Department of Pharmacognosy, College of Pharmacy, Hawler Medical University, 44001, Erbil, Iraq.
Corresponding Author E-mail: zahra_alnajaar@pha.hmu.edu.krd
DOI : https://dx.doi.org/10.13005/bpj/2553
Abstract
Wound healing is a curative process that starts with trauma and finishes with scar formation. Various plant extracts have been used for the treatment and controlling of wounds. In this study Anethum graveolens has been used to accelerate in vivo excision model of wound healing on Sprague Dawley rats and to proliferate the in vitro cell viability model using skin fibroblast cell line through the scratch assay. Results confirm that this plant extract decreases the wound area and increases itswound size reduction percentage, hydroxyproline and nitric oxide levels of the plant extract treated groups were near to the normal control group that indicated effective healing process. On the other hand, in vitro cytotoxicity results should that Anethum graveolens plant extract was safe on skin fibroblast cell lines and induced the normal proliferation and growth of these cells. The migration rate to heal the in vitro wound gaps was 89.1% which indicates a perfect wound size reduction. In conclusion, the results proved that the topical application of Anethum graveolens plant extract quicken the wound healing process.
Keywords
Anethum Graveolens; Hydroxyproline; Nitric Oxide; Wound Healing
Download this article as:| Copy the following to cite this article: Amin Z. A. Anethum Graveolens Leaves Extract Accelerate Wound Healing In Vitro and In Vivo. Biomed Pharmacol J 2022;15(4). |
| Copy the following to cite this URL: Amin Z. A. Anethum Graveolens Leaves Extract Accelerate Wound Healing In Vitro and In Vivo. Biomed Pharmacol J 2022;15(4). Available from: https://bit.ly/3FMFxtv |
Introduction
Wound healing process is a complex sequence of interacion between cells and inflammatory mediators like cytokines, proteases, kinins, nitric oxide, eicosanoids and the other cellular elements 1. Each cell type activity through three phases: proliferation, migration, contraction and matrix synthesis also the growth factor and matrix signals are reported to be present at a wound site 2. The natural healing reaction begins the minute the tissue is injured then blood elements leak into the site of injury. Platelets connect with collagen and other elements of the extracellular matrix then release the clotting factors, cytokines and the growth factors like platelet-derived growth factor (PDGF) and transforming growth factor beta (TGF-ß) afterwards, the neutrophil cells enter the wound location and begin the phagocytotic process to clean out the wound site. Fibroblasts transfer in and begin the proliferative phase later the collagen matrix undergoes the remodelling phase 3. Tissue healing process depend on the balance between the deleterious effects of reactive oxygen species (ROS) which are a group of potent molecules that limits successful tissue regeneration 4.
Many medicinal plant extracts or decoctions had been used by traditions for the treatment of wounds, cuts and burns 5-8. Anethum graveolens, a herb from the celery family Apiaceae. Commonly known as dill, however, in the Middle east region it is named as Ain jaradeh (Grasshopper’s eye) while in Arab countries and Kurdistan region of Iraq it called Shibit. Fresh and dried dill leaves called “dill weed” to differentiate it from dill seed, both has been used widely in traditional medicine for enclosing different activities such as antibacterial effect 9 diuretic, stomachic, appetite stimulant and it reported to stimulate milk flow in a lactating mother 10. Also dill is used to reduce blood cholesterol and lipid levels, menstrual bleeding and dysmenorrhea 11. Studies showed that a tea made from dill seed can be used in the initial stages of labour to reduce labour pain without any side effects on mother and fetus 12. The induction of apoptosis of the fungus “Candida albicans” apoptosis was revealed by Anethum graveolens seed essential oils 13 its antifungal activity was reported as potential food preservative 14. In addition, other studies reported different bioactivities of Antheum graveolens like anti-inflammatory, antimicrobial 15, antioxidant and free radical scavenging activities 16. The current study aimed to test the in vitro wound healing activity of Anethum graveolens and it’s effects on fibroblast cell viability along with the in vivo wound healing activity on Sprague Dawley rats.
Materials and methods
Plant Extraction
For the preparation of ethanol extract of Anethum graveolens, 100 grams of the dried weed part was soaked in 1000 milliters of ethanol for 72 hours. After that, the mixture was filtered by filter paper (Whatman No.1) and extracted under compact pressure in an Eyla rotatary evaporator (Sigma Aldrich, USA). Intrasite gel was used as the reference control (is a trademark for Smith and Nephew Healthcare Limited,UK). Acacia Arabic gum (Sigma Aldrich, USA) was used as the vehicle control following the method of Dhiyaaldeen et al.17 One gram of gum acacia was dissolved in 100 ml of normal saline, from this, 10 ml of solution, which contains 100mg of gum acacia, was used for dissolving 100mg and 200mg of Plant extract each.
Excision Model Of In Vivo Wound Healing
Sprague Dawley (SD) adult rats weighing between (250 -300 g), were gained from the animal house unit of the Faculty of Medicine /University of Malaya/ Malaysia. They were maintained at 22 ± 3 °C in wire bottom cages under a 12 h–12 h light–dark cycle with 50% to 60 % humidity for at least one week former to the test. The study was approved by the ethics Committee for animal experimentation, Faculty of Medicine, University of Malaya, Ethic No. PM/27/07/2009/MAA. For wound healing experiment, the rats were divided randomly into four groups each with eight rats which housed individually. After anesthetizing the animals, the skin was shaved with an electrical shaver and disinfected with 70% alcohol. An identical wound area with 2cm diameter ( Figure 1) was excised from the napes of the rats using a curved stamp, as described by Morton and Malone 18. All rats were treated twice a day, as follows: the vehicle control group was treated with 0.2 ml gum acacia; the reference drug group was treated with 0.2 ml intrasite gel; one of the treated groups was topically administered 0.2 ml of the 100 mg/ml plant extract; and the other treated group was topically administered 0.2 ml of the 200 mg/ml plant extract.
 |
Figure 1: 2cm diameter excision skin wound on the day of surgery. |
Reduction of the wound area was measured on the day of surgery and then on days 5 and 10. An excision edge was traced after wound formation with transparent paper, and the wound area was measured using graphing paper. The tracing paper was placed on 1 mm2 graph sheet and traced out. The squares were counted and the area was recorded. Then the percentage of wound size reduction was calculated following the formula:
Wound size reduction (%) = 1- (Ad/A0)*100 (A0 is wound area on day zero, Ad is wound area on corresponding day)19 .Wound contraction was measured at 5-day-intervals. At day 10, the animals were anesthetized under a high dose then the skin from the restored wound area was excised;The skin tissue was homogenized and the supernatant was used to measure nitric oxide (NO) and hydroxyproline (HXP) determinations using the NO and HXP assay kit (Cayman Chemical Co., USA).
In Vitro Wound Healing
The MTT (3-(4,5-dimethylthiazol-2- yl)-2,5-diphenyltetrazolium bromide) assay (Merck, Germnay) was performed to study Anethum graveolens cytotoxicity. Cells were grown in DMEM medium (Dulbecco’s Modified Eagle’s) (Sigma Aldrich, USA) supplemented with 10% fetal bovine serum (Sigma Aldrich, USA) at 37°C under 5% CO2 in a humidified incubator (NUAIRE laboratory equipment supply, USA). Cells (0.5 × 105 cells/ml) were transmitted into a 96-well plate, and incubated for 48 hours then serial dilutions of Anethum graveolens plant extract was prepared by using 0.25 % DMSO (Dimethyl Sulfoxide) as solvents to give final concentrations of (1.25, 2.5, 5, 10 and 20 mg/ml) then 10µl was injected to each well and incubated for further 48 hours. MTT test was performed following the method of Amin et al 8 then, the absorbance was read by the ELISA plate reader (PowerWave X340, BIO-TEK Instruments Ltd.) at 595 nm. The percentage of cell growth inhibition was calculated as:
Cell viability % = (abs of extract sample – abs of control/abs of control) × 100
Moreover, the cytoselect wound healing assay kit (Cell Biolabs, Inc., USA) was used to assess the in vitro wound healing assay in which specific inserts were used to make wound gaps (0.9 mm), then cells were cultured (0.5 x106 cells/ml) in media containing 10% fetal bovine serum (FBS) and incubated for 48 hours. The inserts detached and the cells were treated with the plant extract (200µg/ml), incubated for further 48 hours. The cells migration into the wound gaps was measured and the wound size reduction was calculated as: Total surface area = 0.9 mm * length
Migrated cell surface area= length of cell migration (mm) * 2 * length
Percent wound size reduction (%) = migrated cell surface area / total surface area * 100 as mentioned by cytoselect wound healing assay kit (Cell Biolabs, Inc., USA)
Statistical Analysis
All data were expressed as Mean ± SEM. The statistical analysis was achieved using one-way ANOVA, Post Hoc Boneferoni test to compare the groups, a p-value ≤ 0.05 as considered significant.
Results
Excision Model Of In Vivo Wound Healing
Table (1) shows the effects of Anethum graveolens ethanol extract on the percentage of wound healed on days post-surgery. Throughout the experiment, the percentage of healing in the vehicle control group gross wounds was significantly lower than those of Anethum graveolens extract treated groups and intrasite gel control groups (Figure 1). On day 10 post-surgery showed that wound dressed with Anethum graveolens extract showed comparatively less scar width at wound size reduction compared to the vehicle -treated group.
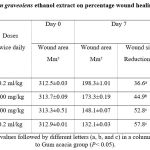 |
Table 1: Effect of Anethum graveolens ethanol extract on percentage wound healing in Sprague Dawley rats. |
Data expressed as Mean ± SEM, Mean values followed by different letters (a, b, and c) in a column are significantly different as compared to Gum acacia group (𝑃< 0.05).
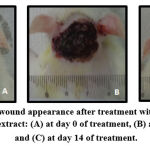 |
Figure 2: Excisional wound appearance after treatment with 200mg/kg Anethum graveolens ethanol extract: (A) at day 0 of treatment, (B) at day 7 of treatment and (C) at day 14 of treatment. |
The hydroxyproline parameter result (HXP) is shown in Figure 3. Generally, the rats dressed with gum accacia showed significant (𝑃< 0.05) decreased levels of HXP compared to the treatment groups. Notably, the animals dressed topically with 200mg/kg Anethum graveolens plant extract showed significantly increased levels of HXP in wound homogenate compared to the animals treated with the gum accacia.
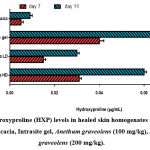 |
Figure 3: Hydroxyproline (HXP) levels in healed skin homogenates treated with 2% gum accacia, Intrasite gel, Anethum graveolens (100 mg/kg), Anethum graveolens (200 mg/kg). |
On the other hand, the nitric oxide (NO) results were higher in rats dressed with Anethum graveolens plant extract and Intrasite gel compared to gum accacia group (Figure 4). Rats dressed with Intrasite gel had the highest NO levels. Dressing the animals with high dose of Anethum graveolens significantly (𝑃< 0.05) increased NO levels.
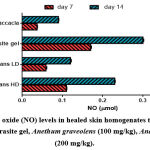 |
Figure 4: Nitric oxide (NO) levels in healed skin homogenates treated with 2% gum accacia, Intrasite gel, Anethum graveolens (100 mg/kg), Anethum graveolens (200 mg/kg). |
In Vitro Fibroblast Cell Proliferation
The Effect of Anethum graveolens extracton human fibroblast cells of the skin showed initially a significant cell viability and proliferation as presented in Figure5. This indicates that the plant extract is safe and non- toxic to normal skin cells. Furthermore, Anethum graveolens ethanol extract showed an increase fibroblast migration rate toward closing the wound gap which assessed in vitro, with a percent wound size reduction of the wound around (89.1%).
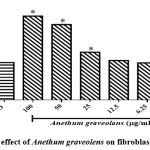 |
Figure 5: The effect of Anethum graveolens on fibroblast cell viability. |
Dıscussıon
During the wound healing procedure, risk factors should be reduced that prevent the wound from healing and causes infections 20. Indeed, plant extracts are inexpensive, affordable, and safe with no or rare side effects 21. The results of the present study showed effective activity of Anethum graveolens in wounds induced in vivo on Sprague dawely rats. The healing process was successful as represented by decreased wound area measurements and increased wound size reduction percentage of the rats treated with the Anethum graveolens ethanol extract as shown in Table 1 and Figure2. There are plenty of evidences that proposes that increased production of lipid peroxidation; reactive oxygen species and ineffective scavenging activity play a vital role in most skin lesions and in modulation of fibroblast proliferation 22. In the present investigation, Anethum graveolens was found to treat wounds through restoring the levels of hydroxyproline and nitric oxide when compared with control (Figures 3 and 4). Nitric oxide (NO) is an essential regulatory molecule in several developmental and physiological processes. It has been demonstrated that NO participates in the wound-healing response 23. It is a small radical, formed from the amino acid L-arginine by three distinct isoforms of nitric oxide synthase. The inducible isoform (iNOS) is synthesized in the early phase of wound healing by inflammatory cells, mainly macrophages 24. However, many cells participate in NO synthesis during the proliferative phase after wounding. NO released through iNOS regulates collagen formation, cell proliferation, and wound contraction in distinct ways in animal models of wound healing 25. Furthermore, there are numerous cells in the wound area that can produce NO including platelets, macrophages, fibroblasts, endothelial cells and keratinocytes 26. In the chronic wound cases, upregulated expression of NO and oxidants will result in constant production of constituents of oxidative and nitroxidative stress 27. The favorable effects of NO on wound repair may be accredited to its functional influences on inflammation, angiogenesis, cell proliferation, remodelling and matrix deposition. These evidences support that NO plays a major role in the wound healing process 26. On the other hand, the protein collagen contains the amino acid (hydroxyproline) which is the major element of extra cellular tissue, it provides support and strength. Breakdown of collagen releases free hydroxyproline and its peptides. Measurement of the hydroxyproline could be used as an index for collagen turnover28. As the Proliferative phase develops, the chief cell in the wound site is the fibroblast. It is responsible for producing the new matrix needed to restore structure and function to the injured tissue. During this process, there is an important step involving hydroxylation of proline deposits. Hydroxyproline in collagen is important because it gives the molecule its stable helical conformation 3. Hydroxyproline content was found to be significantly increased in the skin burns, wounds and many other skin disorders29. The data of hydroxyproline content (Figure 3) showed that the hydroxyproline content of the homogenate tissue of the animals treated with ethanol extract of Anethum graveolens was significantly increased when compared to the control. Furthermore, the wound re-epithelialization potential of many crude plant extracts, isolated compounds or pharmaceutical preparations were investigated. Studies have proved various herbs contributed significantly to the stimulation of fibroblast proliferation and their migration to reduce the wound gap in vitro 30. The same results were seen in this study where Anethum graveolens ethanol extract showed no cytotoxicity against these cells and oppositely it increased the viability rate and increased the wound closure induced by the scratch assay in vitro. These results are supported by the in vitro studies of some plant extracts which demonstrated that these extracts enhanced proliferation of endothelial cells, fibroblastsand keratinocytes also stimulated keratinocyte migration in vitro in a wound assay and inhibited collagen matrix contraction by fibroblasts 31. Previous studies on dill seed extract showed increased expression of Lysyl oxidase–like enzyme (an extracellular enzyme that catalyzes the cross-linking between micro-fibrils and tropo-elastin) in cultured dermal fibroblasts 32. The cytotoxicity of Anethum graveolens is most likely due to its main constituents: carvone and limonene which has been shown toincrease production of reactive oxygen species (ROS) and decrease mitochondria membrane potential in HL60 cells thus inducing apoptosis 33.
Conclusıon
The results demonstrate that the ethanol extract of Anethum graveolens is capable of promoting wound-healing activity. However, it needs further evaluation in clinical settings before consideration for the treatment of wounds. More efforts should be made toward the characterization and isolation of the active constituents of Anethum graveolens and the relation between their activity and structure should be elucidated. The combination of modern and traditional knowledge can yield better medications for wound healing with fewer side effects.
Conflict of Interest
There is no conflict of Interest.
Funding Sources
There is no funding sources.
References
- George Broughton, I.; Janis, J. E.; Attinger, C. E., The basic science of wound healing. Plastic and reconstructive surgery 2006, 117 (7S), 12S-34S.
CrossRef - Martin, P., Wound healing–aiming for perfect skin regeneration. Science 1997, 276 (5309), 75-81.
CrossRef - Diegelmann, R. F.; Evans, M. C., Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci 2004, 9 (1), 283-289.
CrossRef - Bryan, N.; Ahswin, H.; Smart, N.; Bayon, Y.; Wohlert, S.; Hunt, J. A., Reactive oxygen species (ROS)–a family of fate deciding molecules pivotal in constructive inflammation and wound healing. Eur Cell Mater 2012, 24 (249), e65.
CrossRef - Kumar, B.; Vijayakumar, M.; Govindarajan, R.; Pushpangadan, P., Ethnopharmacological approaches to wound healing—exploring medicinal plants of India. Journal of ethnopharmacology 2007, 114 (2), 103-113.
CrossRef - Abdulla, M. A.; Fard, A. A.; Sabaratnam, V.; Wong, K.-H.; Kuppusamy, U. R.; Abdullah, N.; Ismail, S., Potential activity of aqueous extract of culinary-medicinal Lion’s Mane mushroom, Hericium erinaceus (Bull.: Fr.) Pers.(Aphyllophoromycetideae) in accelerating wound healing in rats. International journal of medicinal mushrooms 2011, 13 (1).
CrossRef - Al-Bayaty, F. H.; Abdulla, M. A.; Hassan, M. I. A.; Ali, H. M., Effect of Andrographis paniculata leaf extract on wound healing in rats. Natural product research 2012, 26 (5), 423-429.
CrossRef - Amin, Z. A.; Ali, H. M.; Alshawsh, M. A.; Darvish, P. H.; Abdulla, M. A., Application of Antrodia camphorata promotes rat’s wound healing in vivo and facilitates fibroblast cell proliferation in vitro. Evidence-Based Complementary and Alternative Medicine 2015, 2015.
CrossRef - Rafii, F.; Shahverdi, A. R., Comparison of essential oils from three plants for enhancement of antimicrobial activity of nitrofurantoin against enterobacteria. Chemotherapy 2006, 53 (1), 21-25.
CrossRef - Muna, M.; Mukhls, N. J.; Abass, F.; Ibrahim, S. N.; Noor, M. A.; Doaa, A. A., The role of dill (Anethum greavelon) alcoholic extract on mammary glands performance and some plasma biochemical parameters during lactation period in rats. Diyala Journal for pure science 2016, 12 (3-part 2).
- Yousofvand, N.; Soltany, A., Effects of hydroalcoholic extract of dill (Anethum graveolens) on the serum levels of blood lipids cholesterol, triglycerides, LDL and HDL in male NMRI mice. J Pharmaceut Chem Biol Sci 2015, 3, 114-21.
- Mirmolaee, S. T.; Hekmatzadeh, S. F.; Kazemnazhad, A.; Aidenlou, F.; Shamsi, M., Evaluating the effects of Dill (Anethum graveolens) seed on the duration of active phase and intensity of labour pain. Journal of Herbal Medicine 2015, 5 (1), 26-29.
CrossRef - Chen, Y.; Zeng, H.; Tian, J.; Ban, X.; Ma, B.; Wang, Y., Dill (Anethum graveolens L.) seed essential oil induces Candida albicans apoptosis in a metacaspase-dependent manner. Fungal biology 2014, 118 (4), 394-401.
CrossRef - Tian, J.; Ban, X.; Zeng, H.; Huang, B.; He, J.; Wang, Y., In vitro and in vivo activity of essential oil from dill (Anethum graveolens L.) against fungal spoilage of cherry tomatoes. Food Control 2011, 22 (12), 1992-1999.
CrossRef - Kazemi, M., Chemical composition and antimicrobial, antioxidant activities and anti-inflammatory potential of Achillea millefolium L., Anethum graveolens L., and Carum copticum L. essential oils. Journal of Herbal Medicine 2015, 5 (4), 217-222.
CrossRef - Shyu, Y.-S.; Lin, J.-T.; Chang, Y.-T.; Chiang, C.-J.; Yang, D.-J., Evaluation of antioxidant ability of ethanolic extract from dill (Anethum graveolens L.) flower. Food chemistry 2009, 115 (2), 515-521.
CrossRef - Dhiyaaldeen, S. M.; Amin, Z. A.; Darvish, P. H.; Mustafa, I. F.; Jamil, M. M.; Rouhollahi, E.; Abdulla, M. A., Protective effects of (1-(4-hydroxy-phenyl)-3-m-tolyl-propenone chalcone in indomethacin-induced gastric erosive damage in rats. BMC veterinary research 2014, 10 (1), 961.
CrossRef - Morton, J.; Malone, M., Evaluation of vulneray activity by an open wound procedure in rats. Archives Internationales de Pharmacodynamie et de Therapie 1972, 196 (1), 117-126.
- Niyas, M.; Sastry, T., AN IN VIVO STUDY ON THE WOUND HEALING ACTIVITY OF CELLULOSECHITOSAN COMPOSITE INCORPORATED WITH SILVER NANOPARTICLES IN ALBINO RATS. International Journal of Research in Ayurveda & Pharmacy 2011, 2 (4).
- Nagori, B. P.; Solanki, R., Role of Medicinal Plants in Wound Healing. Research Journal of Medicinal Plant 2011, 5 (4), 392-405.
CrossRef - Raina, R.; Prawez, S.; Verma, P.; Pankaj, N., Medicinal Plants and their Role in Wound Healing. VetScan 2008, 3 (1).
- Shukla, A.; Rasik, A. M.; Dhawan, B. N., Asiaticoside‐induced elevation of antioxidant levels in healing wounds. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives 1999, 13 (1), 50-54.
CrossRef - París, R.; Lamattina, L.; Casalongué, C. A., Nitric oxide promotes the wound-healing response of potato leaflets. Plant Physiology and Biochemistry 2007, 45 (1), 80-86.
CrossRef - Shi, H. P.; Most, D.; Efron, D. T.; Tantry, U.; Fischel, M.; Barbul, A., The role of iNOS in wound healing. Surgery 2001, 130 (2), 225-229.
CrossRef - Witte, M. B.; Barbul, A., Role of nitric oxide in wound repair. The American Journal of Surgery 2002, 183, 406-412.
CrossRef - Luo, J.-d.; Chen, A. F., Nitric oxide: a newly discovered function on wound healing. Acta Pharmacologica Sinica 2005, 26 (3), 259-264.
CrossRef - Soneja, A.; Drews, M.; Malinski, T., Role of nitric oxide, nitroxidative and oxidative stress in wound healing. Pharmacological reports: PR 2004, 57, 108-119.
- Prashanthi, R.; Mohan, N.; Siva, G. V., Wound healing property of aqueous extract of seed and outer layer of Momordica charantia L. on albino rats. Indian Journal of Science and Technology 2012, 5 (1), 1936-1940.
CrossRef - Gurung, S.; Škalko-Basnet, N., Wound healing properties of Carica papaya latex: in vivo evaluation in mice burn model. Journal of ethnopharmacology 2009, 121 (2), 338-341.
CrossRef - Fronza, M.; Heinzmann, B.; Hamburger, M.; Laufer, S.; Merfort, I., Determination of the wound healing effect of Calendula extracts using the scratch assay with 3T3 fibroblasts. Journal of ethnopharmacology 2009, 126 (3), 463-467.
CrossRef - Thang, P. T.; Teik, L. S.; Yung, C. S., Anti-oxidant effects of the extracts from the leaves of Chromolaena odorata on human dermal fibroblasts and epidermal keratinocytes against hydrogen peroxide and hypoxanthine–xanthine oxidase induced damage. Burns 2001, 27 (4), 319-327.
CrossRef - Sohm, B.; Cenizo, V.; André, V.; Zahouani, H.; Pailler‐Mattei, C.; Vogelgesang, B., Evaluation of the efficacy of a dill extract in vitro and in vivo. International journal of cosmetic science 2011, 33 (2), 157-163.
CrossRef - Sharopov, F.; Wink, M.; Gulmurodov, I.; Isupov, S.; Zhang, H.; Setzer, W., Composition and bioactivity of the essential oil of Anethum graveolens L. from Tajikistan. International Journal of Medicinal and Aromatic Plants 2013, 3 (2), 125-130.









