Moushira Zaki1 and Eman R. Youness2*
and Eman R. Youness2*
1Biological Anthropology Department, Medical Research and ClinicalStudies Institute - National Research Centre, Cairo, Egypt.
2Medical Biochemistry Department, Medical Research Division, Medical Research and Clinical StudiesInstitute - National Research Centre, Cairo, Egypt.
Corresponding Author E-mail: hoctober2000@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2465
Abstract
Somatotype is the parameter used to determine the body composition. Our objective was to prove if there are somatotype divergences among metabolically healthy women and women with metabolic syndrome. Study included 100 obese women aged 28.09± 9.21 years with metabolic syndrome (MetS) and 100 healthy control women without MetS matched in age and BMI. Metabolic condition was evaluated according to International Diabetes Federation criteria (IDF), whilst somatotype was achieved via Heath-Carter method. There were notable variations in somatotype between the two groups. Women without metabolic syndrome had obviously higher ectomorph-mesomorph (p<0.03) mesomorph-endomorph (8.31-4.51-0.61) in comparison to women with metabolic syndrome showing higher endomorph-mesomorph 6.67-4.41-0.62. Significant positive correlation was found between HOMA-IR and endomorph component (p = 0.01) as well as between triglycerides and endomorph component in MetS group (p=0.01), but no difference was observed in those without. We concluded that endomorph is more dominant in metabolically obese women and mesomorph in group with MetS. Metabolic abnormalities are directly correlated with the 1 The obtained results imply the important role of the nonadipose components, presented by mesomorphy and ectomorphy, in the distinction between healthy and risky metabolic profile. This study expressed the need for somatotyping in MetS to deal with disease prevention.
Keywords
Biochemical parameters; metabolic syndrome; obese women; somatotyping
Download this article as:| Copy the following to cite this article: Zaki M, Youness E. R. Somatotype Features and Biochemical Characteristics of Patients With and Without Metabolic Syndrome in Obese Women. Biomed Pharmacol J 2022;15(3). |
| Copy the following to cite this URL: Zaki M, Youness E. R. Somatotype Features and Biochemical Characteristics of Patients With and Without Metabolic Syndrome in Obese Women. Biomed Pharmacol J 2022;15(3). Available from: https://bit.ly/3BGIoT3 |
Introduction
Somatotype expressed in three types (ectomorph, mesomorph and endomorph) that experimentally describe diverse features of the body composition: the musculoskeletal development, linearity and the body composition degree hotness 2. Obesity, particularly central category, has been confirmed as independent risk factor for the progress of metabolic and cardiovascular turbulences. Nevertheless, numerous persons with phenotypically obese have naturalistic metabolic profile3,4. Incidence of impediments in obesity is not associated with obesity phenotype however might be imputed to inflammations related the pattern of visceral fat distribution and metabolic abnormalities, dysfunction regardless of total body fats5. The techniques discussing the metabolic turbulences in individuals having metabolically obese normal weight, in addition, avoid the progress of metabolic irregularities in individuals with metabolically healthy obese, are imperfectly comprehend. This could be of great value in the conception of these situations6. Little is known about the association between somatotype obesity and chronic diseases. MetS is an escalating and major clinical challenge and universal public-health in the wake of increasing obesity, sedentary life habits surplus energy intake and urbanization. MetS cause a 5-fold upsurge in the risk of 2-fold the risk of developing cardiovascular disease (CVD) and type 2 diabetes mellitus (T2DM) over the next five to ten years8. Presently, no researches where the somatotype of patients with metabolic abnormalities has been founded, especially in the Egyptian inhabitants. Some studies indicate that 10-25% of obese individuals are actually metabolically healthy 3,4,9.
Consequently, we aimed to create the somatotype of patients with MetS among obese women with and without MetS and assess its relation with metabolic parameters.
Subjects and methods
The study comprises 200 obese women; 100 with MetS and 100 without MetS. A written informed consent was obtained from each participant. The study was approved by National Research Centre Ethics Committee, Egypt (Number 16361), according to the World Medical Association’s Declaration of Helsinki. Exclusion criteria include viral infection within the last 2 months or subjects with a history of any systemic chronic disease. During the past 2 months, subjects that received vitamin supplements systemic steroids and mineral or antibiotics were also omitted. Somatotype was assessed using Heath & Carter method2. Anthropometric measures details have been previously reported
. Anthropometric measurements were recorded in duplicate and on the right side of the body (where appropriate). Height was measured with the patients standing with their backs leaning against the stadiometer of the same scale. Body mass index was calculated as weight in kilograms divided by height in meters squared (kg/m2). Waist circumference was measured with light clothing at a level midway between the lower rib margin and the iliac crest standing and breathing normally. Sum of skinfolds including triceps, biceps,and supraspinale were calculated following the recommendations of the International Biological Program 11
Homeostasis Model Insulin Resistance (HOMA-IR) was used to estimate insulin resistance12. Mean values with t-distribution comparison tests were done where normality could be expected, with a statistical significance of 95%. Medians comparison tests were performed SPSS 20.0 software was used for statistical
analysis.when normality could not be assumed. Plotting somatotypes on 2-D stomato-chart as follows:
X−coordinate = Mesomorphy – Endomorphy
Y−coordinate= 2 x Mesomorphy- (Endomorphy + Ectomorphy)
Results are presented as mean ± standard deviation
Results
Descriptive statistics for the anthropometric variables and age for all cases have been presented in Table 1. There was significant increase in skinfolds and waist circumference in women with MetS compared to those without. Somatotype components for patients with MetS was 6.67-4.41-0.62 in correspondence to an endomorph-mesomorph somatotype and was 8.31-4.51-0.61 in obese cases Mean without MetS, corresponding to ectomorph-mesomorph mesomorph to endomorph (Figure 1). Table 2 shows biochemical parameters of the study groups. Significant increase of serum lipids and HOMA-IR was observed in obese cases with MetS compared to those without. Table 3 shows significant positive correlation between HOMA-IR and endomorph somatotype in group with MetS as well as between TG triglycerides and endomorph. On the other hand, no relations were observed in the other one without MetS. Somatotypes are elucidated and represent an overview of the dominance of each component in Fig. 1.
Table 1: Descriptive statistics of anthropometric the studied women.
| Variables | With MetS | Without MetS | P |
|
Age (years) |
27.19± 3.21 |
26.88±4.12 |
0.067 |
| BMI (kg)/height (m)2 | 31.45± 3.33 | 30.89±3.67 | 0.087 |
| Sum of skinfolds (mm) | 25.88±6.64 | 20.77±5.99 | 0.05 |
| Waist circ. (cm) | 99.78± 5.66 | 91.65±4.66 | 0.05 |
| Arm circ. (cm) | 34.88± 4.44 | 33.23±3.74 | 0.06 |
| Calf circ. (cm) | 38.99±3.21 | 35.44±4.12 | 0.09 |
| Humerus width | 5.88±2.10 | 5.77±2.23 | 0.08 |
| Epicondyle width | 8.28±1.22 | 7.99±1.15 | 0.07 |
Table 2: Biochemical parameters of obese women with and without MetS.
|
Characteristics Parameters |
Obese | P values | |
| With MetS | Without MetS | ||
| HOMA-IR | 6.7 ± 1.3 | 3.4 ± 1.2 | 0.01 |
| FBG (mg/dL) | 133.4±12.7 | 98.0 ±8.9 | 0.01 |
| TC(mg/dL) | 255.3±13.2 | 205.6±9.01 | 0.01 |
| TG (mg/dL) | 247.8± 13.94 | 210.2±10.33 | 0.01 |
| HDL-C (mg/dL) | 68.6±5.8 | 45.84 ±10.8 | 0.01 |
| LDL-C (mg/dL) | 163.89± 25.88 | 153 ± 20.88 | 0.01 |
Table 3: Correlations between somatotype features and biochemical parameters in obese women with and without MetS.
| Variable | with MetS | without MetS | ||
| r | P | r | P | |
| HOMA-IR-Endomorphy | 0.64 | 0.01 | 0.22 | 0.66 |
| HOMA-IR- Mesomorphy | 0.24 | 0.06 | 0.11 | 0.77 |
| HOMA-IR- Ectomorphy | 0.21 | 0.67 | -0.34 | 0. 05 |
| TG- Endomorphy | 0.66 | 0.01 | 0.23 | 0.07 |
| TG- Mesomorphy | 0.21 | 0.07 | 0.24 | 0.06 |
| TG- Ectomorphy | 0.21 | 0.66 | 0.15 | 0.080.03 |
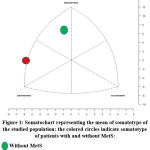 |
Figure 1: Somatochart representing the mean of somatotype of the studied population: the colored circles indicate somatotype of patients with and without MetS. |
Discussion
Different aspects of body composition are described by somatotype. It is utilized for the estimation of the variations in physique throughout physical activity, ageing and growth. Nevertheless, some studies displayed that it could be used to predict specific diseases13 . Obesity not constantly exhibit metabolic complications14. Previously, mesomorph endomorphs suffer from neurosis, lumbosacral radiculitis or digestive system disorders13. It was shown a significant relationship between clinical/subclinical cardiovascular disease and metabolically healthy obese phenotype and all-cause mortality15,16. Metabolically normal-weight persons are recognized to establish metabolic turbulences despite of BMI normal values. Numerous researches revealed low lean mass and higher body fat in the obese persons with metabolically normal-weight 17,18. In normal weight persons, incidence of metabolic aberration is imputed to subcutaneous fat tissue dysfunction due to monocytes/macrophage or infiltration while in these patients, visceral fat tissues are unaffected19. The two phenotypes of obesity are described as metabolically healthy obese and metabolically unhealthy normal weight. The metabolically healthy obese individuals have a favorable lipid profile, normal insulin response, normal blood pressure, and a minor visceral fat despite surplus quantity of body fat4. The variance of these two phenotypes has been argued, conversely, the incidence of well condition in metabolic healthy obese persons has been partially due to normal function of adipose tissue. It is imperative to differentiate them as opposed to just lean versus obese. However, information concerning demographic characteristics of individuals and lifestyle 20of both are contradictory21. Incidence of impediments in obesity is not associated to obesity phenotype but might be due to inflammatory related metabolic dysfunction, aberrations and visceral fat allocation pattern regardless of total fat of the body 5,22. In analyzing the fitness of somatotype to predict metabolic risk, endomorph and mesomorph types were shown as better markers for metabolic abnormalities2312425. In agreement with our findings mesomorph component in diabetic patients was observed1 and waist circumference and TG are main risk factors for insulin resistance26. Obesity and insulin resistance may contribute in the development of metabolic syndrome among the middle-aged women27.
Conclusion
In conclusion, the findings of this study indicate somatotype difference in obese women with MetS and those with without. This is the first reporting on the somatotype study among Egyptian patients with MetS. The greatest predictor of somatotype among metabolically obese was endomorph-mesomorph on the other hand, ectomorph- mesomorph is the greatest predictor in metabolically healthy obese one, as it mainly reveals muscularity. Moreover, significant association of abnormal metabolic parameters with somatotypes features may account for their contribution in MetS risk.
Conflict of Interest
There is no conflict of interest.
Funding Sources
There is no funding sources.
References
- Galić BS, Pavlica T, Udicki M, et al. Somatotype characteristics of normal-weight and obese women among different metabolic subtypes. Arch Endocrinol Metab. 2016;60:60-65.
CrossRef - Carter JEL, Carter JEL, Heath BH. Somatotyping: Development and Applications. Vol 5. Cambridge university press; 1990.
- Karelis AD, Brochu M, Rabasa‐Lhoret R, Garrel D, Poehlman ET. Clinical markers for the identification of metabolically healthy but obese individuals. Diabetes, Obes Metab. 2004;6(6):456-457.
CrossRef - Blüher M. The distinction of metabolically “healthy” from “unhealthy” obese individuals. Curr Opin Lipidol. 2010;21(1):38-43. doi:10.1097/MOL.0b013e3283346ccc
CrossRef - Ding C, Chan Z, Magkos F. Lean, but not healthy: the ‘metabolically obese, normal-weight’phenotype. Curr Opin Clin Nutr Metab Care. 2016;19(6):408-417.
CrossRef - Messier V, Karelis AD, Prud’homme D, Primeau V, Brochu M, Rabasa‐Lhoret R. Identifying metabolically healthy but obese individuals in sedentary postmenopausal women. Obesity. 2010;18(5):911-917.
CrossRef - Consultation WHO. Obesity: preventing and managing the global epidemic. World Health Organ Tech Rep Ser. 2000;894:1-253.
- Alberti KGMM, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international . Circulation. 2009;120(16):1640-1645.
CrossRef - Blüher M. Metabolically Healthy Obesity. Endocr Rev. 2020;41(3):bnaa004. doi:10.1210/endrev/bnaa004
CrossRef - Zaki M, Kamal S, Basha WA, et al. Assessment of DNA damage in obese premenopausal women with metabolic syndrome. Gene Reports. 2018;10. doi:10.1016/j.genrep.2017.10.012
CrossRef - Tanner JM, Hiernaux J, Jarman S, Weiner JS, Lourie JA. Growth and physique studies. Hum Biol A Guid to F methods IBP Handb. 1969;9:1-60.
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412-419.
CrossRef - Koleva M, Nacheva A, Boev M. Somatotype and disease prevalence in adults. Rev Environ Health. 2002;17(1):65-84.
CrossRef - Grundy SM. Metabolic complications of obesity. Endocrine. 2000;13(2):155-165.
CrossRef - Roberson LL, Aneni EC, Maziak W, et al. Beyond BMI: The “Metabolically healthy obese” phenotype & its association with clinical/subclinical cardiovascular disease and all-cause mortality–a systematic review. BMC Public Health. 2014;14(1):1-12.
CrossRef - Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions? A systematic review and meta-analysis. Ann Intern Med. 2013;159(11):758-769.
CrossRef - Ruderman N, Chisholm D, Pi-Sunyer X, Schneider S. The metabolically obese, normal-weight individual revisited. Diabetes. 1998;47(5):699-713.
CrossRef - Tanaka S, Togashi K, Rankinen T, et al. Is adiposity at normal body weight relevant for cardiovascular disease risk? Int J Obes. 2002;26(2):176-183.
CrossRef - Knight ET, Liu J, Seymour GJ, Faggion Jr CM, Cullinan MP. Risk factors that may modify the innate and adaptive immune responses in periodontal diseases. Periodontol 2000. 2016;71(1):22-51.
CrossRef - Brennan SL, Henry MJ, Nicholson GC, Kotowicz MA, Pasco JA. Socioeconomic status and risk factors for obesity and metabolic disorders in a population-based sample of adult females. Prev Med (Baltim). 2009;49(2-3):165-171.
CrossRef - Phillips CM. Metabolically healthy obesity: Definitions, determinants and clinical implications. Rev Endocr Metab Disord. 2013;14(3):219-227. doi:10.1007/s11154-013-9252-x
CrossRef - Ipsen DH, Tveden-Nyborg P, Lykkesfeldt J. Dyslipidemia: Obese or Not Obese—That Is Not the Question. Curr Obes Rep. 2016;5(4):405-412. doi:10.1007/s13679-016-0232-9
CrossRef - Chatterjee P, Bhattacarjee A, Banerjee A, Bandyopadhyay S, Bandyopadhyay A. Some important metabolic markers in blood of trained endomorph, mesomorph and ectomorph male athletes. Int J Physiol Nutr Phys Educ. 2017;2(1):335-338.
- Kalichman L, Livshits G, Kobyliansky E. Association between somatotypes and blood pressure in an adult Chuvasha population. Ann Hum Biol. 2004;31(4):466-476.
CrossRef - Urrutia-García K, Martínez-Cervantes TJ, Salas-Fraire O, Guevara-Neri NP. Somatotype of patients with type 2 diabetes in a university hospital in Mexico. Med Univ. 2015;17(67):71-74.
CrossRef - Ascaso JF, Romero P, Real JT, Lorente RI, Martı́nez-Valls J, Carmena R. Abdominal obesity, insulin resistance, and metabolic syndrome in a southern European population. Eur J Intern Med. 2003;14(2):101-106. doi:https://doi.org/10.1016/S0953-6205(03)00022-0
CrossRef - Lin W-Y, Yang W-S, Lee L-T, et al. Insulin resistance, obesity, and metabolic syndrome among non-diabetic pre- and post-menopausal women in North Taiwan. Int J Obes. 2006;30(6):912-917. doi:10.1038/sj.ijo.0803240
CrossRef








