interact with cell membrane causing a rupture and pumping its composition. Another mechanism stated interaction between AgNPs and DNA of the cell membrane, while another speaks about absorption cell plasma causing dryness and shrinking. For the current research, the expected mechanism for antifungal activity is the new interaction prepared particles and cell membrane. It was expected that the harsh effect on cell membrane caused such rupture. We are looking forward to continuing research work to find the right mechanism of antifungal activity of the prepared AgNPs 25.
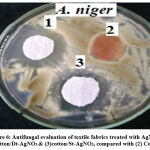 |
Figure 6: Antifungal evaluation of textile fabrics treated with AgNO3; (1) cotton/Dt-AgNO3 & (3)cotton/St-AgNO3, compared with (2) Control. |
Conclusion
Two different shapes of AgNPs were successfully prepared through an ecofriendly method using carbohydrates as reducing and stabilizing agents. A complete evaluation used to confirm AgNPs size and shape has been achieved. The as-prepared AgNPs showed UV spectra at 450 nm which belongs to the AgNPs.TEM confirms two shapes namely sphere and triangular shapes. The AgNPs concentrations were examined using atomic absorption and the concentration was observed low around 2 (ppm/ml). The cotton textile treated with AgNPs showed significantly higher effective antifungal properties at low concentration of (<10 ppm) as compared to the high concentrations of (>100 ppm). Additionally, the two colloidal AgNPs applied onto cotton fabrics demonstrated no changes in color. SEM/EDX showed dispersion and homogeneity of AgNPs on the textile surface. Furthermore, antifungal evaluation against Aspergillus Niger showed powerful activity compared to cotton fabrics loaded with high concentration of AgNPs. From the outcomes, AgNPs of low concentration has a negligible effect on fabric color and would open a new avenue for applications such as antifungal applications.
Conflict of Interests
There are no conflict of interest.
Funding Sources
There is no funding source.
References
- Lamoth, F and T. Calandra. “Let’s add invasive aspergillosis to the list of influenza complications”. The Lancet Respiratory Medicine, 6 (10),733-735(2018).
CrossRef - Garcia-Vidal, C., A. Alastruey-Izquierdo,M. Aguilar-Guisado, J. Carratalà, C. Castro, M. Fernández-Ruiz,J.M. Aguado, J.M.Fernández, J. Fortún, J. Garnacho-Montero, J. Gavaldà, C. Gudiol, J. Guinea, A. Gómez-López,P. Muñoz J. Pemán, M. Rovira, I. Ruiz-Camps and M. Cuenca-Estrella. “Executive summary of clinical practice guideline for the management of invasive diseases caused by Aspergillus”: 2018 Update by the GEMICOMED-SEIMC/REIPI. EnfermInfeccMicrobiolClin, 37 (8):535-541(2019).
CrossRef - Schauwvlieghe, A.F.A.D., B.J.A. Rijnders, N. Philips, R. Verwijs, L. Vanderbeke, C.V.Tienen, K. Lagrou, P.E. Verweij, F.L.V. de Veerdonk, D. Gommers, P. Spronk, D.C.J.J. Bergmans, A. Hoedemaekers, E.R. Andrinopoulou, C.H.S.B. den Berg, N.P. Juffermans, C.J. Hodiamont, A.G. Vonk and J. Wauters.“Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study”. The Lancet Respiratory Medicine, 6 (10),782-792(2018).
CrossRef - Atchade, E., S. Jean-Baptiste, S. Houzé, C. Chabut, L. Massias, Y. Castier, O. Brugière, H. Mal and P. Montravers. “Fatal invasive aspergillosis caused by Aspergillusniger after bilateral lung transplantation”. Medical Mycology Case Reports, 17,4-7(2017).
CrossRef - Moussa, A.R., A.M. El-Kady, A.N. Elboraey, D.Y. Zaki, H.M. Elgamily, M.I.Ramzy and G.T. El-Bassyouni. “Antimicrobial properties of tissue conditioner containing silver doped bioactive glass nanoparticles: in vitro study”. Advances in Natural Sciences: Nanoscience and Nanotechnology, 9(3):035003 (10pp).(2018).
CrossRef - Abd El Aty, A.A., G.T. El-Bassyouni, N.A. Abdel-Zaher and O.W. Guirguis. “Experimental Study on Antimicrobial Activity of Silk Fabric Treated with Natural Dye Extract from Neem (Azadirachtaindica). Leaves”, Fibers and Polymers, 19 (9):1880-1886(2018).
CrossRef - Chaturvedi, V.K., S.N. Rai,N.Tabassum, N. Yadav, V. Singh, R.A. Bohara, M.P. Singh. “Rapid eco-friendly synthesis, characterization, and cytotoxic study of trimetallic stable nanomedicine: A potential material for biomedical applications”.Biochemistry and Biophysics Reports, 24, 1-7(2020).
CrossRef - Yazdanshenas, M.E., M.S. Khalilabad and M.R. Nateghi. A New Method Based on Exhaustion for Immobilization of Silver Nanoparticles on the Surface of Cotton Fabrics”. Asian Journal of Chemistry, 21(7),5741-5748(2009).
- Mabrouk M., S.M. Mousa, W.A. AbdElGhany, M.T. Abo‑elfadl and G.T. El‑Bassyouni. “Bioactivity and cell viability of Ag+ ‑ and Zr4+‑co‑doped biphasic calcium phosphate”. Applied Physics A127, 948(2021).
CrossRef - Khatami, M., R.S. Varma, N. Zafarnia, H. Yaghoobi, M. Sarani and V.G. Kumar. “Applications of green synthesized Ag, ZnO and Ag/ZnO nanoparticles for making clinical antimicrobial wound-healing bandages”. Sustainable Chemistry and Pharmacy, 10, 9-15(2018).
CrossRef - Abdel-Zaher, N.A., G.T. El-Bassyouni, M.A., Esawy and O.W. Guirguis. “Amendments of the Structural and Physical Properties of Cotton Fabrics Dyed with Natural Dye and Treated with Different Mordants”. Journal of Natural Fibers,18(9), 1247-1260(2021).
CrossRef - El-Batal, F.H., A.A. El-Kheshen, G.T. El-Bassyouni and A.A. Abd El Aty. “In Vitro Bioactivity Behavior of some Borate Glasses and their Glass-Ceramic Derivatives Containing Zn2+, Ag+ or Cu2+ by Immersion in Phosphate Solution and their Anti-Microbial Activity.Silicon, 10,943-957(2018).
CrossRef - Maddinedi, S.B., B.K. Mandal and S.K. Maddili. “Biofabrication of size controllable silver nanoparticles – A green approach”. Journal of Photochemistry and Photobiology B: Biology, 167, 236-241(2017).
CrossRef - Jebril, S., R.K. Ben Jenana and C. Dridi. “Green synthesis of silver nanoparticles using Meliaazedarach leaf extract and their antifungal activities: In vitro and in vivo”. Materials Chemistry and Physics, 248,122898(2020).
CrossRef - Rajan, R., K. Chandran, S.L. Harper, S.I. Yun and P.T. Kalaichelvan. “Plant extract synthesized silver nanoparticles: An ongoing source of novel biocompatible materials”. Industrial Crops and Products, 70,356-373(2015).
CrossRef - Montaser, A.S., M.A. Ramadan and A.A. Hebeish. “Facile way for synthesis silver nanoparticles for obtaining antibacterial textile fabrics”. Journal of Applied Pharmaceutical Science, 6 (6),139-144(2016).
CrossRef - Rehan, M., S. Zaghloul, F.A.Mahmoud, A.S. Montaser and A.A. Hebeish. “Design of multi-functional cotton gauze with antimicrobial and drug delivery properties”. Materials Science and Engineering: C, 80,29-37(2017).
CrossRef - Montaser, A.S., M. Rehan, W.M. El-Senousy and S. Zaghloul. “Designing strategy for coating cotton gauze fabrics and its application in wound healing”. Carbohydrate Polymers,244(1),116479(2020).
CrossRef - Tania, I.S., M. Ali and M.S. Azam. “In-situ synthesis and characterization of silver nanoparticle decorated cotton knitted fabric for antibacterial activity and improved dyeing performance”. SN Applied Sciences, 1 (1), 64 (2019).
CrossRef - Schiesaro, I., C. Battocchio, I. Venditti, P. Prosposito, L. Burratti, P. Centomo and C. Meneghini. “Structural characterization of 3d metal adsorbed AgNPs”. Physica E: Low-dimensional Systems and Nanostructures, 123(3),114162(2020).
CrossRef - Ramadan, M.A., S. Sharawy, M.K. Elbisi and K. Ghosal. “Eco-friendly Packaging Composite Fabrics based on in situ synthesized Silver nanoparticles (AgNPs) & treatment with Chitosan and/or Date seed extract”. Nano-Structures & Nano-Objects,22(10-11),100425(2020).
CrossRef - Bhutiya, P.L., N. Misra, M. Abdul Rasheed and S.Z. Hasan. “Silver Nanoparticles Deposited Algal Nanofibrous Cellulose Sheet for Antibacterial Activity”. BioNanoScience, 10 (1), 23-33(2020).
CrossRef - Khodashenas, B and H.R. Ghorbani..“Synthesis of silver nanoparticles with different shapes”. Arabian Journal of Chemistry, 12(8), 1823-1838(2015).
CrossRef - Kumar, A., J. Dalal, S. Dahiya, R. Punia, K.D. Sharma, A. Ohlan, A and A.S. Maan. “In situ decoration of silver nanoparticles on single-walled carbon nanotubes by microwave irradiation for enhanced and durable anti-bacterial finishing on cotton fabric”. Ceramics International, 45 (1),1011-1019(2019).
CrossRef - Yin, I.X., J. Zhang, I.S. Zhao, M.L. Mei, Q. Li and C.H. Chu. “The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry”. International journal of Nanomedicine, 15, 2555-2562(2020).
CrossRef








