Imad K A AlSabri1, 3 , Amina B Aldujele2
, Amina B Aldujele2 and Zuhair Allebban3*
and Zuhair Allebban3*
1Middle Euphrates Cancer Therapy Center, Najaf, Iraq
2Department of Physiology, College of Medicine, Kufa University, Najaf, Iraq
3Middle Euphrates Unit for Cancer Research, College of Medicine, University of Kufa, Najaf, Iraq
Corresponding Author E-mail: zuhair.allabban@uokufa.edu.iq
DOI : https://dx.doi.org/10.13005/bpj/2568
Abstract
Background. Two recent major wars and a 12-year economic embargo as well as several years of war on terrorism have had a damaging effect on Iraq’s land, air, water, food, and health infrastructure. The presence of depleted uranium (DU) in Iraqi soil, water and the overall food chain is documented by measuring the DU in animal organs and fish as well as the water in the most populated cities in the middle and south of Iraq. Breast cancer is the most common tumor type among Iraqi women living in war zones, and triple-negative breast cancer (TNBC) constitutes the most aggressive molecular subtype among breast tumors. The objective of this pilot study is to determine the prevalence and prognostic target of androgen receptor (AR) positivity in TNBC patients living in war regions polluted with a high level of DU. Methods: This observational, retrospective pilot study included 50 cases of TNBC patients living in the war region. The expression of AR, CK5/6, and CK8/18 biomarkers was evaluated using an immunohistochemistry study on formalin-fixed paraffin-embedded tumor samples from TNBC patients. The serum level of CA-153 and vitamin D was measured. Results: AR was positive (IHC>12%) in 12% of TNBC patients. K5/6 expression was considered if the score was >2. This expression of K5/6 was positive in 80% of cases, and CK8/18 was negative in 80% of cases. Serum vitamin D level was significantly lower in TNBC patients compared to controls. Since the two Gulf wars, there has been a steady increase in the incidence of breast cancer in Iraq. Conclusion: The middle and south of Iraq contain a heavily war-related, DU-polluted environment. Based upon the findings of this study, in regions exposed to high levels of DU, AR overexpression in TNBC patients is similar to studies that have been conducted on populations not exposed to DU.
Keywords
Breast Cancer; Depleted Uranium; Gulf War
Download this article as:| Copy the following to cite this article: AlSabri I. K. A, Aldujele A. B, Allebban Z. Androgen Receptor Marker among Iraqi Patients with Triple-Negative Breast Cancer Exposed to Depleted Uranium. Biomed Pharmacol J 2022;15(4). |
| Copy the following to cite this URL: AlSabri I. K. A, Aldujele A. B, Allebban Z. Androgen Receptor Marker among Iraqi Patients with Triple-Negative Breast Cancer Exposed to Depleted Uranium. Biomed Pharmacol J 2022;15(4). Available from: https://bit.ly/3iZJF0i |
Introduction
Cancer in the developing world is projected to increase by 70% over the next 20–25 years. Breast cancer (BC) represents 20–30% of cancer among women and is likely to account for a major part of that increase1. Cancer is largely considered a disease of older people, and the change in lifestyle from a traditional to a Westernized lifestyle exposes women to higher individual risk2. In general, the categorization of breast cancer is based on receptor expression, including progesterone receptor (PR), estrogen receptor (ER), and Her2/neu receptor (Her2) expression. TNBC is characterized as tumors that do not express ER, PR, and HER-2 genes, which account for 10-24% of invasive high-grade breast cancers with different histological types. Patients with TNBC tend to have a short, disease-free interval, a higher recurrence rate after diagnosis, a relatively higher rate of relapse, and a worse overall survival rate than other forms of breast cancer3. Reduced overall survival, especially for the lack of targeted therapies, tends to be more common in younger women4. The relatively high rate of relapse of TNBC has intensified interest in understanding its pathogenesis and the research of new molecular signatures tailored to this specific BC subtype. Furthermore, studies attempted to further classify TNBC through a detailed analysis of gene expression profiling, receptor expression, and histopathological features to identify potential new therapeutics for TNBC patients. This approach has led to the adoption of seven subtypes of TNBC in addition to the androgen receptor as a prognostic marker and therapeutic target for TNBC patients5,6,7. The AR is expressed by approximately 10-15% of TNBC8.
Garay et al have demonstrated, both in vitro and in vivo, that AR-positive (AR+) TNBC is dependent on androgens for growth, and inhibition of AR signaling results in growth inhibition of the target cells9,10. Androgen deprivation therapy (ADT) is a standard-of-care for prostate cancer and Enzalutamide is an ADT agent and has shown a growth inhibitory effect on AR+ TNBC11.
Immunohistochemical and molecular analyses demonstrated that a subset of TNBC expresses the AR, while the low or lack of AR expression prognostic marker correlated with aggressive tumor behavior and poorer overall survival4.
In the first years of diagnosis, TNBC patients with the residual disease tend to have a poor prognosis with a high probability of relapse and lower overall survival. Recent evidence suggests a significant heterogeneity within the TNBC subtype. Meanwhile, more studies have focused on genetic targets and demonstrated high rates of altered expression and genotoxicity due to DU exposure. This is expected to worsen the campaign to combat cancer in Iraq, including TNBC. Currently, in clinical practice and clinical trials, targeted treatment approaches based on the expression of molecular markers including androgen receptors are being implemented12.
Briefly, it is critically important that readers of this pilot study understand this overview of the seriousness of DU contamination in Iraq and the damaging effects on Iraqi civilians’ health. Iraq suffered from two major and tragic wars where munitions containing DU were used. The Royal Society of London estimated that more than 350 tons of DU were used in the 1991 war on Iraq by the US and Coalition forces13. Other reports indicated that DU levels were estimated to be around 320–800 tons in the aftermath of the first Gulf War in 1991 with further comparable levels occurring in 200314,15. During the US invasion of Iraq in 2003, both the USA and UK governments acknowledged that at least 150 tons of DU munitions had been used in Iraq14,15.
Studies indicated that DU was found in the Iraqi soil and food chain. This was documented by measuring the uranium in sheep organs, fish, and also in seasonal dust storms affecting the most populated Iraqi cities in middle and southern Iraq16,17. Additionally, it was reported that the concentration of DU in the blood of leukemia patients and the teeth of healthy children was significantly higher than in clinically healthy control samples18,19. It was also reported that the uranium concentration in clinically healthy human blood samples of people living in the southern city of Basra (heavily bombarded by the US and allied forces in 1991 and 2003) was higher than in people living in Babylon city in the middle of Iraq, which did not see the same level of bombardment20. Meanwhile, DU concentration was found high in nursing mothers’ milk and in the hair of Iraqi parents of children with congenital anomalies21,22. Additionally, DU was also found in the semen and body fluids of US veterans who served in the wars in Iraq 23,24. Bombardments were consistently in heavily populated areas in the middle and southern Iraq, extensively exposing individuals to DU, although this was challenging to document accurately. At the time of publishing, breast cancer is the most common tumor type among Iraqi women living in these previous war zones25,26.
Since the 1990s, reports from southern Iraq reported a steep rise in the incidence of cancers, especially in children27. According to Iraq’s High Commission for Human Rights, the city of Basra is registering 800 new cases of cancer each month, attributing the cause to “multiple reasons, including environmental pollutants in the water and soil, that resulted from the effects of wars”28.
Cellular DNA and specifically proto-oncogenes and tumor suppressor genes are sensitive to damage from environmental factors such as DU. This may lead to uncontrolled cell division that may form tumors. DU is known to induce genomic instability such as DNA double-strand breaks, chromosome aberrations, and micronuclei formation29.
This pilot study is the first to look into the prevalence of the biomarker, AR positivity, and to evaluate its prognostic target in TNBC cancer in women living in the Middle Euphrates region of Iraq exposed to high levels of DU. The results of this work are compared to findings on the co-expression of these biomarkers in studies on breast cancer from other parts of the world, in a population not exposed to DU. Reports have demonstrated an association between AR expression and vitamin D serum levels. Vitamin D may act directly in tumorigenesis in breast tissue, expressing the CYP27B1 enzyme, which is responsible for converting the inactive precursor of vitamin D to the active form 1,25-dihydroxycholecalciferol [1,25(OH)2D3]. This vitamin can have autocrine or paracrine activity, protecting the breast tissues from malignant transformation30. The normal serum value of 1,25(OH)2D3 is 20 ng/ml (50 nmol/L). Values under 20 ng/ml are classified as deficient. Low sun exposure, reduced dietary vitamin D intake, and older age are the most common factors associated with vitamin D deficiency31. The serum level of vitamin D may be linked with the recurrence and survival of cancer, which makes it a possible target for treatment and a prognostic tool for TNBC. Meanwhile, other reports suggested using AR agonists in breast cancer treatment will depend on ER and HER-2 status, and a combination of AR and vitamin D receptor (VDR) agonists can be additive with chemotherapy32.
Subjects and Methods
Patients and Specimens
This prospective study included 50 consecutive cases of TNBC. From 2016 to 2017, patients who met the criteria of ER-, PR- and HER-2 status and were confirmed by two experienced pathologists based on immunohistochemical staining were enrolled in this study as TNBC. In our institution, the percentage of tumors classified as TNBC is approximately 15-20% of the total number of breast cancer cases treated. All cases of TNBC and non-TNBC breast samples were reviewed according to WHO classification criteria using standard tissue sections and appropriate immunohistochemical slides. Medical records for all cases of TNBC samples were reviewed for clinical information, including histologic parameters that were determined from the H&E slides. The following clinical and pathological parameters were evaluated for each tumor included in the study: patient age at initial diagnosis, tumor size, histologic subtype, histologic grade, nuclear grade, nodal status, number of positive lymph nodes, tumor stage, and tumor recurrence. In addition, all specimens were characterized for all routine diagnostic immunophenotypic parameters.
Measurement of Vitamin D
Vitamin D levels in the form of 1,25(OH)2D3 were measured in TNBC patients and healthy control samples using a combination of enzyme immunoassay competition methods with final fluorescent detection. Quantitative determination of 1,25(OH)2D3 concentrations was performed using the enzyme-linked fluorescent assay (ELFA) based on the VIDAS System (bio Mérieux-Lyon, France), following the manufacturer’s protocol7. Briefly, the blood samples were mixed with a pre-treatment reagent to separate vitamin D from its binding protein. The pretreated sample is then collected and transformed into the well that contains the alkaline phosphates labeled anti-vitamin D antibody (conjugate). The vitamin D antigen present in the sample and the vitamin D coating the interior of the solid phase receptacle compete for binding sites on the anti-vitamin D antibody alkaline phosphate conjugate. In the final detection step, the substrate (4-methyl-umbelliferyl phosphate) is cycled in and out of the solid phase receptacle. The conjugate enzyme catalyzes the hydrolysis of this substrate into a fluorescent product (4-Methyl-umbelliferone). Fluorescence was measured at 450 nm. The intensity of the fluorescence is inversely proportional to the concentration of vitamin D antigen present in the sample.
Immunohistochemistry Analysis
Immunohistochemical staining was done on slides from formalin-fixed, paraffin-embedded tissues to evaluate the expression of ER, PR, HER-2, K5/6, K8/18, and AR markers according to the method described by Collina et al, 201633 with slight modification. Paraffin slides were de-paraffinized in xylene and rehydrated through graded alcohols. Antigen retrieval was performed with slides heated in 0.01M citrate buffer (pH 6.0) in a 97°C bath for 20 minutes. After antigen retrieval, the slides were allowed to cool. The slides were rinsed with Tris-Buffered Saline (TBS) and the endogenous peroxidase was inactivated with 3% hydrogen peroxide. After protein block (Bovine Serum Albumin 5% in Phosphate-Buffered Saline, 1x), the slides were incubated with primary antibody to human ER (Monoclonal Mouse Anti-Human ER, Clone ID5, dilution 1:35, Dako North America, Inc., Carpinteria, CA, USA), PR (Monoclonal Mouse Anti-Human PR, Clone 636, dilution 1:50, Dako North America, Inc., Carpinteria, CA, USA), and K5/6 (Monoclonal Mouse Anti-Human K5/6 Ag Clone MIB-1, dilution 1:75, Dako North America, Inc., Carpinteria, CA, USA) for 30 minutes. For CK 8/18, mouse monoclonal antibody keratin 8/18 Ab-2 ready-to-use (clone K8.8+ DC10; like 5D3, Lab Vision, Neo Marker) and AR (monoclonal mouse anti-human AR antibody clone AR441, dilution 1:75, # M3562; Dako North America, Inc., Carpinteria, CA, USA) were used. The sections were rinsed in TBS and incubated for 20 minutes with a biotinylated secondary antibody (Novocastra RE7103), a biotin-conjugated secondary antibody formulation that recognizes mouse and rabbit immunoglobulins. Afterward, the sections were rinsed in TBS and incubated for 20 minutes with Streptavidin-HRP (Novocastra RE7104) and peroxidase reactivity was visualized using a 3,3’-diaminobenzidine (DAB). Finally, the sections were counterstained with hematoxylin and mounted. The results were interpreted using a light microscope.
Evaluation of Immunohistochemistry
Antigen expression was evaluated independently by a pathologist using light microscopy. The observer was unaware of the clinical outcome. For each sample, at least five fields (inside the tumor and in the area exhibiting tumor invasion) and >500 cells were analyzed. Using a semi-quantitative scoring system microscopically and referring to each antigen scoring method in other studies, an observer evaluated the intensity, extent, and subcellular distribution.
Statistical Analysis
Data analysis was done by using SPSS (Statistical Package for Social Sciences) version 20 (SPSS, Inc., Chicago, USA), where we used mean plus standard deviation and percentages as descriptive statistics. For analysis, we used an independent sample t-test for continuous variables and Yates corrected the chi-square test for categorical data. Fisher’s exact test was used to analyze the correlation between the triple-negative phenotype and AR expression. P-values <= 0.05 were regarded as significant.
Results
The incidence of TNBC in the Middle Euphrates region was 15-20%, with 12% of this incidence being AR-positive. TNBC was strongly associated with younger age. The number of patients with various types of cancer and those with breast cancer is demonstrated in Figure 1.
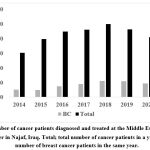 |
Figure 1: Number of cancer patients diagnosed and treated at the Middle Euphrates Cancer Therapy Center in Najaf, Iraq. |
The majority of patients were middle-aged between 41 and 65 years old and had a mean age of 49.2 ± 9.65 (30-65) years. The median age was 48 years. Most of the cases were invasive ductal carcinoma (IDC), accounting for 78.5% of all cases. Useful immunohistochemical markers for characterizing basal-like carcinomas are CK5/6 (Figure 2), androgen receptor (Figure 3), and CK8/CK18 (Figure 4).
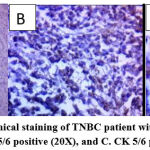 |
Figure 2: Immunohistochemical staining of TNBC patient with CK5/6. A. CK 5/6 control (10X), B. CK 5/6 positive (20X), and C. CK 5/6 positive (40X). |
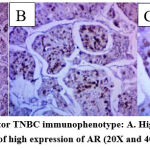 |
Figure 3: Androgen Receptor TNBC immunophenotype: A. High expression of AR (10X); B and C. Details of high expression of AR (20X and 40X respectively). |
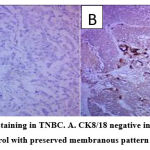 |
Figure 4: CK8/18 immunostaining in TNBC. A. CK8/18 negative internal control (10X); B. CK 8/18 positive internal control with preserved membranous pattern in invasive duct carcinoma. |
The demographic characteristics of the study participants are presented in Table 1. Five years of survival with various variables are shown in Table 2. No significant difference was observed with various variables when comparing the presence or absence of androgen receptors as indicated in Table 3. An evaluation of the association between 5-year survival and androgen receptor, a trend toward increased survival of AR+ vs AR- is shown in Table 4. Serum vitamin D level was tested in both the TNBC patients and controls and the results indicated a significantly low level of serum vitamin D in TNBC patients compared to controls, while no significant difference in the BMI of patients versus healthy controls was found. Figure 4 demonstrates immunostaining of the CK8/18 in negative and positive TNBC patients included in this study.
Table 1: Demographic characteristics of studied patients.
| Variable | Values
Mean + SD (range) |
|
| Age/years | 49.2±9.65 (22-72) | |
| BMI Kg/m2 | 25.8±2.6 (18.7-32.9)
|
|
| Type | Ductal | 41 (82%) |
| Lobular
|
9 (18%)
|
|
| 5-year survival | Yes | 46 (92%) |
| No | 4 (8%) | |
Table 2: Five-years survival among different variables.
| Parameter | 5-years Survival | N | Mean | Std. Deviation | P value |
| Vitamin D ng/dl | Yes | 46 | 11.9 | 4.7 | 0.364 |
| No | 4 | 14.2 | 4.7
|
||
| CA 15-3 | Yes | 46 | 78.6 | 104.6 | 0.897 |
| No | 4 | 71.8 | 13.9 | ||
| BMI Kg/m2 | Yes | 46 | 25.6 | 2.6 | 0.093 |
| No | 4 | 27.8 | 1.3 | ||
| Serum cholesterol mg/dl | Yes | 46 | 247.9 | 30.9 | 0.932 |
| No | 4 | 249.3 | 28.4 | ||
| TG mg/dl | Yes | 46 | 205.1 | 31.2 | 0.992 |
| No | 4 | 205.3 | 20.9 | ||
| LDL mg/dl | Yes | 46 | 59.4 | 11.6 | 0.378 |
| No | 4 | 64.8 | 11.1 | ||
| HDL mg/dl | Yes | 46 | 41.8 | 12.3 | 0.231 |
| No | 4 | 34.0 | 12.1 | ||
| UREA mg/dl | Yes | 46 | 44.3 | 10.8 | 0.131 |
| No | 4 | 56.3 | 11.6 | ||
| CREATININE mg/dl | Yes | 46 | .99 | 2.2 | 0.626 |
| No | 4 | .4 | .4 | ||
| Hb g/dl | Yes | 46 | 11.6 | 1.3 | 0.221 |
| No | 4 | 11.8 | 1.4 | ||
| WBC | Yes | 46 | 6.9 | 5.9 | 0.509 |
| No | 4 | 6.1 | 1.6 | ||
| Platelet (1000/ul) | Yes | 46 | 267.8 | 54.7 | 0.785 |
| No | 4 | 259.0 | 16.8 |
Table 3: Androgen receptor and different variables.
| Parameter | AR | N | Mean | Std. Deviation | P value |
| Vitamin D | positive | 6 | 11.5 | 4.6 | 0.728
|
| Negative | 44 | 12.2 | 4.7 | ||
| CA 15-3 | positive | 6 | 75.7 | 30.9 | 0.951
|
| Negative | 44 | 78.4 | 106.5 | ||
| BMI Kg/m2 | positive | 6 | 26.7 | 2.8 | 0.366
|
| Negative | 44 | 25.6 | 2.6 | ||
| Serum cholesterol mg/dl | positive | 6 | 245.0 | 33.4 | 0.801
|
| Negative | 44 | 248.4 | 30.4 | ||
| TG mg/dl | positive | 6 | 206.2 | 37.2 | 0.928
|
| Negative | 44 | 205.0 | 29.9 | ||
| LDL | positive | 6 | 55.8 | 13.2 | 0.376
|
| Negative | 44 | 60.3 | 11.5 | ||
| HDL | positive | 6 | 36.2 | 11.6 | 0.297
|
| Negative | 44 | 41.8 | 12.5 | ||
| UREA | positive | 6 | 47.7 | 16.5 | 0.589
|
| Negative | 44 | 45.0 | 10.6 | ||
| CREATININE | positive | 6 | 0.5 | 0.2 | 0.556
|
| Negative | 44 | 1.0 | 2.3 | ||
| Hb g/dl | positive | 6 | 11.2 | 1.6 | 0.446
|
| Negative | 44 | 11.6 | 1.2 | ||
| WBC (1000/ul) | positive | 6 | 5.4 | 1.0 | 0.509
|
| Negative | 44 | 7.0 | 6.0 | ||
| Platelet (1000/ul) | positive | 6 | 261.5 | 46.5 | 0.785
|
| Negative | 44 | 267.8 | 53.9 |
Table 4: Association between 5-year survival and androgen receptor
| 5-years Survival | ||||
| Yes | No | P value | ||
|
AR |
Negative | 42 | 2 |
0.101 |
| 91.3% | 50.0% | |||
| positive | 4 | 2 | ||
| 8.7% | 50.0% | |||
| Total | 46 | 4 | ||
| 100.0% | 100.0% | |||
|
Yates chi square = 2.677 |
||||
Discussion
For the last six years, the number of cancer patients increased steadily, with most patients coming from battleground areas of the 1991 and 2003 US-led military invasion of Iraq where DU was used in armor-piercing rounds to disable enemy tanks during these wars34. Inhalation, ingestion, dermal contact, and open wounds are the most likely routes of DU intake into the human body. It is a fact that there is a link between DU and the increase in the incidence of cancer14. In Iraq, there has been an increase in the number of cancer patients, specifically in the south and middle of Iraq where the war was most intense35. Seasonal dust storms carrying DU-contaminated dust moving from the south to the north of the country made it difficult to find an area in Iraq devoid of DU contamination.
Several studies showed that in ER-negative tumors, AR expression is associated with significantly better disease-free survival than in AR-negative tumors. Gonzalez-Angulo et al have evaluated the impact of the AR expression on treatment outcomes of 347 patients with breast cancer and demonstrated that high levels of AR were associated with higher age at diagnosis, the presence of low nuclear grade, and the expression of ER and PR. Among different subtypes of breast cancer, a significant difference in AR expression was observed. Additionally, it was discovered that high expression levels of AR were observed in ER and/or PR positive subtypes and low expression levels in the TNBC36, which is similar to the results of our study. Meanwhile, our results indicated no significant difference in overall survival between AR- and AR+ of TNBC patients, while both the disease-free survival and overall survival were similar in AR+ and AR- patients and were similar to previously reported studies37.
Our TNBC patients’ results with a low level of serum vitamin D are similar to other reported studies38. Other reports indicated that the ratio of patients with TNBC was higher in the region with more sunlight exposure39. This conclusion is different from our results; while our region in Iraq is exposed to long hours of sunlight, the ratio of TNBC patients is similar to the ratio reported in other parts of the world40. Since the majority of women in Iraq wear clothing that covers their entire body, this may interfere with skin exposure to direct sunlight and, in turn, vitamin D deficiency. Further study is recommended to evaluate the effect of sunlight and vitamin D on the development of breast cancer in Iraq. Other studies also suggested that using a combination of AR and Vitamin D receptor (VDR) agonists can be additive with chemotherapy in the treatment of TNBC32. In our study, even though the serum level of vitamin D is significantly lower than controls, giving vitamin D supplementation had no significant effect on the status of TNBC patients. With the limited resources of our study, we were unable to confirm a direct link between the increase in the incidence of breast cancer and the exposure of patients to depleted uranium.
Conclusion
Current research strategies are aimed at better understanding both the risk factors and the biology underlying triple-negative breast cancer to develop preventive measures and improve treatment strategies for this challenging subtype of breast cancer in women living in DU-polluted areas. Limitations of this study are the small sample size, the lack of resources to affirm the direct link between DU and breast cancer, and the political sensitivity of the topic. Our recommendation is to initiate a comprehensive program to investigate the Iraqi environment – especially war zones where DU ammunition was used – to identify DU-contaminated areas and assess the health risks. This investigation would inform a remediation plan with the help of UNEP and WHO, similar to the plan executed in Serbia and Kosovo. A long-term plan should be available to assess the long-term health risks to Iraqi citizens living in DU-polluted areas similar to that used by the Depleted Uranium Follow-up Program at the US Department of Veteran Affairs.
Conflict of Interest
There is no conflict of interest.
Funding Sources
There is no funding source.
References
- American Cancer Society. Global cancer facts and figures. 2nd ed. Atlanta: American Cancer Society; 2011. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/global-cancer-facts-and-figures/global-cancer-facts-and-figures-2nd-edition.pdf.
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
- Tan AR, Swain SM. Therapeutic strategies for triple-negative breast cancer. Cancer J. 2008; 14:343–351.
- Elsamany S, Abdullah S. Triple-negative breast cancer: future prospects in diagnosis and management. Med Oncol 2014; 31: 834.
- Abramson VG, Lehmann BD, Ballinger TJ and Pietenpol JA. Subtyping of triple-negative breast cancer: implications for therapy. Cancer. 2015; 121(1):8-16.
- Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011; 121:2750-2767.
- Gasparini P, Fassan M, Cascione L, Guler G, Balci S, Irkkan C, et al. Androgen receptor status is a prognostic marker in non-basal triple negative breast cancers and determines novel therapeutic options. PLoS One. 2014; 9: e88525.
- Safarpour D, Pakneshan S and Tavassoli FA. Androgen receptor (AR) expression in 400 breast carcinomas: is routine AR assessment justified? Am J Cancer Res. 2014; 4:353-368.
- Garay JP, Karakas B, Abukhdeir AM, Cosgrove DP, Gustin JP, Higgins MJ, et al. The growth response to androgen receptor signaling in ER alpha-negative human breast cells is dependent on p21 and mediated by MAPK activation. Breast Cancer Res. 2012; 14: R27.
- Lu-Yao GL, Albertsen PC, Moore DF, Shih W, Lin Y, DiPaola RS, et al. Fifteen-Year Survival Outcomes Following Primary Androgen Deprivation Therapy for Localized Prostate Cancer. JAMA Intern Med. 2014; 174(9): 1460–1467.
- Barton VN, D’Amato NC, Gordon MA, Lind HT, Spoelstra NS, Babbs BL, et al. Multiple molecular subtypes of triple negative breast cancer critically rely on androgen receptor and respond to enzalutamide in vivo. Mol Cancer Ther. 2015; 14:769-778.
- Moszynski P. Royal Society warns of risks from depleted uranium. BMJ 326:952, 2003
- Al-Azzawi SN. Depleted uranium radioactive contamination In Iraq: An overview. Global Res. 2006; 1:4.
- Al-Azzawi SN.Depleted Uranium and Radioactive Contamination in Iraq: An Overview. Global Res, Dec 28, 2019. https://www.globalresearch.ca/depleted-uranium-and-radioactive-contamination-in-iraq-an-overview/5605215
- Tawfiq NF, Yas RM, Alrawi NB, Elias MM. Determination of Uranium Concentration in Sheep Organs for Some Iraqiۥ s Cities. Baghdad Sci J 2011; 8(3): 766-771.
- Hassan AA. Determination of uranium in fish’s samples from selected regions in Iraq using neutron activation technique for nuclear track detectors AL-Qadisiyah J of pure Sci 2017; 22(2): 47-59.
- Al-Dabbas M, Khafaji RA. Some Geochemical, textural and radioactive characteristics of the sandstorms loads blown over Baghdad and Ramadi cities, middle Iraq. Iraqi J Sci 2012;53 (Special Issue):57–66.
- Al-Hamzawi AA. Uranium concentration in blood samples of Southern Iraqi leukemia patients using CR-39 track detector J Radioanal Nucl Chem 2014; 299:1267–1272.
- Kadim NH, Tawfiq NF, Abdurazak A. Determination of Uranium Concentration in Child Teeth by Track Detector CR-39 in the Middle and South of Iraq. Badhdad J of Sci 2010; 8(4): 909-913. file:///C:/Users/lebba/Desktop/Emad-AR-2020/MEJC/Middle%20East%20J%20of%20Cancer/Depleted%20uranium-Tawfiq-teeth-2010.pdf
- Al-Hamzawi AA, Jaafar MS, Tawfiq NF. The Measurements of Uranium Concentration in Human Blood in Selected Regions in Iraq Using CR-39 Track Detector. Advanced Materials Research 2014; 925:679-683.
- Kadim NH. Using of Nuclear Detector CR-39 to measure depleted uranium concentrations in mother’s milk. Baghdad Sci J 2004; 1(2): 229-233. http://bsj.uobaghdad.edu.iq/index.php/BSJ/article/view/551/482
- Alaani S, Tafash M, Busby C, Hamdan M, Blaurock-Busch E. Uranium and other contaminants in hair from the parents of children with congenital anomalies in Fallujah, Iraq Conflict and Health 2011; 5:1-15.
- McDiarmid MA, Gucer P, Centeno JA, Todorov T, Squibb KS. Semen Uranium Concentrations in Depleted Uranium Exposed Gulf War Veterans: Correlations with Other Body Fluid Matrices. Biol Trace Element Res 2019;190(1):45-51.
- 24.Mendiola J, Moreno JM, Roca M, Vergara-Juárez N, Martínez-García MJ, García-Sánchez A, et al. Relationships between heavy metal concentrations in three different body fluids and male reproductive parameters: a pilot study. Environ Health 2011; 10(6):1-7. https://doi.org/10.1186/1476-069X-10-6
- Results of Iraqi cancer registry 1997-2002: Iraqi Cancer Board, Iraqi Cancer Registry Ministry of Health, Baghdad Iraq.
- William D. 2002. “Hazards of Uranium weapons in proposed war on Iraq”, sept. 22nd, 2002. The Eos life-work resource center. Updated 27 October 2002. http://www.eoslifework.co.uk/u231.html.
- Shelleh HH. Depleted Uranium. Is it potentially involved in the recent upsurge of malignancies in populations exposed to war dust? Saudi Med J.2012; 33(5):483-488.
- Read more: https://www.al-monitor.com/pulse/originals/2019/06/iraq-health-basra-cancer.html#ixzz6OJLffqAc…
- Asic A, Kurtovic-Kozaric A, Besic L, Mehinovic L, Hasic A, Kozaric M, et al. Chemical toxicity and radioactivity of depleted uranium: the evidence from in vivo and in vitro studies. Environ Res. 2017; 156:665–673.
- Gaugris S, Heaney RP, Boonen S, Kurth H, Bentkover JD, Sen SS. Vitamin D inadequacy among post-menopausal women: A systematic review. QJM. 2005; 98:667–676.
- Christodoulou S, Goula T, Ververidis A, Drosos G. Vitamin D and bone disease. Biomed Res Int 2013; 396541.
- Thakkar A, Wang B, Picon-Ruiz M, Buchwald B and TA Ince. Vitamin D and androgen receptor-targeted therapy for triple-negative breast cancer. Breast Cancer Res Treat 2016; 157:77–90
- Collina F, Cerrone M, Peluso V, Laurentiis MD, Caputo R, Cecio RD, et al. Down regulation of androgen receptor is strongly associated with diabetes in triple negative breast cancer patients. Am J Transl Res 2016; 8(8):3530-3539.
- Marshall, AC. Gulf war depleted uranium risks. J Expos Sci and Environ Epid. 2008; 18: 95–108.
- Fathi RA, Matti LY, Al-Salih HS, Godbold D. Environmental pollution by depleted uranium in Iraq with special reference to Mosul and possible effects on cancer and birth defect rates (2013). Med Confl Surviv.; 29(1):7-25.
- Gonzalez-Angulo AM, Stemke-Hale K, Palla SL, Carey M, Agarwal R, Meric-Berstam F .et al. Androgen receptor levels and association with PIK3CA mutations and prognosis in breast cancer. Clin Cancer Res.2009;15(7):2472–2478.
- He J, Peng R, Yuan Z, Wang S, Peng J, Lin G, et al. Prognostic value of androgen receptor expression inoperable triple-negative breast cancer: a retrospective analysis based on at issue microarray. Med Oncol. 2012; 29(2):406–410.
- Marcinkowska E, Wallace GR and G Brown. The Use of 1α,25-Dihydroxyvitamin D3 as an Anticancer Agent. Int. J. Mol. Sci. 2016; 17:729-745.
- Mutlu H, Büyükçelik A, Çolak T, Özdoğan M, Erden A, Aslan T, et al. Is Sunlight a Predisposing Factor for Triple Negative Breast Cancer in Turkey? Asian Pacific J Cancer Prev 2013;14 (2):801-803.
- Shekarriz-Foumani S and F Khodaie. The Correlation of Plasma 25-Hydroxy vitamin D deficiency with risk of breast neoplasms: A systematic review. Iran J Cancer Prev. 2016; 9(3): e4469tep the substrate (4-methyl-umbelliferyl phosphate) is cycled in and out of solid phase receptacle. The conjugate enzyme catalyzes the hydrolysis of this substrate into a fluorescent product (4-Methyl-umbelliferone). Fluorescence was measured at 450 nm. The intensity of the fluorescence is inversely proportional to the concentration of vitamin D antigen present in the sample.








