Sharmila Devi Veeraswamy1 , Ilavarasan Raju2
, Ilavarasan Raju2 and Sumithra Mohan1*
and Sumithra Mohan1*
1Department of Pharmacology, SRM College of Pharmacy, SRMIST, Kattankulathur - 603203, Tamil Nadu, India.
2Department of pharmacology, Captain Srinivasa Murthy Central Ayurveda Research institute, CCRAS, Chennai- 600106, Tamil Nadu, India
Corresponding Author E-mail: sumi26379@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2544
Abstract
In the current scenario, there is a thirst for research against emerging microorganisms, and it becomes challenging to introduce new drugs against organism virulence are pretty interesting. Herbal medicines are now gaining popularity as a treatment option for various diseases worldwide. The present study analyzes the antifungal effect of a polyherbal formulation through in vitro well diffusion method using fungal strains such as Candida albicans, Aspergillus niger, Aspergillus fumigatus, Cryptococcus neoformans, and Sporothrix schenckii. Molecular docking is done using the Auto dock vina tool to predict the mechanism of action of the phytomolecules present in the polyherbal formulation. The molecular interactions are visualized using molecular modelling (PyMOL) software. The antifungal effect was observed in a concentration-dependent manner with a significant zone of inhibition. Also, phytomolecules in polyherbal formulation showed potential inhibition on CYP450 Lanosterol 14 α-demethylase 1, 3 β-Glucan synthase, and Thymidylate synthase from docking analysis.
Keywords
Anti-Fungal; Binding energy; Molecular Docking; Polyherbal formulation; well diffusion; zone of inhibition
Download this article as:| Copy the following to cite this article: Veeraswamy S. D, Raju I, Mohan S. An Approach to Antifungal Efficacy through Well Diffusion Analysis and Molecular Interaction Profile of Polyherbal Formulation. Biomed Pharmacol J 2022;15(4). |
| Copy the following to cite this URL: Veeraswamy S. D, Raju I, Mohan S. An Approach to Antifungal Efficacy through Well Diffusion Analysis and Molecular Interaction Profile of Polyherbal Formulation. Biomed Pharmacol J 2022;15(4). Available from: https://bit.ly/3CJi7mj |
Introduction
Polyherbal combination due to its various phytochemical constituents found effective against various disorders in which it becomes an approach for developing the potential and promising traditional therapy 1. Predominantly, polyherbal drugs are used in the Ayurveda system to treat numerous infections like Indukantha Ghritha (IG), a polyherbal formulation containing 17 different phytochemical components, is widely prescribed by ayurvedic physicians to treat a variety of ailments 2. The relevance of antifungals in medical advances has grown significantly over last 30 years. Because of overwhelming amount of reality fungal diseases influence individuals with weakened immune systems, a rise in the number of people living with innate immunity circumstances or therapies can be connected to an increasing number of fungal infections.3 Invasive Fungal infections in the population are currently posing a threat to treatment. New drug development for treating fungal infections has become more challenging, particularly with post-covid patients.4 The fungal cell wall is an essential structure which is absent in mammalian hosts that gives easy access to drug targets against fungi. The proteins anchoring to the plasma membrane act as a potential target for the drug having an antifungal activity.5
Currently available anti-fungal drugs like fluconazole, itraconazole, voriconazole, and posaconazole act by C14-Demethylase inhibition that blocks ergosterol synthesis.6 The polyenes like amphotericin B inhibit ergosterol synthesis in the cell membrane.7 The drugs that act on (1, 3)-6-D glucan synthase, which is responsible for cell wall synthesis, are echinocandins such as caspofungin, micafungin, and anidulafungin. The degradation of fungi can also be achieved by fluoropyrimidine i.e., 5-fluorocytosine acts by thymidylate synthase for nucleic acid synthesis.8 The receptors involved in the pathogenesis of fungal infections can targeted to develop a new drug against fungal infections.9 Microorganisms like Candida albicans, a polymorphic fungus, can cause infections ranging from superficial skin to life-threatening infections in the systemic circulation. Oral candidiasis is caused by Candida albicans which affects around 70 % of the population in which immune system is affected mainly oropharynx and esophagus.10
Aspergillus species mainly Aspergillus fumigatus and Aspergillus niger cause morbidity and mortality due to infection, specifically causing otomycosis, cutaneous infections, and pulmonary diseases. Aspergillus niger mainly causes Pulmonary aspergillosis that affects about 3.6% of Chronic obstructive pulmonary disease (COPD) patients.11 Similarly, Cryptococcus neoformans is a human fungal pathogen that causes symptomatic infections highly in immune compromised patients with immunity defects.12 And also, Sporotrichosis affects humans and animals mainly due to the hyphomycete genus Sporothrix, and among Sporothrix species, Sporothrix schenckii was found to have high genetic viability.13 The polyherbal formulation consists of aqueous extracts of eleven herbs, namely Aerva lanata (L.) (Whole plant), Boerhavia diffusa L. (Whole plant), Hemidesmus indicus (L.) (Root), Salacia reticulata Wight (root), Berberis aristata DC. (Stem), Gymnema sylvestre (Retz.) (Leaves), Tinospora cordifolia (Willd.) (Stem and leaves), Camellia sinensis (L.) (Leaves), Vitis vinifera L. (seeds), Curcuma longa L. (rhizome), Moringa oleifera Lam. (leaves) and it was subjected to antifungal activity by well diffusion method against Candida albicans, Aspergillus niger, Aspergillus fumigatus, Cryptococcus neoformans and Sporothrix schenckii. Auto dock vina has been used to predict the mechanism of action of the phytomolecules present in the polyherbal formulation.
Materials and Methods
Polyherbal formulation
The polyherbal formulation is a proprietary preparation which consists of eleven different parts of medicinal herbs aqueous extract mixed in different ratios and it is coded as DNF11.
Determination of antifungal activity by well diffusion method
Fungal Strains, Chemicals, and Reagents
Fungal strains were purchased from MTCC, Candida albicans (MTCC 183), Aspergillus niger (MTCC 545), Aspergillus fumigatus (MTCC 2550) purchased from MTCC, Cryptococcus neoformans was purchased from Himedia, Cat No: 0291P, and Sporothrix schenckii isolated from the environment. Potato dextrose agar (HiMedia) and Amphotericin B (Zydus) were used to carry out the in-vitro antifungal activity.
Preparation of culture media
The 3.9 g potato dextrose agar medium was dissolved in 100 ml of distilled water and autoclaved at 15 lbs. pressure at 121°C for 15 minutes. The autoclaved medium was mixed well and poured onto 100 mm Petri plates (25-30 ml/plate) while still molten.
Measurement of Zone of inhibition
The well diffusion method was the standard method for carrying out the antimicrobial analysis using 100 µl of a suspension containing 106 spores/ml of fungal organisms which spread on Potato dextrose agar medium.14 Petri plates containing 20 ml potato dextrose agar medium were seeded with a 72 hr. culture of a different fungal strain. The wells were made at the dimension of 8mm and different concentrations of test sample polyherbal formulation (500, 250, 100, and 50μg/ml) were added to their respective wells. Amphotericin B 100 units were used as a positive control. The experiment was carried out in triplicates and the plates were incubated by inverting at 37°C for 72 hours. The antifungal effect was assessed by measuring the diameter of the inhibition zone formed around the wells and mean and SD were calculated using Graph Pad Prism 6.0 software (USA). Figure 1 represents the methodology of well diffusion method.
 |
Figure 1: Methodology for well diffusion method |
In silico Molecular docking
Selection and Preparation of the target protein
The targets of antifungal agents were selected based on the literature survey and the 3D structure of CYP450 Lanosterol 14 α-demethylase (PDB ID: 1EQ1), 1,3-glucan synthase ((PDB ID: 1EQP), and Thymidylate synthase (PDB ID: 1HZW) was retrieved from the Research Collaboratory for Structural Bioinformatics (RCSB) Protein data bank and saved in program database (PDB) format for docking elucidation. Then, with the exception of metals, all water molecules, and hetero groups are removed and converted into PDBQT format.
Selection and preparation of ligand molecules
The polyherbal preparation constitutes aqueous extracts of eleven herbs, phytocompounds have been selected random from each herb based on its importance from previous individual herb literature with its biological activity given in table 1 and its molecular formula in table 2. To compare the affinity and interacting residues, standard antifungal drugs such as isavuconazole (Triazole), caspofungin (echinocandins) and 5-fluorocytosine (anti-metabolite) were docked against their respective receptor based on their mechanism of action. The canonical smiles are obtained from the Pubchem database and converted into PDB (Program database) format or Protein Data Bank, Partial Charge (Q), & Atom Type (T) (PDBQT) format using appropriate tools.
Table 1: List of selection of phytocompounds and its biological activity.
| Herbs | Parts used | Phytocompounds | Biological activity |
| Aerva lanata (L.) (whole plant) | Whole plant | Kaempferol-3- Rhamnoside15 | Anti-Inflammatory16 |
| Boerhavia diffusa L. (whole plant) | Whole plant | Punarnavine17 | Immunomodulatory18,Antiangiogenic19 |
| Hemidesmus indicus (L.) (Root) | Root | 2-Hydroxy-4-Methoxy Benzaldehyde20 | Antivenom 21 |
| Salacia reticulata Wight (root) | Root | Salacinol22 | Alpha-glucosidase inhibitory activity 23 |
| Mangiferin24 | Antifungal Antioxidant 25,26 | ||
| Berberis aristata DC. (stem) | Stem | Berberine27 | Anti-inflammatory28 ,Antifungal 29,Anti-convulsant 30 |
| Gymnema sylvestre (Retz.) (leaves) | Leaves | Gymnemic acid I 31 | Antiviral 32,Anti-sweet 33 |
| Quercetin34 | Antioxidant, Anti-inflammatory35,Anti mutagenic36 | ||
| Tinospora cordifolia (Willd.) (stem and leaves) | Stem and leaves | Tinosporin A37 | Antibacterial 38 |
| Camellia sinensis (L.) (leaves) | Leaves | Epigallocatechin-3-Gallate 39 | Anti-cancer 40 |
| Vitis vinifera L. (seed) | Seed | Gallic acid41 | Anti-cancer 42 |
| Epicatechin43 | Anti-oxidant 44 | ||
| Curcuma longa L. (rhizome) | Rhizome | Curcumin45 | Anti-inflammatory46, Immunomodulatory47 , Antioxidant 48 |
| Moringa oleifera Lam. (leaves) | Leaves | N-α-L-Rhamnopyranosyl vincosamide49 | Cardio protective 50 |
Table 2: List of phytoconstituents that are selected as ligands for molecular docking.
| Name of the Herb | Phytocompounds | Pubchem ID | Molecular formula | Molecular weight |
| Aerva lanata (L.) (whole plant) | Kaempferol-3- Rhamnoside | 5835713 | C21H20O10 | 432.4 |
| Boerhavia diffusa L. (whole plant) | Punarnavine | 442922 | C18H15NO4 | 469.31 |
| Hemidesmus indicus (L.) (Root) | 2-Hydroxy -4- Methoxybenzaldehyde | 358341 | C8H8O3 | 328.4 |
| Salacia reticulata Wight (root) | Salacinol | 6451151 | C9H18O9S2 | 333.4 |
| Mangiferin | 5281647 | C19H18O11 | 422.3 | |
| Salacia reticulata Wight (root) | Salacinol | 6451151 | C9H18O9S2 | 333.4 |
| Mangiferin | 5281647 | C19H18O11 | 422.3 | |
| Berberis aristata DC. (stem) | Berberine | 2353 | C20H18NO4+ | 336.4 |
| Gymnema sylvestre (Retz.) (leaves) | Gymnemic acid I | 11953919 | C43H66O14 | 807 |
| Quercetin | 5280343 | C15H10O7 | 302.23 | |
| Tinospora cordifolia (Willd.) (stem and leaves) | Tinosporin A | 122206355 | C21H26O8 | 406.4 |
| Camellia sinensis (L.) (leaves) | Epigallocatechin-3-Gallate | 65064 | C22H18O11 | 458.4 |
| Vitis vinifera L. (seed) | Gallic acid | 370 | C7H6O5 | 170.12 |
| Epicatechin | 72276 | C15H14O6 | 290.27 | |
| Curcuma longa L. (rhizome) | Curcumin | 969516 | C21H20O6 | 368.4 |
| Moringa oleifera Lam. (leaves) | N-α-L-Rhamnopyranosylvincosamide | 71717770 | C32H40N2O13 | 660.66 |
Prediction of molecular docking interactions
The docking experiments between the ligands and the target were carried out with the AutoDock Vina 4.2.6 programme (The Scripps Research Institute), which has been used in medicinal chemistry. Based on the Lamarckian Genetic Algorithm, which combines energy evaluation with affinity potential grids to discover the best binding location for a ligand on a certain protein target. 51 The software was used to anticipate protein-ligand interactions, and it is known for its speed and flexibility in performing docking operations to demonstrate that the ligand binds to the target protein. The docking process begins with the ligand and receptor to identify potential binding sites on the target protein in order to anticipate the ligand-binding mode. Polar hydrogen atoms were introduced to the protein targets as per the usual technique, and Kollman unified atomic charges were computed. Hydrogen atoms were added to the ligands before the Gastiger partial charges were applied. The bond orders were examined after the current crystal ligand removal. To cover the entire protein, the target’s grid map was generated and set with proper grid spacing. The target molecule’s grid box was properly adjusted to cover the active residues, and the typical docking process was followed. 52 Finally, independent docking runs were carried out for each ligand, and results were retrieved as binding energies. The poses that showed high free energy values and less RMSD were tabulated and the molecular interactions are visualized using PyMOL 1.7.4.5.
Results and Discussion
Antifungal activity of polyherbal formulation
Previously, polyherbal formulation which contains five herbs has been studied for antifungal efficacy like against Candida albicans, Trichophyton rubrum, Microsporum gypseum, Epidermophyton floccosum showed significant effect in a dose-dependent manner.53 From the existing literature, some of the individual phytochemicals like quercetin, epicatechin, epigallocatechin has been proved for their antifungal effect against Cryptococcus neoformans, dermatophytes, and Candida species.54-57 Similarly, mangiferin showed potential inhibition against Aspergillus flavus and Aspergillus fumigatus. 58 and berberine, which is an isoquinoline alkaloid proven to have potential antifungal effect against fluconazole resistant Candida, Cryptococcus neoformans, and other Candida species. Likewise, Curcumin was also studied for its antifungal activity in the form of silver nanoparticles. 59 From the results figure 2 and 3, Anti-fungal activity of polyherbal formulation assessed by a well diffusion method against different fungal strains comparing with standard drug Amphotericin B through zone of inhibition was found as concentration-dependent antifungal effect.
Table 3: Zone of inhibition against Candida albicans, Aspergillus fumigatus, Aspergillus niger, Cryptococcus neoformans and Sporothrixschenckii.
| S.No | Name of the test organism | Zone of inhibition (mm)
|
||||
| 500 µg/ml | 250 µg/ml | 100 µg/ml | 50 µg/ml | Positive Control | ||
| 1. | Candida albicans | 11±1.41 | 8.45±0.63 | 0 | 0 | 22±1.41 |
| 2. | Aspergillus fumigatus | 11.5±0.7 | 7.75±0.35 | 0 | 0 | 12.5±0.7 |
| 3. | Aspergillus niger | 12.25±1.76 | 8.25±0.35 | 6.2±0.28 | 0 | 16.5±0.7 |
| 4. | Cryptococcus neoformans | 13.5±0.7 | 7.25±0.35 | 0 | 0 | 26±1.41 |
| 5. | Sporothrixschenckii.
|
10.5±0.7 | 8.35±0.49 | 6.25±0.35 | 0 | 29±1.41 |
SD ± Mean, SD – Standard Deviation.
Among the fungal organisms, The zone of inhibition at various concentration (500-50 µg/ml) as given in table 3 the Cryptococcus neoformans showed 13.50 ± 0.70 mm zone of inhibition at the concentration of 500 µg/ml followed by Aspergillus niger showed 12.50 ± 0.70 mm, Aspergillus fumigates showed 11.50 ± 0.70 mm, Candida albicans showed 11.00 ± 1.41 mm and Sporothrix schenckii showed 10.50 ± 0.70 mm zone of inhibition. Compared to other organisms, the highest zone of inhibition was found in Candida albicans (8.45± 0.63 mm) with a concentration of 250 µg/ml. Sporothrix schenckii showed 6.25 ± 0.35 mm and Aspergillus niger showed 6.20 ± 0.28 mm of the zone of inhibition at 100 µg/ml whereas the remaining organisms did not respond. At the concentration of 50 µg/ml, no inhibition was observed in all five organisms. Positive control, Amphotericin B showed an effective zone of inhibition in all the fungal organisms. Among all the fungal strains, the polyherbal formulation has effectively inhibited the growth of Cryptococcus neoformans, Aspergillus niger, and Aspergillus fumigatus at higher concentrations. This antifungal potency can be better to take into a therapeutic advantage against fungal infections due to phytoconstituents present in the polyherbal formulation.
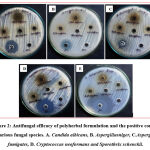 |
Figure 2: Antifungal efficacy of polyherbal formulation and the positive control in various fungal species. |
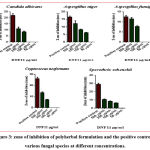 |
Figure 3: zone of inhibition of polyherbal formulation and the positive control in various fungal species at different concentrations. |
In silico molecular docking prediction
The phytoconstitutents present in each herb of polyherbal formulation selected based on marker estimation and solubility from existing literature has been studied for the molecular interaction to predict the pathway behind the mechanism of that particular phytomolecule. The fungal metabolic enzymes were considered as antifungal targets and the results obtained from the study are given as follows.
CYP450 Lanosterol 14 α-demethylase
The fungal species like Candida albicans contain cytochrome p450 that converts N-alkanes to alkanols and grows with N-alkanes as its carbon source. Cytochrome p450 comes under the class of protoheme proteins showing Soret absorption band at 450 nm in reduction co-complex. This is due to thiolate anion coordination in cysteine residue present in apoprotein to heme protein. It has thiolate-ligated iron protoporphyrin IX as its prosthetic group 60. Among 14 phytomolecules present in polyherbal formulation, N α-l-rhamnopyranosyl vincosamide which is mainly present in Moringa oleifera has a higher binding energy of -9.8 Kcal/mol with seven hydrogen bonding interacting with Asp 151, Tyr 153, Gly 143, Arg 309, Tyr 317 amino acids. It forms a good affinity with Arg 309 with 3 hydrogen bonds. Salacinol present in Salacia reticulata interacted with the target enzymes, formed 10 hydrogen bonds with the binding energy of -7.0, Kcal/mol. Since this is one of the major ingredients in the polyherbal formulation, this could be the reason for the inhibition of fungal growth. Other phytoconstituents binding interactions and their energy values are given in table 4 and the evidence for the interactions was given in figure 4.
Table 4: Interaction between Phytomolecules and Standard against Cyp450 Lanosterol 14 α-demethylase (PDB ID: 1EA1).
| S.No | Phytomolecules | Binding energy (Kcal/mol) | Interacting residues in the target protein | Number of Hydrogen bonds |
| 1 | Standard – Isavuconazole | -9.8 | His253 | 1HB |
| 2 | N-α-L-Rhamnopyranosylvincosamide | -10.6 | Asp151, Tyr153, Gly143, Arg309(3), Tyr317 | 7HB |
| 3 | Epigallocatechin-3-Gallate | -10.5 | Arg309, Asp145, Asp145, Asn146, Tyr29, Tyr255 | 6HB |
| 4 | Kaempferol-3- Rhamnoside | -10.4 | Glu192(2), Tyr317, Asn305, Arg309, Leu304 | 6HB |
| 5 | Mangiferin | -10.1 | Glu27, Tyr29, Glu292, Tyr255, Arg309, Tyr317(2), Arg312(2) | 9HB |
| 6 | Punarnavine | -9.9 | Arg309, Tyr29, Asn146 | 3HB |
| 7 | Berberine | -9.8 | Asn146 | 1HB |
| 8 | Quercetin | -9.5 | Glu292, Tyr255, Asn146, Asp145, Arg309, Arg312 | 6HB |
| 9 | Epicatechin | -9.0 | Tyr255, Glu292, Glu27, Asn305, Arg305, Tyr29 | 6HB |
| 10 | Gymnemic Acid | -9.0 | Tyr317, Arg309, Asn305, Trp27(2),Tpr255(3) | 7HB |
| 11 | Tinosporin A | -8.4 | Asn146, Asp145, Asp145, Leu304, Tyr317 | 5HB |
| 12 | Curcumin | -8.2 | Glu27, Tyr317, Arg309 | 3HB |
| 13 | Salacinol | -7.0 | Tyr255, Glu292(2), Asn146(2), Asp145, Tyr29, Glu27, Arg312, Asn305 | 10HB |
| 14 | Gallic Acid | -6.5 | Glu192, Glu292, Tyr255, Tyr29, Glu27, Asn146, Asp145 | 7HB |
| 15 | 2-Hydroxy -4-
Methoxybenzaldehyde |
-5.5 | Glu192, Asn146(2), Asp145 | 4HB |
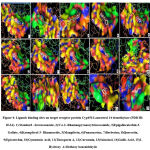 |
Figure 4: Ligands binding sites on target receptor protein Cyp450 Lanosterol 14 demethylase (PDB ID: 1EA1). |
1,3 β-Glucan synthase
β (1,3)-D-glucan is a polysaccharide component present in the cell wall of fungi that plays a major role in cell wall synthesis. The enzyme 1,3 β-Glucan synthase was suspected to be a target for many natural products like Aculeacin A, B, and Paulacandin. The inhibition of β (1,3)-D-glucan in the organisms such as Candida albicans, Aspergillus fumigatus, and Cryptococcus neoformans appeals to have a potential broad-spectrum fungal specific target that brings interest in new drug development. 61 Among all phytomolecules against 1,3 β-Glucan synthase enzyme, N-α-L Rhamnopyranosyl vincosamide showed higher binding energy of -10.6 Kcal/mol with 5 hydrogen bonds followed by Epigallocatechin-3-Gallate -10.5 Kcal/mol with 6 hydrogen bonds and Kaempferol -10.3 Kcal/mol with 6 hydrogen bonds respectively. Caspofungin, a standard compound interacts with Ser259, Tyr255, Trp277, Phe229 with 5 hydrogen bonds, Similarly, Epigallocatechin-3-Gallate, Mangiferin, Curcumin, Salacinol, and Gallic acid interact with Tyr255 amino acid. Trp277 amino acid interaction was found in Gymnemic acid similar to that of Standard drug with 1 hydrogen bond. The binding affinity of gymnemic acid was also found higher which has a binding energy of about -9.0 Kcal/mol with 9 hydrogen bonds compared with caspofungin standard. Other phytoconstituents binding interactions and their energy values are given in table 5 and the evidence for the interactions was given in figure 5.
Table 5: Interaction between Phytomolecules and Standard against 1,3β-Glucan Synthase (PDB ID: 1EQP).
| S.No | Phytomolecules | Binding energy (Kcal/mol) | Interacting residues in target protein | Number of Hydrogen bonds |
| 1 | Standard-Caspofungin | -7.8 | Ser259, Tyr255, Trp277, Phe229(2) | 5HB |
| 2 | N-α-L Rhamnopyranosyl vincosamide | -10.6 | Tyr317, Arg309(3), Asp151, Tyr153 | 6HB |
| 3 | Epigallocatechin-3-Gallate | -10.5 | Asn146, Asp145, Leu304, Asp145, Tyr255, Tyr29 | 6HB |
| 4 | Kaempferol-3- Rhamnoside | -10.3 | Tyr317, Arg309, Glu192, Leu304, Asp145 | 5HB |
| 5 | Mangiferin | -10.1 | Arg309,Tyr317(2), Arg312(2), Glu292, Tyr255, Tyr29, Glu27 | 9HB |
| 6 | Berberine | -10.0 | Asp146(2), Tyr317, Arg312 | 4HB |
| 7 | Punarnavine | -9.9 | Arg309, Asn145, Tyr29 | 3HB |
| 8 | Quercetin | -9.5 | Arg312, Arg309, Asp145, Tyr255, Glu292, Asn146 | 7HB |
| 9 | Epicatechin | -9.0 | Arg309, Asn305,Tyr255, Tyr29 | 4HB |
| 10 | Gymnemic Acid | -9.0 | Trp277, His254, Tyr255(2), Arg309, His253, Tyr317, Asn305, Asp318 | 9HB |
| 11 | Tinosporin A | -8.4 | Tyr317, Asn146, Asp145(2) | 4HB |
| 12 | Curcumin | -8.2 | Tyr255, Asn146(2) | 3HB |
| 13 | Salacinol | -7.1 | Tyr29(2), Tyr255, Glu292, Glu27, Asn146(2), Asn305, Arg309 | 9HB |
| 14 | Gallic Acid | -6.5 | Glu27, Tyr29, Tyr255, Glu192, Glu192, Asp145, Asn146 | 7HB |
| 15 | 2-Hydroxy -4-
Methoxy benzaldehyde |
-5.5 | Asp145, Glu192, Asn146(2) | 5HB |
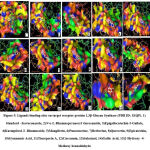 |
Figure 5: Ligands binding sites on target receptor protein 1,3β-Glucan Synthase (PDB ID: 1EQP). |
Thymidylate synthase
Thymidylate synthase (5, 10-methylenetetrahydrofolate dUMP C-methyltransferase) has a key role in DNA synthesis in mammals.62 It binds with dUMP and 5,10-methylenetetrahydrofolate as a co-factor that catalyzes the process called reduction methylation in substrate and forms dTMP and dihydrofolate.63 5-fluorocytosine is an antifungal; it has been used as an oral drug and by injection in combination with Amphotericin B for the treatment of Candida infections, chromomycosis, and cryptococcosis. Some common side effects include bone marrow suppression, vomiting, loss of appetite, diarrhea, and psychosis was observed while using this drug.64 Docking analysis showed Standard 5-Flurocytosine has -4.9 Kcal/mol binding energy interacting with Met149, Ser151, Ser154, His141, Tyr153 with 5 hydrogen bonds whereas N-α-L-Rhamnopyranosylvincosamide showed higher binding affinity towards target protein of -9.0 Kcal/mol has interacted with Ile108, Tyr258, Arg215, Asn226, Ser216, Arg215 with 7 hydrogen bonds. Salacinol has a higher binding affinity of -5.6 Kcal/mol with similar interacting residues Ser216, Asp218, His196, Tyr135, Glu87, Asn226 as the standard 5-Fluorocytosine which shows that a better antifungal effect with the same mechanism as standard to inhibit the thymidylate synthase enzyme. Other phytoconstituents binding interactions and their energy values are given in table 6 and the evidence for the interactions was given in figure 6.
Table 6: Interaction between Phytomolecules and Standard against Thymidylate synthase (PDB ID: 1HZW).
| S.NO | Phytomolecules | Binding energy (Kcal/mol) | Interacting residues in the target protein | Number of Hydrogen bonds |
| 1 | Standard 5-Fluorocytosine | -4.9 | Met149, Ser151, Ser154, His141, Tyr153 | 5HB |
| 2 | N-α-L-Rhamnopyranosyl vincosamide | -9.0 | Ile108, Tyr258, Arg215, Asn226(2), Ser216, Arg215 | 7HB |
| 3 | Kaempferol-3- Rhamnoside | -8.7 | Tyr258, His256, Gln214, His196, Asn226, Asp218, Tyr135, Ile108, Asn226 | 11HB |
| 4 | Epigallocatechin-3-Gallate | -8.6 | Ser216, Asp218, Gln214, Leu221, His196 | 5HB |
| 5 | Punarnavine | -8.5 | His196,Glu87(2) | 3HB |
| 6 | Tinosporin A | -8.3 | Leu221, His196, Asn226 | 9HB |
| 7 | Gymnemic Acid | -8.2 | Arg215, Leu251 | 2HB |
| 8 | Mangiferin | -7.9 | Lys77, Phe80, His196, Glu87 | 4HB |
| 9 | Berberine | -7.7 | Phe80 | 1HB |
| 10 | Epicatechin | -7.6 | Ala293, Arg140, Ile92 | 3HB |
| 11 | Quercetin | -7.2 | Phe80, Asn226 | 2HB |
| 12 | Curcumin | -6.8 | Tyr135, His196, Asn226 | 3HB |
| 13 | Gallic Acid | -5.8 | Glu100, Ser95, Thr96(3), His141 | 8HB |
| 14 | Salacinol | -5.6 | Ser216, Asp218, His196(2), Tyr135, Glu87(2), Asn226(2) | 9HB |
| 15 | 2-Hydroxy -4-
Methoxy benzaldehyde |
-5.0 | Asn226(2), His126 | 3HB |
 |
Figure 6: Ligands binding sites on target receptor protein Thymidylate synthase (PDB ID: 1HZW). |
Conclusion
Based on the results from in vitro and in silico studies, it is acknowledged that the polyherbal formulation acts as a potential antifungal effect against various fungal strains in dose dependent manner. In-silico analysis, the phytomolecules selected showed an affinity towards target enzymes, high binding energy, more hydrogen bond formation, and amino acid interactions which become additional evidence for the antifungal effect and also exert its mechanism of action through inhibition of various fungal metabolic enzymes. Further phytochemical present in polyherbal formulation have to be quantified to evaluate the concentration of each phytomolecules present in it which is under progress.
Acknowledgment
The author is thankful to Dr. K. Jayachandra, Research Scientist, Department of Clinical Chemistry, Sri Ramachandra Institute of Higher education and Research, Porur, Chennai for providing necessary guidance during the research work. The author is also thankful to the Dean and the management of SRM College of Pharmacy for the given opportunity to carry over the research work.
Conflict of Interest
All authors involved in this research work declared that there is no conflict of interest.
Funding Source
The research work has been Self-funded and has not received any external funds from any source.
References
- Umadevi A, Kumari C, Kumar, Am HK, Divya, Hisana V. Development And Evaluation of Polyherbal Gel for Antifungal Activity. Int. J. Curr. Pharm. Res., 2018; 10(5):40-43. 10. 40. 10.22159/ijcpr.2018v10i5.29694.
CrossRef - Aslam MS. An Update Review on Polyherbal Formulation: A Global Perspective. Systematic Reviews in Pharmacy., 2016;7(1):35-41
CrossRef - Roemer T, Krysan DJ. Antifungal drug development: challenges, unmet clinical needs, and new approaches. Cold Spring Harb Perspect Med. 2014;4(5):a019703. doi: 10.1101/cshperspect.a019703. PMID: 24789878; PMCID: PMC3996373.
CrossRef - Bhatt K, Agolli A, Patel MH, Garimella R, Devi M, Garcia E, Amin H, Domingue C, Guerra Del Castillo R, Sanchez-Gonzalez M. High mortality co-infections of COVID-19 patients: mucormycosis and other fungal infections. Discoveries (Craiova). 2021; 9(1):e126. doi: 10.15190/d.2021.5. PMID: 34036149; PMCID: PMC8137279.
CrossRef - Moriyama B, Gordon LA, Mccarthy M, Henning SA, Walsh TJ, Penzak SR. Emerging Drugs and Vaccines for Candidemia. Mycoses.2014; 57(12):718-733.
CrossRef - Parker JE, Warrilow AG, Price CL, Mullins JG, Kelly DE, Kelly SL. Resistance to antifungals that target CYP51. J Chem Biol. 2014;7(4):143-61. doi: 10.1007/s12154-014-0121-1. PMID: 25320648; PMCID: PMC4182338.
CrossRef - Mazu TK, Bricker BA, Flores-Rozas H, Ablordeppey SY. The Mechanistic Targets of Antifungal Agents: An Overview. Mini Rev Med Chem. 2016; 16(7):555-78. doi: 10.2174/1389557516666160118112103. PMID: 26776224; PMCID: PMC5215921.
CrossRef - Grover ND. Echinocandins: A ray of hope in antifungal drug therapy. Indian J Pharmacol. 2010;42(1):9-11. doi: 10.4103/0253-7613.62396. PMID: 20606829; PMCID: PMC2885632.
CrossRef - McCarthy, M. W., Kontoyiannis, D. P., Cornely, O. A., Perfect, J. R., & Walsh, T. J. Novel Agents and Drug Targets to Meet the Challenges of Resistant Fungi. J. Infect. Dis., 2017; 216(3):S474–S483. https://doi.org/10.1093/infdis/jix130
CrossRef - Mayer FL, Wilson D, Hube B. Candida albicans pathogenicity mechanisms. Virulence., 2013; 4(2):119–28.
CrossRef - Person AK, Chudgar SM, Norton BL, Tong BC, Stout JE. Aspergillusniger: An unusual cause of invasive pulmonary aspergillosis. J Med Microbiol., 2010;59(7):834–8.
CrossRef - Alspaugh JA. Virulence mechanisms and Cryptococcus neoformans pathogenesis. Fungal Genet Biol., 2015; 78:55–8.
CrossRef - Rodrigues AM, De Hoog S, De Camargo ZP. The emergence of pathogenicity in the Sporothrix schenckii complex. Med Mycol., 2013; 51(4):405–12.
CrossRef - Jorgensen JH & Ferraro MJ. Antimicrobial Susceptibility Testing: Special Needs for Fastidious Organisms and Difficult-to-Detect Resistance Mechanisms, Clin. Infect. Dis., 2000; 30(5):799–808. https://doi.org/10.1086/313788
CrossRef - Lekha GS. Evaluation of nephroprotective activity of sirupeelai kudineer (Aerva lanata decoction) in rats. J. Chem. Pharm. Res., 2015; 7: 522-530.
- Chung MJ, Pandey RP, Choi JW, Sohng JK, Choi DJ, Park YI. Inhibitory effects of kaempferol-3-O-rhamnoside on ovalbumin-induced lung inflammation in a mouse model of allergic asthma. In Immuno pharmacol., 2015; 25(2):302-10. doi: 10.1016/j.intimp.2015.01.031. Epub 2015 Feb 16. PMID: 25698556.
CrossRef - Kaur H. Boerhaavia diffusa: Bioactive Compounds and Pharmacological Activities. Biomed. Pharmacol. J., 2019; 12: 1675-1682. 10.13005/bpj/1797.
CrossRef - Aher VD, Chattopadhyay P, Patra A. Immunomodulatory Activity of Punarnavine Alkaloid from Boerhaavia diffusa. Curr. Bioact. Compd., 2020; 16(4):460-468.
CrossRef - Saraswati S, Abdulqader AA, Agrawal SS. Punarnavine, an alkaloid from Boerhaavia diffusa exhibits anti-angiogenic activity via down regulation of VEGF in vitro and in vivo. Chem Biol Interact., 2013; 206(2):204-13.
CrossRef - Deena Raj KM, Sujatha S. A Review on medicinal properties of Hemidesmus indicus. Adv. Biores., 2021; 12 (3) :238-247
- Alam MI, Alam MA, Alam O, Nargotra A, Taneja SC, Koul S. Molecular modeling and snake venom phospholipase A2 inhibition by phenolic compounds: Structure-activity relationship. Eur. J. Med. Chem., 2016; 114(23):209-219.
CrossRef - Arunakumara K, Subasinghe S. Salacia reticulata Wight: A Review of Botany, Phytochemistry and Pharmacology. Tropical Agricultural Research and Extension. 2011; 13(2):41–47. DOI: http://doi.org/10.4038/tare.v13i2.3137
CrossRef - Muraoka O, Ying S, Yoshikai K, Matsuura Y. Synthesis of a Nitrogen Analogue of Salacinol and Its α-Glucosidase Inhibitory Activity. Chem. Pharm. Bull., 2001; 49(11):1503-5.
CrossRef - Karunanayake EH, Sirimanne SR. Mangiferin from the root bark of Salacia reticulata. J Ethnopharmacol., 1985 ; 13(2):227-8. doi: 10.1016/0378-8741(85)90010-8. PMID: 4021520.
CrossRef - Shen J, Lu R, Cai Q, Fan L, Yan W, Zhu Z, Yang L, Cao Y. Mangiferin enhances the antifungal activities of caspofungin by destroying polyamine accumulation. Virulence., 2021; 12(1):217–230. https://doi.org/10.1080/21505594.2020.1870079
CrossRef - Dar A, Faizi S, Naqvi S, Roome T, Zikr-ur-Rehman S, Muhammad Ali, Firdous S, Moin ST. Analgesic and antioxidant activity of mangiferin and its derivatives: the structure-activity relationship. Biol Pharm Bull., 2005; 28(4):596-600
CrossRef - Saxena V, Lal N Rana M, Thomas A. Pharmacognostic and phytochemical analysis of Berberisaristata stem and standardization of berberine by HPLC, HPTLC and IR Spectra, IJSDR., 2021; 6(7):378-385
- Li Z, Geng Y, Jiang JD, Kong WJ. Antioxidant and anti-inflammatory activities of berberine in the treatment of diabetes mellitus. Evid Based Complement Alternat Med. 2014:289264.
CrossRef - Da Silva AR, Neto JBA, Da Silva CR, Campos RS, Costa Silva RA, Freitas DD, do Nascimento FB, de Andrade LN, Sampaio LS et al. Berberine Antifungal Activity in Fluconazole-Resistant Pathogenic Yeasts: Action Mechanism Evaluated by Flow Cytometry and Biofilm Growth Inhibition in Candida spp. Antimicrob. Agents Chemother., 2016; 60(6), 3551–3557. https://doi.org/10.1128/AAC.01846-15
CrossRef - Bhutada P, Mundhada Y, Bansod K, Dixit P, Umathe S, Mundhada D. Anticonvulsant activity of berberine, an isoquinoline alkaloid in mice. Epilepsy Behav., 2010; 18(3):207-210.
CrossRef - Manohar SH, Naik PM, Praveen N, Murthy HN. Distribution of gymnemic acid in various organs of Gymnema sylvestre. J. For. Res., 2009; 20: 268-270. 10.1007/s11676-009-0046-7.
CrossRef - Sinsheimer JE, Subba Rao G, McIlhenny HM, Smith RV, Maassab HF, Cochran KW. Isolation and antiviral activity of the gymnemic acids. Experientia., 1968; 24(3):302-303.
CrossRef - Kurihara Y. The anti-sweet activity of gymnemic acid A1 and its derivatives. Life Sci., 1969; 8(9):537-43.
CrossRef - Parveen S., Ansari M., Parveen R., Khan W., Ahmad S, Husain, S. A. Chromatography Based Metabolomics and In Silico Screening of Gymnema sylvestre Leaf Extract for Its Antidiabetic Potential. Evid. Based Complementary Altern. 2019:7523159. https://doi.org/10.1155/2019/7523159
CrossRef - Lesjak M, Beara I, Simin N, Pintac D, Majkic T, Bekvalac K, Orčić D, Mimica-Dukić N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives, J. Funct. Foods., 2018; 40:68-75,
CrossRef - Geetha T, Malhotra V, Chopra K, Kaur IP. Antimutagenic and antioxidant/prooxidant activity of quercetin. Indian J Exp Biol., 2005; 43(1):61-7.
- Tiwari P, Nayak P, Prusty SK, Sahu PK. Phytochemistry and Pharmacology of Tinospora cordifolia: A Review. Syst. Rev. Pharm., 2018; 9:70-78. 10.5530/srp.2018.1.14.
CrossRef - Priyanka M, Preya J, Sharav D, Dhara P, Dhananjay M. Phytochemical analysis and assessment of in vitro antibacterial activity of Tinospora cordifolia. Int. J. Curr. Microbiol.App.Sci., 2014; 3. 224-234.
- Anand J, Upadhyaya, B, Rawat P, Rai N. Biochemical characterization and pharmacognostic evaluation of purified catechins in green tea (Camellia sinensis) cultivars of India. Biotech.2015; 5(3): 285–294. https://doi.org/10.1007/s13205-014-0230-0
CrossRef - Chen PN, Chu SC, Kuo WH, Chou MY, Lin JK, Hsieh YS. Epigallocatechin-3 Gallate Inhibits Invasion, Epithelial−Mesenchymal Transition, and Tumor Growth in Oral Cancer Cells. J. Agric. Food Chem., 2011; 59(8): 3836–3844.
CrossRef - Ignat I, Stingu A, Volf I, Popa VI. Characterization of grape seed aqueous extract and possible applications in biological systems. Cellul. Chem. Technol., 2011; 45:205-209.
- Zhang T, Ma L, Wu P, Li W, Li T, Gu R, Dan X, Li Z, Fan X, Xiao Z. Gallic acid has anticancer activity and enhances the anticancer effects of cisplatin in non‑small cell lung cancer A549 cells via the JAK/STAT3 signaling pathway. Oncol Rep., 2019; 41(3):1779-1788.
CrossRef - Ma Z. F, Zhang, H. Phytochemical Constituents, Health Benefits, and Industrial Applications of Grape Seeds: A Mini-Review. Antioxidants (Basel, Switzerland)., 2017; 6(3):71. https://doi.org/10.3390/antiox6030071
CrossRef - Duangyod T, Palanuvej C, Ruangrungsi N. (+)-Catechin and (-)-Epicatechin contents and antioxidant activity of commercial black catechu and pale catechu. J. Chem. Pharm. Res., 2014; 6(7):2225-2232
CrossRef - Sanghvi K, Chandrasheker K. S, Vasudev Pai, Aswatha Ram H. N. Review on Curcuma longa: Ethnomedicinal uses, Pharmacological Activity, and Phytochemical constituents. Research J. Pharm. and Tech., 2020; 13(8):3983-3986. doi: 10.5958/0974-360X.2020.00704.0
CrossRef - Chainani N. Safety and Anti-inflammatory activity of Curcumin: A component of Turmeric (Curcuma longa). J Altern Complement Med., 2004; 9(1):161-168.
CrossRef - Antony S, Kuttan R, Kuttan G. Immunomodulatory activity of curcumin. Immunol Invest., 1999; 28(5-6):291-303. doi: 10.3109/08820139909062263. PMID: 10574627.
CrossRef - Asouri M, Ataee R, Ahmadi Aa, Amini A, Moshaei MR. Antioxidant and Free Radical Scavenging Activities of Curcumin. Asian J. Chem., 2013; 25(13)7593-7595
CrossRef - Panda S, Kar A, Sharma P, Sharma A. Cardioprotective potential of N, a-l-rhamnopyranosylvincosamide, an indole alkaloid, isolated from the leaves of Moringaoleifera in isoproterenol induced cardiotoxic rats: in vivo and in vitro studies. Bioorg Med ChemLett., 2013; 23:959–962. doi:10.1016/j.bmcl. 2012.12.060
CrossRef - Nadia Noble-Daoud Aniss, Yasser H. Abdel Rahman, Asmaa M. Zaazaa. Cardioprotective effect of Moringaoleifera against doxorubicin cardiotoxicity in leukemia rat model. International Journal of Pharmaceutical and Phytopharmacological Research., 2020; 10(2), pp.148-161.
- Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olsen AJ. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem., 1998; 19(14):1639–62.
CrossRef - Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem., 2010; 31(2), 455–461. https://doi.org/10.1002/jcc.21334
CrossRef - Kuncha J, Thirugnanasambantham P., Shanmugam K, Narayanan N. In vitro antibacterial and antifungal activity of hydro-alcoholic extract of polyherbal formulation. J. Pharm. Sci. Res., 2019; 11(3), 721-725.
- Oliveiraa VM, Carraroa E, Aulerb ME, Khalil NM. Quercetin and rutin as potential agent antifungal against Cryptococcus spp, Braz. J. Biol., 2016; 76(4):1029-1034.
CrossRef - Chen M, Zhai L, Arendrup MC. In vitro activity of 23 tea extractions and epigallocatechingallate against Candida species. Med Mycol., 2015; 53(2):194-8.
CrossRef - Da Silva CR, De Andrade Netoa JB, de Sousa CR, Figueiredo NS, Sampaio LS, Magalhãesa HI, Cavalcanti BC, Gaspar DM, de Andrade GM, Lima ISP et al. Synergistic Effect of the Flavonoid Catechin, Quercetin, or EpigallocatechinGallate with Fluconazole Induces Apoptosis in Candida tropicalisResistant to Fluconazole. Antimicrobial Agents and Chemotherapy,2014; 8(3):1468-1478.
CrossRef - Tempesti TC, Alvarez MG, de Araújo MF, Junior FEAC, de Carvalho MG, Durantini EN. Antifungal activity of a novel quercetin derivative bearing a trifluoromethyl group on Candida albicans. Med Chem Res., 2011; 21: 2217-2222. 10.1007/s00044-011-9750-x.
CrossRef - Stoilova I, Jirovetz L, Stoyanova A, Krastanov AI. Antioxidant activity of the polyphenol mangiferin. Elec. J. Env. Agricult. Food Chem., 2008; 7. 2706-2716.
- Paul S, Mohanram K, Kannan I. Antifungal activity of curcumin-silver nanoparticles against fluconazole-resistant clinical isolates of Candida species. Ayu., 2018; 39(3):182–186.
CrossRef - Yoshida Y. Primary Target for Azole. Curr Top Med Mycol., 1988; (51):388–418.
CrossRef - Douglas CM. Fungal β (1,3)-D-glucan synthesis. Med Mycol Suppl., 2001; 9(1):55–66.
CrossRef - Lockshin A, Moran RG, Danenbergt P V. Thymidylate synthetase purified to homogeneity from human leukemic cells (affinity chromatography/fluorinated pyrimidines/enzyme-inhibitor complex/neoplastic tissue/amino acid analysis). Proc Natl Acad Sci U S A., 1979; 76(2):750-4.
CrossRef - Anderson AC, Perry KM, Freymann DM, Stroud RM. The crystal structure of thymidylate synthase from Pneumocystis carinii reveals a fungal insert important for drug design. J Mol Biol., 2000; 297(3):645–57.
CrossRef - Bennett JE, Dismukes WE, Duma RJ et al. A comparison of amphotericin B alone and combined with flucytosine in the treatment of cryptococcal meningitis. N Engl J Med., 1979; 301:126-31.
CrossRef








