Faten Farid Awdallah1 , Islam Hassan Abulnaga2*
, Islam Hassan Abulnaga2* , Suzy Fawzy Michael1
, Suzy Fawzy Michael1 , Hassan Khaled Nagi1
, Hassan Khaled Nagi1 and Mohamed Hosny Abdallah1
and Mohamed Hosny Abdallah1
1Department of Critical care, Cairo University, Cairo, Egypt.
2Department of Critical care, National heart institute, Cairo, Egypt.
Corresponding Author E-mail: islamhassanabulnaga@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2506
Abstract
Rapid recognition of elevated intracranial pressure (ICP) is essential to avoid brain stem herniation and death. Brain stem herniation is a very serious disorder in which an excess of intracranial pressure causes part of the brain to be squeezed through the foramen magnum at the base of the skull. Increased pressure on the brain stem can lead to blood pressure and breathing problems and brain death, respiratory or cardiac arrest, permanent brain damage, coma and death. The aim of this work was to evaluate the diagnostic accuracy of using Optic Nerve Sheath Diameter (ONSD) measurements to detect elevated ICP compared to computed tomography (CT) brain scanning. A prospective, observational cross-sectional study of 100 randomly selected ICU patients with suspected elevated ICP at Cairo university hospitals and cardiothoracic surgical ICU of the National Heart Institute (NHI), Cairo, Egypt. Optic nerve ultrasonography (US) was performed on all patients; CT brain scanning was chosen as the gold standard for detecting elevated ICP. Results: the cause of elevated ICP, 51.3% of patients were admitted with head trauma P-value = 0.000, clinical signs of elevated ICP, 31.3% of patients developed convulsions and 26.3% showed Cushing reflex (P=0.004 and 0.010 respectively). Besides the Glasgow Coma Scale (GCS), patients with elevated ICP had a mean GCS score of 7 and patients without elevated ICP had mean GCS of 13 (P˂0.001). Regarding ONSD, mean average ONSD was 6 in patients with elevated ICP and 3.1 in patients without elevated ICP (P˂0.01), receiver operating characteristic (ROC) curve analysis revealed that ONSD greater than 4.31 mm was significant in prediction of elevated ICP compared to CT brain scanning with 94.8% sensitivity and 90.11% specificity. Conclusion: patients’ ONSD is a simple bedside technique that can accurately diagnose of elevated ICP compared to CT brain scanning. The diagnostic accuracy of ONSD using a 4.31 mm cut-off value can diagnose of elevated ICP with 94.8% sensitivity and 90.11% specificity.
Keywords
Elevated Intracranial Pressure; Optic Nerve Sheath Diameter; Sonographic Measurement
Download this article as:| Copy the following to cite this article: Awdallah F. F, Abulnaga I. H, Michael S. E, Nagi H. K, Abdallah M. H. Sonographic Measurement of the Optic Nerve Sheath Diameter to Improve Detection of Elevated Intracranial Pressure. Biomed Pharmacol J 2022;15(3). |
| Copy the following to cite this URL: Awdallah F. F, Abulnaga I. H, Michael S. E, Nagi H. K, Abdallah M. H. Sonographic Measurement of the Optic Nerve Sheath Diameter to Improve Detection of Elevated Intracranial Pressure. Biomed Pharmacol J 2022;15(3). Available from: https://bit.ly/3PY0XXt |
Introduction
Elevated ICP is critical problem in patients with head trauma, ischemic or hemorrhagic stroke, post-cardiac arrest or post-cardiopulmonary bypass (CPB). There is a significant association between elevated ICP and poor outcome, with elevated ICP carrying a mortality rate of about 20%1-4. Consequently, to minimize brain stem herniation and mortality, prompt detection of increased ICP is critical for implementing therapeutic options such as osmotic therapy, decompression craniectomy, craniotomy, and haemorrhage evacuation, or cerebrospinal fluid (CSF) diversion5-6. ICP monitoring is used to guarantee appropriate cerebral perfusion pressure (CPP), however it is associated with a high risk of infection and mortality 7-9. Because the optic nerve sheath and its contents are continuous with the dura mater and the subarachnoid space. As a result, increased ICP causes an increase in the ONSD 10-12. Because it is a straightforward and noninvasive procedure that can be used for critically ill patients, CT brain is the gold standard for diagnosing increased ICP. Brain edoema, constriction or removal of sulci, periventricular lucency (areas of hypodensity or lower attenuation around ventricles), and mass effect (change in ventricular size) are all common signs of increased ICP in CT brain scans 13,14. As a result, the goal of this study was to compare the diagnostic accuracy of ONSD measures vs CT brain scans in detecting increased ICP.
Patients and Methods
A prospective observational study was conducted on 100 patients with suspected elevated ICP and planned to do CT scan from the ICU of Cairo university hospitals and cardiothoracic surgical ICU of the National Heart Institute (NHI), Cairo, Egypt, during the period between January 2017 and July 2018.
Ethical consideration
Both the critical care department at Cairo university hospitals and National Heart Institute Institute’s ethical committees gave their approval (NHI). In addition, clinicaltrials.gov was used to register the current study (Registration ID: NCT03529370). All participants or their surrogates signed a written informed consent form. Patients who were suspected of having an increased ICP and scheduled to undergo a CT scan were included in the study.
Inclusion criteria
Patients with a high ICP who were scheduled for a CT scan. Intracranial bleeding with mass effect, changes in ventricular size (dilatation or compression), changes in rate of grey/white matter (brain edoema), constriction or elimination of sulci (brain edoema), periventricular lucency were all considered indicators of elevated ICP (areas of hypodensity or lower attenuation around ventricles).
Exclusion criteria
Individuals with glaucoma history, eyelid injuries, and orbital edoema After receiving consent to participate in the study, medical records were used to acquire a complete medical history (age, gender, BMI risk factors such as diabetes mellitus, hypertension, and ischemic heart disease), as well as a neurological examination for all participants.
Following the CT scan, the same operator performed an optic nerve ultrasonography (US) on all patients (the US operator did not know whether the patient scheduled for medical, surgical, or even the result of CT scan).
Patients were placed in a supine position with their eyes closed, a generous amount of sterile gel was applied to the closed eyelid, and a Siemens ultrasound machine (ACUSON S3000TM) was used to apply a 6–12 MHz linear probe to the superior and lateral aspects of the upper eyelid, then the probe was angled slightly medially and caudally until the oblique hypoechoic tract of the optic nerve could be visualized with clear margins posterior to the retina’s diameter should be measured 3 mm behind it.
For all patients, optic nerve US was performed immediately after the first CT scan and within 48 hours following brain lowering measures after the second scan (conservative medical therapy and surgical therapy).
Sample size calculation
Power Analysis and Sample Size (PASS) Software version 11 (NCSS, LLC, Kaysville, Utah, USA) was used to compute the required sample size. According to local institutional data, approximately 75% of patients clinically suspected of having elevated ICP had elevated ICP based on CT results. As a result, a sample of 100 patients clinically suspected of having elevated ICP would be expected to contain 80 patients with elevated ICP based on CT results (positive group) and 20 patients without elevated ICP (negative group), resulting in a negative group/positive group ratio of 0.33. This sample size achieved 90% power to detect a change in the area under the receiver operating characteristic curve (ROC) for predicting elevated ICP using ONSD from a null value of 0.5 to an alternative value of 0.7, which is considered the smallest AUC for a predictor to have clinical value, this calculation used a two-sided z-test with a significance level, alpha = 0.05.
Statistical analysis
IBM SPSS version 23 (IBM Corp, Armonk, NY) was used to analyse the data. Categorical data were presented as numbers and percentages. The unpaired t-test was used to compare intergroup differences. Numerical data were provided as means with standard deviations (SDs). The paired-samples t-test was used to compare numerical data, the Pearson correlation was used to evaluate correlations, and the receiver-operating characteristic (ROC) curve analysis was used to look at the diagnostic/predictive value of ONSD. A P-value of 0.05 or less was declared statistically significant.
Results
In the current study, 120 patients were suspected of having elevated intracranial pressure, but 7 patients were excluded due to facial trauma and orbital edoema, 4 patients with tear in the eyelid, 4 patients who failed to obtain consent from their relatives, and 5 patients who refused to sign consents, leaving 100 adult patients with suspected elevated ICP. Optic nerve US was performed on both eyes of the studied population to measure ONSD after CT scan. The study’s population included 100 adult patients with suspected increased ICP, with 80 of them showing evidence of elevated ICP in CT brain scans (these 80 patients were named as group A in this study) and 20 of them did not show signs of elevated ICP in CT brain (these 20 patients were named as group B where we did not repeat the test). In CT brain scanning, 56 patients in Group A showed symptoms of brain edoema, 25 showed mass effect, and 25 showed signs of hydrocephalus and periventricular lucency. The 56 patients with signs of brain edoema received conservative medical treatment, including osmotherapy, to decrease raised ICP. In follow-up CT brain scanning, 14 of them still showed signs of elevated ICP, while 42 of them did not. 38 patients with signs of elevated ICP underwent surgical intervention to relieve elevated ICP, either decompression craniectomy (DC), craniotomy and evacuation of intracranial haemorrhage, or ventriculoperitoneal shunt (VPS), and all of these patients did not show signs of elevated ICP in follow-up CT brain scanning. (Figure 1).
 |
Figure 1: Flowchart diagram of the studied population. |
Table 1 reveals that there was no statistically significant difference between the two groups of patients based on demographic data and risk variables of the examined population (Table 1).
The most common cause of increased ICP in the current study was head trauma, which was responsible for 41 of group A’s patients (51.3%), as well as ischemic or hemorrhagic stroke, post-cardiac arrest, post CPB, and hepatic encephalopathy. Convulsions and the Cushing reflex were common in group A patients, according to clinical symptoms in the study population, (Table 2)
Table 1: Demographic data and risk factors in the studied population.
|
Demographic data |
CT brain | Test value | P-value | ||
| Group A | Group B | ||||
| Age/year | Mean ± SD | 42.11 ± 15.37 | 48.80 ± 12.38 | -1.803• | 0.074 |
| Range | 18 – 74 | 27 – 62 | |||
| Sex | Male | 58 (72.5%) | 14 (70.0%) |
0.050* |
0.824 |
| Female | 22 (27.5%) | 6 (30.0%) | |||
| DM
|
Yes | 36 (45.0%) | 5 (25.0%) | 0.684 | |
| No | 48 (60.0%) | 11 (55.0%) | |||
| HTN | Yes | 36 (45.0%) | 5 (25.0%) | 2.646 * | 0.104 |
| No | 44 (55.0%) | 15 (75.0%) | |||
| IHD | Yes | 32 (40.0%) | 10 (50.0%) | 0.657* | 0.418 |
| No | 48 (60.0%) | 10 (50.0%) | |||
| BMI | Mean ± SD | 32.50 ± 5.64 | 33.35 ± 4.88 | -0.618• | 0.538 |
| Range | 22 – 42 | 25 – 38 | |||
*: Chi-square test; •: Independent t-test, DM: diabetes mellitus, HTN: hypertension, IHD: ischemic heart disease, BMI: body mass index.
Table 2: Clinical signs in the studied population.
|
Clinical signs |
CT brain | Test value | P-value | ||
| Group A | Group B | ||||
| No. = 80 | No. = 20 | ||||
| Pupils | Equal & reactive | 68 (85.0%) |
20 (100.0%) |
3.409* | 0.182 |
| Dilated fixed | 5 (6.3%) | 0 (0.0%) | |||
| Unequal | 7 (8.8%) | 0 (0.0%) | |||
| Convulsions | Yes | 25 (31.3%) | 0 (0.0%) | 8.333* | 0.004 |
| No | 55 (68.8%) | 20 (100.0%) | |||
| Cushing reflex | Yes | 21 (26.3%) | 0 (0.0%) | 6.646* | 0.010 |
| No | 59 (73.8%) | 20 (100.0%) | |||
| MAP | Mean ± SD | 84.71 ± 2.26 | 84.35 ± 1.81 | 0.664• | 0.508 |
| Range | 81 – 89 | 81 – 88 | |||
| Signs of lateralization | Yes | 7 (8.8%) | 0 (0.0%) | 1.882* | 0.170 |
| No | 73 (91.3%) | 20 (100.0%) | |||
*: Chi-square test; •: Independent t-test
The mean average ONSD in group A patients was 61.6, but in group B patients it was 3.1 (difference =-2.9, 95% CI=-2.8 to -3, P<0.000), indicating that average ONSD is useful in detecting increased ICP. The optimal cut off for ONSD was 4.310 mm, with an area under the ROC curve (AUC) of 0.975 (95 percent CI 0.80-0.929, p=0.014) for detection of high ICP using average ONSD. ONSD had a sensitivity and specificity of 94.8 percent and 90.11%, respectively, for detecting high ICP, (Table 3, Figure 2).
Table 3: ROC curve for the prediction of elevated ICP using average ONSD.
| Area | Asymptotic Sig. | cut-off | Sensitivity | Specificity | Asymptotic 95% Confidence Interval |
| 0.975 | .014* | >4.31 mm | 94.8% | 90.11% | 0.80-0.929 |
 |
Figure 2: shows the ROC curve for the prediction of elevated ICP using average ONSD. |
There was a significant reduction in ONSD after receiving osmolar therapy and after surgical intervention as shown in (Table 4, Figure 3).
Table 4: The Comparison of average ONSD before and after conservative medical therapy and surgical intervention.
| Pre-medical conservative therapy | Post-medical conservative therapy | Paired differences | ||||||||
| n | Mean | SD | Mean | SD | Mean | SD | 95% CI | P-value* | ||
| Average ONSD (mm) | 56 | 6 | 1.6 | 4 | 1.5 | -2 | 1.4 | -2.37 to -1.76 | <0.0001 | |
| Pre-surgical intervention | Post-surgical intervention | Paired differences | ||||||||
| Average ONSD (mm) | n | Mean | SD | Mean | SD | Mean | SD | 95% CI | P-value* | |
| 38 | 6 | 1.6 | 3.1 | 1.5 | -2.9 | 2.7 | -2.98 to -2.81 | <0.0001 | ||
n = number, SD = standard deviation, 95% CI = 95% confidence interval. *Paired t-test.
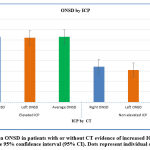 |
Figure 3: Mean ONSD in patients with or without CT evidence of increased ICP. Error bars represent the 95% confidence interval (95% CI). Dots represent individual observations. |
Discussion
To our knowledge, this is the first study to look into the utility of ONSD in Egyptians. The findings of our study are divided into two categories: first, determining the diagnostic accuracy of ONSD in comparison to the gold standard, and second, determining the clinical utility of ONSD in following ICP deceasing mode. To begin with, our findings revealed that ONSD is a simple bedside approach that can accurately diagnose ICP when compared to CT brain scanning, which is considered the gold standard. With 94.8 percent sensitivity and 90.11 percent specificity, ONSD greater than 4.31 mm was considered evidence of elevated ICP, according to ROC analysis. The current findings are consistent with those of other studies 14. The majority of studies have suggested a 5 mm cut-off point 15-16.
Several studies have found that the cutoff for ONSD correlation with increased intracranial pressure (EICP) is between 4.8 mm and 5.9 mm when compared to invasive ICP monitoring 17-21. An ONSD cutoff of 4.8 mm was found to be 96 percent sensitive and 94 percent specific for elevated ICP by Rajajee et al. 21. Moretti and Pizzi,22 compared ultrasound assessment of the ONSD to invasive ICP monitoring and reported that a cutoff of 5.2 mm for increased ICP was 93 percent sensitive and 74 percent specific. Lim et al., in a 2017 study on patients with traumatic brain injury, found that ONSD higher than 5.5 mm (measured on CT) was a good indication of midline shift, with a sensitivity of 89.9% and a specificity of 80%, 23, 24.
Second, our findings showed that osmolar therapy reduced ICP, as evidenced by a significant reduction in ONSD among patients in group A who had symptoms of excessive ICP disappear in CT brain scans. These findings are consistent with those of a prospective observational study conducted by Launey et al. in 2014, which included patients with a severe acute brain injury who were monitored with an invasive ICP monitor and the ONSD was measured for each patient who had a sustained elevated ICP. ICP and CPP were also recorded at the same time. All measures were taken before and after a 20 percent mannitol infusion for 20 minutes. In all cases, the ONSD was greater than 5.8 mm before osmotherapy, and the ONSD significantly decreased after mannitol infusion from 6.3 (6.1-6.7) mm to 5 mm. (5.5-6.3) mm (p=0.0007). Concomitantly, the ICP decreased from 35 (32-41) to 25 (22-29) mmHg (p=0.001) and the CPP increased from 47 (50-60) to 66 (59-69) mmHg (p=0.003), 25.
Finally, the study found that surgical intervention, such as craniotomy and cerebral haemorrhage evacuation, craniectomy, or VPS insertion, reduced ICP. ONSD was considerably higher in those individuals before surgical intervention than after surgery, indicating that surgical intervention was effective in reducing ONSD in patients who had no symptoms of raised ICP in CT brain after surgery. The ONSD was measured to determine VPS malfunction in a study conducted by Zaidi & Yamamoto in 2014, and similar results were reported. They discovered that VPS dysfunction increased the growth of the optic nerve 226. Gao et al. in 2018 on the other hand, looked at patients after a hemicraniectomy in a prospective observational analysis. Within 6 hours of surgery, all patients had invasive ICP monitoring and ocular ultrasonography. They followed the patients for 6 months and analyzed the ONSD in relation to ICP and neurological result, concluding that the ONSD assessed with ultrasound was unreliable for determining ICP following hemicraniectomy, albeit it did have potential predictive value for a bad neurological outcome 27.
Several factors could account for their findings. First, the impact of decompressive craniectomy (DC) consequences on CSF hydrodynamics, such as subdural hygroma and post-traumatic hydrocephalus, was overlooked 28. CSF secretion may be reduced, outflow may be affected, or CSF may be redistributed as a result of surgical craniectomy. Such changes in CSF hydrodynamics could prevent the ONSD from decreasing after DC was used to treat intracranial hypertension 29. Damage to the optic nerve sheath may also cause the reversal of ONSD distension to be delayed or prevented 30. Because they evaluated patients who had undergone hemicraniectomy surgery, their data differed from the results of the current study. Patients who received any surgical intervention to treat increased ICP, such as decompressive craniectomy, craniotomy, and drainage of cerebral haemorrhage, or CSF drainage by a VPS, were included in the current study, 31.
Limitations of the Study
The effect of surgical intervention to relieve elevated ICP was observed whether this surgical intervention was decompression craniectomy or craniotomy and evacuation of intracranial hemorrhage or VPS, but the effect of each type of surgical intervention on ICP was not included in this study. Also, the study was done on Egyptian patients only who belong to the Caucasian race and was not done on different races.
Conclusion
ONSD is a simple bedside technique that can accurately diagnose of elevated ICP compared to CT brain scanning. The diagnostic accuracy of ONSD using a 4.31 mm cut-off value can predict of elevated ICP with 94.8% sensitivity and 90.11% specificity.
Conflict of Interest
There is no conflict of interest.
Funding Sources
There is no funding Source.
References
- Miller JD, Becker DP, Ward JD, Sullivan HG, Adams WE, Rosner MJ. Significance of intracranial hypertension in severe head injury.J Neurosurg; 47(4):503-16 (1977)
CrossRef - Miller JD, Butterworth JF, Gudeman SK, Faulkner JE, Choi SC, Selhorst JB, et al. Further experience in the management of severe head injury. J Neurosurg; 54(3):289-99 (1981)
CrossRef - Marshall LF, Smith RW, Shapiro HM. The outcome with aggressive treatment in severe head injuries. Part I: the significance of intracranial pressure monitoring. J Neurosurg; 50(1):20-5 (1979)
CrossRef - Fakhry SM, Trask AL, Waller MA, Watts DD. Management of brain-injured patients by an evidence-based medicine protocol improves outcomes and decreases hospital charges.J Trauma; 56(3):492-9 (2004)
CrossRef - Saul TG, Ducker TB. Effect of intracranial pressure monitoring and aggressive treatment on mortality in severe head injury.J Neurosurg; 56(4):498-503 (1982)
CrossRef - Hakim S, Venegas JG, Burton JD. The physics of the cranial cavity, hydrocephalus, and normal pressure hydrocephalus: mechanical interpretation and mathematical model.Surg Neurol; 5(3):187-210 (1976)
- Lane PL, Skoretz TG, Doig G, Girotti MJ. Intracranial pressure monitoring and outcomes after traumatic brain injury. Can J Surg; 43(6):442-8 (2000)
- Cremer OL, van Dijk GW, van Wensen E, Brekelmans GJ, Moons KG, Leenen LP, et al. Effect of intracranial pressure monitoring and targeted intensive care on functional outcome after severe head injury. Critical care medicine; 33(10):2207-13 (2005)
CrossRef - Dang Q, Simon J, Catino J, Puente I, Habib F, Zucker L, et al. More fateful than fruitful? Intracranial pressure monitoring in elderly patients with traumatic brain injury is associated with worse outcomes.J Surg Res; 198 (2):482-8 (2015)
CrossRef - Shapiro HM. Intracranial hypertension: therapeutic and anesthetic considerations.Anesthesiology; 43(4): 445-71 (1975)
CrossRef - Lidofsky SD, Bass NM, Prager MC, Washington DE, Read AE, Wright TL, et al. Intracranial pressure monitoring and liver transplantation for fulminant hepatic failure. Hepatology;16(1):1-7 (1992)
CrossRef - Bingaman WE, Frank JI. Malignant cerebral edema and intracranial hypertension.Neurol Clin; 13(3):479-509 (1995)
CrossRef - Hansen HC, Helmke K. The subarachnoid space surrounding the optic nerves: an ultrasound study of the optic nerve sheath. Surg Radiol Anat; 18:323–328 (1996)
CrossRef - Hansen HC, Helmke K, Kunze K. Optic nerve sheath enlargement in acute intracranial hypertension. Neuro-Ophthalmology; 14:345–354 (1994)
CrossRef - Helmke K, Hansen HC. Fundamentals of transorbital sonographic evaluation of optic nerve sheath expansion under intracranial hypertension, I: an experimental study. Pediatr Radiol; 26:701–705 (1996)
CrossRef - Tayal VS, Neulander M, Norton HJ, Foster T, Saunders T, Blaivas M. Emergency department sonographic measurement of optic nerve sheath diameter to detect findings of increased intracranial pressure in adult head injury patients. Ann Emerg Med; 49:508–514 (2007)
CrossRef - Harden SP, Dey C, Gawne-Cain ML. Cranial CT of the unconscious adult patient. Clin Radiol; 62(5):404-415 (2007)
CrossRef - Wang LJ, Yao Y, Feng LS, Wang YZ, Zheng NN, Feng JC, et al. Noninvasive and quantitative intracranial pressure estimation using the ultrasonographic measurement of optic nerve sheath diameter. Scientific reports; 7:42063 (2017)
CrossRef - Blaivas M, Theodoro D, Sierzenski PR. Elevated intracranial pressure detected by bedside emergency ultrasonography of the optic nerve sheath. Academic emergency medicine; 10(4):376-81 (2003)
CrossRef - Tsung JW, Blaivas M, Cooper A, Levick NR. A rapid noninvasive method of detecting elevated intracranial pressure using bedside ocular ultrasound: application to 3 cases of head trauma in the pediatric emergency department. Pediatric emergency care; 21(2):94-8 (2005)
CrossRef - Rajajee V, Vanaman M, Fletcher JJ, Jacobs TL. Optic nerve ultrasound for the detection of raised intracranial pressure. Neurocritical care; 15(3):506-15 (2011)
CrossRef - Moretti R, Pizzi B. Optic nerve ultrasound for detection of intracranial hypertension in intracranial hemorrhage patients: confirmation of previous findings in a different patient population. J Neurosurg Anesthesiol; 21:16 (2009)
CrossRef - Lim TK, Yu BC, Ma DS, Lee GJ, Lee MA, Hyun SY, et al. Correlation between optic nerve sheath diameter measured by computed tomography and elevated intracranial pressure in patients with traumatic brain injury. Journal of Trauma and Injury; 30(4):140-4 (2017)
CrossRef - Novkoski M, Gvozdenović A, Kelečić M, Gopčević A, Mazul-Sunko B, Širanović M, et al. Correlation between Glasgow Coma Scale Score and intracranial pressure in patients with a severe head injury. Acta clinica Croatica; 40(3):191-5 (2001)
- Launey Y, Nesseler N, Le Maguet P, Mallédant Y, Seguin P. Effect of osmotherapy on optic nerve sheath diameter in patients with increased intracranial pressure. Journal of neurotrauma; 31(10):984-8 (2014)
CrossRef - Zaidi SJ, Yamamoto LG. Optic nerve sheath diameter measurements by CT scan in ventriculoperitoneal shunt obstruction. Hawai’i Journal of Medicine & Public Health; 73(8):251 (2014)
- Gao Y, Li Q, Wu C, Liu S, Zhang M. Diagnostic and prognostic value of the optic nerve sheath diameter with respect to the intracranial pressure and neurological outcome of patients following hemicraniectomy. BMC neurology; 18(1):199 (2018)
CrossRef - Czosnyka M, Copeman J, Czosnyka Z, McConnell R, Dickinson C, Pickard JD. Post-traumatic hydrocephalus: influence of craniectomy on the CSF circulation. J Neurol Neurosurg Psychiatry; 68(2):246–8 (2000)
CrossRef - Shapiro K, Fried A, Takei F, Kohn I. Effect of the skull and dura on neural axis pressure-volume relationships and CSF hydrodynamics. J Neurosurg; 63(1):76 (1985)
CrossRef - Rajajee V, Fletcher JJ, Rochlen LR, Jacobs TL. Comparison of accuracy of optic nerve ultrasound for the detection of intracranial hypertension in the setting of acutely fluctuating vs stable intracranial pressure: posthoc analysis of data from a prospective, blinded single-center study. Crit Care; 16(3): R79 (2012)
CrossRef - Chen H, Ding GS, Zhao YC, Yu RG, Zhou JX. Ultrasound measurement of optic nerve diameter and optic nerve sheath diameter in healthy Chinese adults. BMC Neurol;15(1):106 (2015).
CrossRef







