Manuscript accepted on :13-10-2022
Published online on: 27-10-2022
Plagiarism Check: Yes
Reviewed by: Dr. Ankur Singh
Second Review by: Dr. Ana Golez
Final Approval by: Dr. Ayush Dogra
Alfaqih Hussain Omar1 , Khalid Hajissa2
, Khalid Hajissa2 , Jarrar Qais Bashir3
, Jarrar Qais Bashir3 , Alfaqih Sirin Omar4, Aldoghachi Ahmed Faris5
, Alfaqih Sirin Omar4, Aldoghachi Ahmed Faris5 , Abu Bakar Nurhidanatasha1*
, Abu Bakar Nurhidanatasha1*
1Biomedicine Programme, School of Health Sciences, Health Campus, Universiti Sains Malaysia, 16150 Kubang Kerian, Kelantan, Malaysia.
2Department of Medical Microbiology and Parasitology, School of Medical Sciences, Health Campus, Universiti Sains Malaysia, 16150 Kubang Kerian, Kelantan, Malaysia.
3Department of Pharmaceutical Science, Faculty of Pharmacy, Al-Isra’a University, Amman, Jordan.
4Department of Internal Medicine, Al Noor Specialist Hospital, Mecca 20424, Saudi Arabia.
5Faculty of Medicine and Health Sciences, Universiti Tunku Abdul Rahman, Cheras, 43000 Kajang, Selangor, Malaysia.
Corresponding Author E-mail:hussain.omar.alfaqih@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2521
Abstract
Artemisinin and its derivatives, a class of antimalarial drugs, were first isolated from Artemisia annua. Artemisinin can alter the pH of the malaria parasite’s digestive vacuole from acidic to alkaline, leading to parasite death. However, the precise mechanism of artemisinin action in changing the digestive vacuole pH has not yet been confirmed. Previous studies reported that artemisinin and its derivatives could kill the parasites through the generation of oxidative stress by the free radicals they generate. This review aims to provide a better understanding of the possible mechanism of action of artemisinin, focusing on the antimalarial activity caused by the generated free radicals through the induction of mutation in the genes that encode the proton pump of the Plasmodium falciparum digestive vacuole.
Keywords
Artemisinin; Free radicals; Proton pump; Plasmodium falciparum; V-type H+-ATPase; VMA gene
Download this article as:| Copy the following to cite this article: Omar A. H, Hajissa K, Bashir J. Q, Omar A. S, Faris A. A, Nurhidanatasha A. B. A Postulated Mechanism of the Antimalarial Effect of Free Radicals Generated by Artemisinin on Plasmodium falciparum. Biomed Pharmacol J 2022;15(4). |
| Copy the following to cite this URL: Omar A. H, Hajissa K, Bashir J. Q, Omar A. S, Faris A. A, Nurhidanatasha A. B. A Postulated Mechanism of the Antimalarial Effect of Free Radicals Generated by Artemisinin on Plasmodium falciparum. Biomed Pharmacol J 2022;15(4). Available from: https://bit.ly/3DaxIeY |
Introduction
Malaria is a life-threatening disease caused by parasites of the genus Plasmodium that is transmitted to humans by female mosquitoes of the genus Anopheles 1. The infection has been identified as one of the major causes of morbidity and mortality globally2. Efforts have been made to identify effective antimalarial drugs over decades of years; however, drug-related problems have emerged in parallel with these discoveries 3,4.
Multiple types of effective antimalarial drugs have since been introduced as alternatives to the original treatments, such as artemisinin. Artemisinin, a sesquiterpene lactone, was first isolated from the Chinese plant, Artemisia annua in 1970 5. The 1,2,4-trioxane system contains an endoperoxide bridge, the active pharmacophore, which plays an important role in the antimalarial activity of artemisinin 6. Although it has been considered one of the most important drugs for the treatment of malaria, the exact mechanism of action remains controversial 7.
Materials and Methods
Methods: We conducted an extensive review of the literature on the antimalarial effects and mechanisms of action of artemisinin, focusing on in vitro studies conducted to evaluate the pH alteration of the P. falciparum digestive vacuole after treatment with this compound.
Results and Discussion
Previous studies reported that antimalarial drugs, such as artemisinin and its derivatives consist of free radicals that are a source of oxidation, which may cause parasite death 8. Artemisinin produces reactive oxygen species (ROS) in the digestive vacuole through the activation of the endoperoxide bridge, and free Fe2+ can increase the generation of ROS via the Fenton process. Thus, it has been reported that artemisinin-activated ROS may lead to parasite death by reducing the ability of the parasite’s antioxidant defence system to remove free radicals 9.
On the other hand, it has been reported that artemisinin can alter the pH of the P. falciparum digestive vacuole from acidic to alkaline 10 (Figure 1). Thus, the protease enzyme that functions only within the digestive vacuole with a pH ranging from 3.7–6.5 11, and which is responsible for the digestion of haemoglobin to release the amino acids as a nutrient source for the parasite, is inhibited, leading to the parasite death 12. However, the precise mechanism behind this phenomenon remains controversial 13.
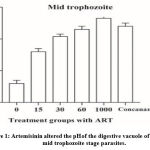 |
Figure 1: Artemisinin altered the pH of the digestive vacuole of the mid trophozoite stage parasites. |
Permeabilized resealed erythrocytes infected with mid trophozoite stage parasites containing FITC-dextran, a ratiometric pH indicator, treated with 15, 30 (sub-lethal concentrations), 60 (a concentration near the IC50-4 hours), 1000 nM (a positive control), and concanamycin A (a standard proton pump inhibitor), confirming the induction of acid-base transition. A non-treated condition was used as a negative control. The data are expressed as mean ± SEM from three independent experiments done in triplicates. The bar graph was generated using GraphPad Prism (version 7). Modified from Ibrahim (2020) 10.
We hypothesized that the alteration of the digestive vacuole pH from acidic to alkaline after treatment with artemisinin might result from the induction of mutation by the free radicals, such as superoxide anion (O2-.) generated from this class of antimalarial drugs in the gene (VMA) encoding the proton pump (V-type H+-ATPase) that is responsible for providing an acidic environment for the parasite’s digestive vacuole, as well as for other microorganisms, including yeast 14,15,21.
Previous studies have reported that the biochemical and genetic properties of yeast V-ATPases are similar to those of other eukaryotic cells; these sharing features encourage the use of yeast V-ATPase as a model for the study of the V-ATPases located at the P. falciparum digestive vacuole’s membrane 16.
Yeast V-ATPase consists of a cytosolic V1 domain, which catalyzes the ATP hydrolysis and the integral membrane Vo domain, which forms the transmembrane proton pore. V-ATPases are responsible for controlling the acidification of yeast and other microorganisms via reassembling and disassembling the V-ATPase complex 17 (Figure 2).
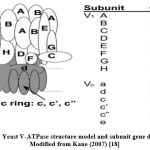 |
Figure 2: Yeast V-ATPase structure model and subunit gene designations. |
Modified from Kane (2007)18
The V1 domain consists of eight subunits, labeled as A, B, C, D, E, F, G, and H. Whereas the V0 domain consists of six subunits labeled as a, c, c’, c”, d, and e. Each of these subunits is encoded by the following genes; VMA1, VMA2, VMA3, VMA4, VMA5, VMA6, VMA7, VMA8, VMA9, VMA10, VMA11, VMA13, and VMA16 19 (Figure 2).
The free radicals have the capability of inducing oxidative damage that leads to a cellular DNA mutation 20. Once a mutation occurs in the genes encoding the yeast V-ATPase, the vacuolar acidification will be lost 21. This postulates that a mutation can also be induced in the gene encoding the V-type H+– ATPase of the P. falciparum digestive vacuole via free radicals generated from artemisinin, ultimately leading to parasite death.
Conclusion
In summary, the present review provides a better understanding of the antimalarial activity of artemisinin and illustrates how artemisinin may alter the pH of the P. falciparum digestive vacuole through the induction of mutation in the gene that encodes the proton pump, V-type H+-ATPase of this organelle through the subsequently generated free radicals.
However, the lack of experimental evidence might have somewhat limited the demonstration of a true cause-and-effect relationship in this study. Therefore, further studies are needed to identify the specific genes that encode the proton pump V-type H+-ATPase of the parasite digestive vacuole, as well as to confirm the induction of mutation in those genes by the free radicals generated from artemisinin.
Acknowledgement
The authors wish to thank the Ministry of Higher Education, Malaysia for providing the Fundmental Research Grant Scheme (FRGS) (FRGS/1/2019/STG03/USM/03/03).
Conflict of Interest
All authors declare that they have no conflict of interest.
References
- World Health World malaria report. Geneva(GE) (2019).
- Talapko J, Škrlec I, Alebić T, Jukić M, Včev A. Malaria: The past and the Microorganisms. 7(6): 179 (2019).
CrossRef - Chu C, White Management of relapsing Plasmodium vivax malaria. Expert Rev. Anti Infect Ther., 14(10): 885-900 (2016).
CrossRef - Thu A, Phyo A, Landier J, Parker D, Nosten F. Combating multidrug‐resistant Plasmodium falciparum FEBS J. 284(16): 2569-2578 (2017).
CrossRef - Liu C. Discovery and Development of Artemisinin and Related Chinese Herbal Medicines. 9: 101-114 (2017).
CrossRef - Rudrapal M, Chetia Endoperoxide antimalarials: development, structural diversity and pharmacodynamic aspects with reference to 1,2,4trioxane-based structural scaffold. Drug Des., Devel. Ther., 10: 3575-3590 (2016).
CrossRef - Pooley S, Krishna S, Gerisch M, Haynes R, Wong H, Staines M, et Artemisone uptake in Plasmodium falciparum-infected erythrocytes. Antimicrob. Agents Chemother., 55(2): 550-556 (2011).
CrossRef - Percário S, Moreira D, Gomes B, Ferreira M, Laurindo P, Green M, et Oxidative stress in malaria. Int. J. Mol. Sci., 13(12): 16346-16372 (2012).
CrossRef - Ismail H, Barton V, Phanchana M, Charoensutthivarakul S, Wong M, Hemingway J, Biagini G, Ward S, et al. Artemisinin activity-based probes identify multiple molecular targets within the asexual stage of the malaria parasites Plasmodium falciparum Proc. Natl. Acad. Sci. U. S. A., 113(8): 2080-2085 (2016).
CrossRef - Ibrahim N, Roslee A, Azlan M, Abu-Bakar Sub-lethal concentrations of artemisinin alter pH of the digestive vacuole of the malaria parasite, Plasmodium falciparum. Trop. Biomed., 37(1): 1-14 (2020).
- Hayward R, Saliba K, Kirk K. The pH of the digestive vacuole of Plasmodium falciparum is not associated with chloroquine J. Cell Sci., 119(6): 1016-1025 (2006).
CrossRef - Wunderlich J, Rohrbach P, Dalton J. The malaria digestive Front. Biosci., 4: 1424-1448 (2012).
CrossRef - Klonis N, Crespo-Ortiz M, Bottova I, Abu-Bakar N, Kenny S, Rosenthal P, & Tilley Artemisinin activity against Plasmodium falciparum requires hemoglobin uptake and digestion. Proc. Natl. Acad. Sci. U. S. A., 108(28): 11405-11410 (2011).
CrossRef - Oluwatosin Y, Kane P. Mutations in the yeast KEX2 gene cause a Vma(-)-like phenotype: a possible role for the Kex2 endoprotease in vacuolar acidification. Cell. Biol., 18(3): 1534–1543 (1998).
CrossRef - Diab HI, Kane PM. Loss of vacuolar H+-ATPase (V-ATPase) activity in yeast generates an iron deprivation signal that is moderated by induction of the peroxiredoxin TSA2. Biol. Chem., 288(16): 11366-11377 (2013).
CrossRef - Graham LA, Finnigan GC, Kane PM. Some assembly required: Contributions of Tom Stevens’ lab to the V‐ATPase field. Traffic, 19(6): 385-390 (2018).
CrossRef - Parra KJ, Chan CY, Chen J. Saccharomyces cerevisiae vacuolar H+-ATPase regulation by disassembly and reassembly: one structure and multiple signals. Cell, 13(6): 706-714 (2014).
CrossRef - Kane PM. The long physiological reach of the yeast vacuolar H+-ATPase. Bioenerg. Biomembr., 39(5): 415-421 (2007).
CrossRef - Sherr GL, Shen C. The Interplay of key phospholipid biosynthetic enzymes and the yeast V-ATPase pump and their role in programmed cell death. In: Tutar Y. (Ed.), Regulation and dysfunction of apoptosis. IntechOpen (2021). https://www.intechopen.com/chapters/76861 doi: 10.5772/intechopen.97886
CrossRef - Pourahmad J, Salimi A, Seydi E. Role of oxygen free radicals in cancer development and treatment. In Ahmad R. (Ed.), Free radicals and diseases. (2016). https://www.intechopen.com/chapters/51903 doi: 10.5772/64787
CrossRef - Beyenbach K, Wieczorek H. The V-type H+-ATPase: molecular structure and function, physiological roles and regulation. Exp. Biol. 209(4): 577-589 (2006).
CrossRef







