Mutah University, Faculty of Sciences, Department of Medical Lab Sciences, Mutah Street, AL-KaraK City, Jordan.
Corresponding Author E-mail: nafitawa77@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2476
Abstract
Context: Treating wounds of various ailments is a large part of the public health care budget. The Artemisia jordanica (Aj) and Achillea fragrantissima (Af) plants are folk medicinal plants that are still increasingly used to treat wounds by healers. Objective: The purpose of this study was to investigate the activity and wound healing by using the singular and combined aqueous extracts of both herbs Aj and Af. Wound healing activity was followed through excision, incision, and burn wound models. Methods: Animals were divided into eight groups (n = 8), each group was divided into two sub-groups (n = 4), one for incision and the second for both excision and burn models. Groups were treated with either 5% or 10% w/w of combined or single herb(s). Betadine and petroleum gel were used as positive and negative control, respectively. Wound contraction rate, tensile strength, period of epithelization and histological transformations were used as scores to evaluate the effect of treatments on wound healing in wounds models. Results and discussion: Wound healing activity of 10% combined aqueous extracts of (Aj-Af) has shown a worthy attainment which has evidenced by inducement of tensile strength on the 22.1 ± 1.34 day of the treatment as compared with positive control on the 21.8 ± 0.73 day of the treatment. The wound contraction rate was occurred on the 17.4 ± 0.7 day of the treatment (p < 0.05) compared with positive and negative control on the 18.5 ± 0.14 and 24.5 ± 0.7 days of the treatment, respectively. Histological observation indicates that the wounds treated with 10% Aj-Af extract have showed thickening of epidermis and formation of granulation tissue with more prominent collagenation and blood vessels formation. Conclusions: Singular and combined aqueous extracts of Aj and Af exhibited good healing activities compared with negative and positive control (p < 0.05). Both singular and combined extracts induced significant healing markings; tensile strength, wound contraction rate, granulation and collagenation compared with negative and positive controls. Moreover, healing potential activity of combined extract (mainly 10%) revealed better marginal healing activity regarding single-herb use.
Keywords
Artemisia jordanica; Achillea fragrantissima; Herbal therapy; Tensile strength; Wound contraction rate
Download this article as:| Copy the following to cite this article: Al-Tawarah N. M. Singular and Combined Healing Activity of Aqueous Extract of Artemisia Jordanica and Achillea Fragrantissima in Rabbit’s Incision, Excision and Burn Models. Biomed Pharmacol J 2022;15(3). |
| Copy the following to cite this URL: Al-Tawarah N. M. Singular and Combined Healing Activity of Aqueous Extract of Artemisia Jordanica and Achillea Fragrantissima in Rabbit’s Incision, Excision and Burn Models. Biomed Pharmacol J 2022;15(3). Available from: https://bit.ly/3qRFgxb |
Introduction
Skin wound means any disruption of epidermal layer of skin by physical, chemical or microbial infection 1. Skin wounds are classified to different categories according to level of contamination and either if open or closed; one of these types are incision (sharp edges wound), excision (removed a part of skin) and burn wound 2,3. In which, differing from incision (i.e. just a cut of skin), excision involves partial removing of epidermis and parts of the dermis 4. Specifically, burn wounds classification depends upon causative agents: chemical, radiative, electrical, and finally the thermal burn injuries that all cause rupturing of cutaneous barrier 5. Wound healing is a complex process, compromise inflammatory series starting with a vasoconstrictive to control bleeding, following with releasing of many inflammatory mediators, and finally proliferative step consists migration and proliferation of different cells to restore integrity of destructive area 6,7.
Each year, several millions of people suffering from acute wounds, by which prevention of septic and healing complications require proper management and treatment 8. Nowadays, researchers looking toward immense potential effects of herbal uses for all medical ailments including management of healing process 9,10. Traditional medication is globally one of the main sources for treating diseases 11–15. In developing countries, more than 70% of the population still depends on traditional medicine. In Jordan, over 65% of the population believes that traditional medicine treatment is effective in curing many diseases 16. Two of these traditional herbs Artemisia jordanica (Aj) and Achillea fragrantissima (Af) used by traditional healers and local people long time ago for management of the wounds 17. Both Aj and Af belongs to the same family Asteraceae 18,19. Traditionally both herbs are processed by decoction; boiling the plant, then it is cooled to be used directly, either alone or by mixing herbs together with different gradients 20. Then both are used, as single herb or mixture, with effective prognosis in various medical conditions such as colic, anthelmintic, and wound healing 21. In many cultures, worldwide, herbal medicine serves potential therapeutic tools in treatment of many skin disorders, including injuries and burns 22.
Animal models were used to study wide variety of antimicrobial strategies of skin injuries, as well as healing capacities of some therapeutic tools 23. In fact, since a while ago rabbits were used as wound healing model, in which assessment of epithelization degree, collagenation and vascularization are the main ways used for assessment of healing efficiency 24. In fact, many studies revealed the potential therapeutic effectiveness of herb-herb combination for treatment of many ailments regardless single herb 25. In view of traditional investigation of both herbs; Aj and Af, in wounds management, by herb-herb combination versus single herb, present work was designed to evaluate the potential healing effects of liquid extract of Aj and Af, in three wound models; incision, excision and burn, in a rabbit.
Materials and methods
Plants material and preparation of plant extract
Both plants, Aj and Af, were selected in this study based on their potential therapeutic use in traditional medicine in wound management by rural population and traditional healers. Leaves, the part normally used by traditional healers of both plants, were collected from the southern part of Jordan, in May 2020. Both plants were recognized and validated by plant taxonomist Prof. Dr. Salih Al-Quraan, Department of Biology, Faculty of Science, Mutah University. Leaves were cleaned with a potable water, and then dried in a dark place without direct exposure to direct sunshine for one week. For each plant, about 500 g were weighed, grounded and macerated in 2 l distilled water overnight at room temperature. Decoction was filtered using Buchner funnel and Whatman filter no. 10 and evaporated above water bath to achieve about 500 ml of aqueous extract, and then were stored in a refrigerator at 2–4 °C. Filtered aqueous extract was then lyophilized (freeze drying) using a freeze drying system to yield the extracts of both investigated plants named aqueous lyophilized Aj and aqueous lyophilized Af 26–28. Preparation of topical ointments (gel) by mixing lyophilized extracts and petroleum gel w/w details present in Table.1.
Table 1. Preparation of topical ointment from lyophilized extracts of both herbs mixed with petroleum gel.
| Ointment formula (w/w) | AJ lyophilized extracts | Af lyophilized extracts | Petroleum gel |
| Aj 5% gel | 50 mg | – | 1 g |
| Af 5% gel | – | 50 mg | 1 g |
| Aj 10% gel | 100 mg | – | 1 g |
| Af 10% gel | – | 100 mg | 1 g |
| Aj-Af 5% gel | 25 mg | 25 mg | 1 g |
| Aj-Af 10% gel | 50 mg | 50 mg | 1 g |
Experimental animals
Sixty-four males’ rabbits, weighing 1.8 ± 0.2 kg were investigated during present study. In fact, to minimize the animal factor that may make differences during the test, we synchronized the animal used in this test as much as possible including breed, age, weight, and health conditions. Physiological parameters (i.e. temperature, hair characteristics, respiration rate, feeding rate, etc.) have assessed for all rabbits to make sure that all animals with a good health status. Animals were housed in clean cages with proper ventilation, maintained on a 12 h light/dark interval, fed with proper ad libitum standard fed. Animals were acclimatized for three successive days in these new conditions before starting the study. In all parts of the present work: handling, anesthesia, induction of wounds’ models were performed by a registered veterinarian (the author himself), as well as animal care facilities were maintained as recommended by international guidelines. Animals that exhibited normal behavior and showed no signs of infection were accepted in the study only, else were excluded. Present work was reviewed and approved by institutional animal ethics committee (Decision number 2012021), as per committee for the purpose of supervision of in-vivo experimentations on animal guidelines in Mutah University.
Animal groupings
Animals were randomly divided into eight groups (n = 8) as following:
Group-1 (Negative control group): Animals were treated with Vaseline (i.e. petroleum gel) only.
Group-2 (Positive control group): Animals were treated with povidone iodine ointment as recommended by Khan et al. (2015).
Group-3 (Treatment group Aj 5%): Animals were treated with Aj 5% ointment.
Group-4 (Treatment group Af 5%): Animals were treated with Af 5% ointment.
Group-5 (Treatment group Aj 10%): Animals were treated with Aj 10% ointment.
Group-6 (Treatment group Af 10%): Animals were treated with Af 10% ointment.
Group-7 (Treatment group Aj-Af 5%): Animals were treated with Aj-Af 5% ointment.
Group-8 (Treatment group Aj-Af 10%): Animals were treated with Aj-Af 10% ointment.
All groups were subdivided into two subgroups (n = 4 for each), in which first sub-group specific for incision model only, while the 2nd sub-group was specific for excision and burn sub-groups.
Wound healing activity
Three wound models were performed; incision, excision, and burn wound in rabbits. Therefore, we can evaluate healing activity of each plant, as well as herb-herb combination with the minimum number of animals 29. Creation of all three models in the animals were carried out under general and local anesthesia. It was taken into consideration suitable size of each wound and enough distances between them (i.e. excision and burn), that met study requirement and decrease the animal suffering as much as possible.
Wound models and treatment
Anesthesia
Creation of all three forms of wounds (i.e. incision, excision and burned) made in the paravertebral region in each animal. For anesthetization purpose, animal was intramuscularly administered with ketamine (40 mg/kg), xylazine (5 mg/kg) and locally using lignocaine 30. About 0.04 mg/kg atropine (0.2%) was administered subcutaneously as a parasympatholytic agent to aid breathing. Dorsal hair were shaved and sterilized with povidone- iodine 10% 31.
Incision wound model
Using a surgical blade (0.1 mm), 1 cm lateral to the mid-line of vertebral column over the paravertebral area a longitudinal incision (4 cm) was made (Fig. 1). After 12 h from the onset of surgery, the edges of the incision kept together by two simple sutures, using a silk thread. In fact, previous step as we think will simulate the natural worst conditions, as that the incision will not suture before hour(s) in most wound cases. In each treatment, groups (group 3–7), prepared ointment was applied topically over the incision once a day. Sutures were removed eight days post wound day. In the last day, Wound Breaking Strength (WBS) (i.e. maximum tensile strength) was measured (in grams) by using S-shaped hock and standard weight, method as described by both 32 and 33.
Excision wound model
Using a surgical blade (0.1 mm), 1 cm lateral to the mid-line of vertebral column over the paravertebral, a circle excision (diameter about 2.5 cm; area about 400 mm2) has made using a toothed forceps and blades (Fig. 1). Internal full thickness of skin was excised and removed. Hemostasis was achieved using sterile gauze soaked in normal saline with pressure 34,35.
Burn wound model
Burned wound was performed in parallel and in the opposite site for excision wound, using the same rabbit Fig.1. Wound performed by pouring a 2 g of hot molten wax (80 °C) on the targeted site using a metal cylinder (diameter 2.4 cm2), and left till solidification. After the pouring, with about 10 min, solidified wax along with that adhere to the skin was removed carefully, process left 1st or 2nd degree burns 36. In the burn model, wounds were assessed as the same manner used in the excision one, by estimation of the contraction rate on the days 3-, 7-, 11-, 14-, 17-, 18- , 19- post wound day.
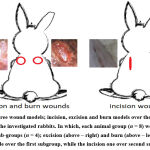 |
Figure 1: Three wound models; incision, excision and burn models over the dorsal back region of the investigated rabbits. |
Wound contraction rate
To assess the degree of wound healing in the excision model, wound contraction rate (WCR); closuring rate, was measured from the day zero until completion of epithelialization process. WCR was assessed planimetrically by measuring wound diameter (mm2) on 3rd, 7th, 11th, 14th, 17th 18th and 19th days after the first wounding day using a transparent paper, permanent marker and 1 mm2 graph paper. Finally, the percentage of wound contraction was quantified using the below equation 33,37,38.
Percentage of wound contraction in day (n) % =
Histopathological assays for burn and excision subgroup
In the days 3rd, 7th, 16th, and 22nd after first wounding day, one rabbits from excision and burn sub-group were euthanized using excessive doses of sodium pentobarbital (100 mg/kg I.P.) (Humane Society of the United States, 2013). From each euthanized rabbit, completely the two skin wound models, excision and burn wound, were excised and cut-of each one alone. Excised skins were fixed in 10% neutral-buffered formalin. All samples were sent to the histopathological lab (Prince Hamzah Hospital, Department of Pathology), after which each specimen was embedded in paraffin, processing 5 mm thickness, and was stained with hematoxylin and eosin. (H&E) dye, as well as Masson Trichrome Staining (MTS). Histopathological pictures were taken by compound and stereomicroscope (Leica microsystem) to monitor histological variations through screening fibroblast proliferation, collagen formation, epithelization, and keratein formation. In the healing process fibroblasts responsible for collagen deposition, in turn maintain skin integrity and strength (Ashoka Babu et al., 2015).
Statistical analysis
Between the groups, healing percentages were considered as the healing factor. One-way analysis of variance (ANOVA) followed by Tukey test, Graph Pad were run. Harvested results were expressed as mean ± standard error of mean (SEM), in which p < 0.05 was considered as significant result.
Results
Wound healing activity
It was obviously noted an improvement of healing process in all three models by using Aj and Af treatment comparing with positive and negative control, with more markedly results achieved by Ajf 10%. Healing performance by using Aj-Af (mainly 10%) combination was significant comparable with the standard drug (i.e. povidone iodine).
Effect of Aj and Af treatment on WBS of the incision model
The entire single-herb and herb-herb combination revealed a significant tensile strength compared with the negative control, more prominently by Aj rather than Af Table 2. Maximum tensile strength achieved with synergistic of both herbs; Aj-Af 10% (22.1 ± 1.34 g), which is similar or more than that achieved with adopted positive control during the study (i.e. povidone iodine) (21.8 ± 0.73 g). Results obviously revealed the significant potential healing activity of herb-herb combination with 10% concentration in the incision wounds.
Table 2: Wound breaking strength (gram) in control and experimental groups.
| Group | N | Mean | SD | Maximum
|
Minimum |
| NC | 6 | 15.5 | 1.57 | 17 | 13 |
| PC | 6 | 21.8*** | 1.3 | 25 | 17 |
| Aj 5% | 6 | 19.3** | 1.63 | 21 | 17 |
| Af 5% | 6 | 16.3 | 1.73 | 19 | 15 |
| Aj 10% | 6 | 18.4* | 1.79 | 21 | 16 |
| Af 10% | 6 | 17.3 | 1.93 | 20 | 15 |
| Aj-Af 5% | 6 | 19.3** | 1.58 | 22 | 18 |
| Aj-Af 10% | 6 | 22.1*** | 2.8 | 26 | 19 |
Notes: NC: Negative Control, PC: Positive control. Data were analyzed using One-way ANOVA followed by post-hoc Tukey test. * is statistically a significant (p < 0.05) difference in comparison with negative control group, ** is statistically a significant (p < 0.01) difference in comparison with negative control group and *** is statistically a significant (p < 0.0001) difference in comparison with negative control group in which all values represent as Mean ± SD.
Effect of both Aj and Af on WCR and rate of epithelization in the excision model
During assessment the healing potential activity of both excision and burn wounds, as wound closuring time is less as the WCR is high. Results showed obviously maximum significant healing capacity of Aj-Af 10% mixture on WCR (17.4 ± 0.7 day) comparing with negative control (24.5 ± 0.7 day) and positive control (18.5 ± 0.4 day) (Table 3) and (Fig. 2). In fact, this significant result achieved by herb-herb combination with 10% w/w concentration, that is better than achieved by Aj-Af 5% w/w concentration (19.1 ± 0.5 day). Consequently, epithelization rate was markedly more significant by herb-herb combination (Aj-Af) with 10% concentration, than by other treatment groups and positive one. In addition, results demonstrated that both Aj and Af have nearly the same epithelization potential activity; same WCR, in which WCR achieved by 10% concentration of both Aj and Af were 21.3 ± 0.10 and 21.5 ± 0.8, days after wounding day, respectively.
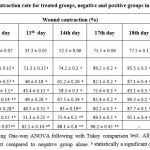 |
Table 3: Wound contraction rate for treated groups, negative and positive groups in the excision model. |
Note: Data were analyzed using One-way ANOVA following with Tukey comparison test.. All values are expressed as means ± SD. a statistically a significant compared to negative group alone. b statistically a significant compared to positive group.
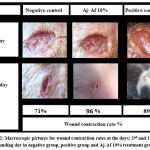 |
Figure 2: Macroscopic pictures for wound contraction rates at the days; 2nd and 17th post-wounding day in negative group, positive group and Aj-Af 10% treatment group. |
Burn wound model
Results showed good healing capacities of all treatment groups, with more marked increase in the healing capacity of the herb-herb combination compared with positive and negative groups (Table 4). As in excision model, the best epithelization rate was achieved by Aj-Af 10 % (17.1 ± 0.27 days) regarding positive control and negative control (18 ± 0.4, 24.3 ± 0.3 days, respectively) (Table 4).
Table 4: Wound healing rate (percentage) by topical application of all treatment groups, positive group and negative group in the burn wound model.
|
Groups |
Wound contraction (%) | Period of
Complete epithelization (days) |
||||||
| 3rd day | 7th day | 11th day | 14th day | 17th day | 18th day | 19th day | ||
| Negative control | 4.5 ± 0.17 | 16.4 ± 0.26 | 35.3 ± 0.2 | 54.3 ± 0.6 | 75.5 ± 0.6 | 81.5 ± 0.5 | 87.8 ± 1.1 | 24.3 ± 0.23 |
| Positive control | 10.7 ± 0.05a | 28.5 ± 0.7 a | 56.3 ± 0.24 a | 77 ± 0.02 a | 91 ± 0.05 a | 97.3 ± 1.1 a | – | 18 ± 0.14 a |
| AJ 5% | 8.2 ± 0.07 a | 27 ± 0.05 a | 45.3 ± 0.37 a | 56.4 ± 1.2 a | 78 ± 0.04 a | 84.1 ± 0.5 a | 91 ± 0.3 a | 22.6 ± 0.3 a |
| Af 5% | 7.1 ± 0.03 a | 20 ± 0.09 a | 36.6 ± 0.15 a | 51.4 ± 0.2 | 68.7 ± 0.5 | 77.4 ± 0.1 | 84.7 ± 0.4 | 23.1 ± 0.25a |
| Aj 10% | 8.8 ± 0.26 a | 27.3 ± 0.06 a | 48.6 ± 0.35 a | 63.6 ± 0.3 a | 81.6 ± 0.4 a | 90.1 ± 0.6 a | 97.5 ± 0.2 a | 20 ± 0.11 a |
| Af 10% | 7.3 ± 0.05 a | 22.5 ± 0.16 a | 39 ± 0.07 a | 50.6 ± 0.7 | 72 ± 0.02 | 81.6 ± 0.3 | 88.6 ± 1.1 a | 22.5 ± 0.25 a |
| Aj-Af 5% | 8.5 ± 0.04 a | 25.4 ± 0.37 a | 49.6 ± 0.08 a | 72.4 ± 0.4 a | 87 ± 0.05 a | 91.2 ± 0.2 a | 97.2 ± 0.4 a | 20.1 ± 0.34 a |
| Aj-Af 10% | 9.4 ± 0.2 a | 30.2 ± 0.4 a b | 55 ± 0.07 a | 86.6 ± 0.3 a b | 98.6 ± 0.4 a b | – | – | 17.1 ± 0.27 ab |
Note: Data were analyzed using One-way ANOVA following with Tukey comparison test.. All values are expressed as means ± SD. a statistically a significant compared to negative group alone. b statistically a significant compared to positive group.
Histopathological results
Histopathological findings of the wound sections (excised and burn model) are shown in both (Fig. 3) and (Fig. 4). Two stains were used: Hematoxylin and Eosin (H&E) stains and Masson’s Trichrome stains (MTS) for general morphology. Results showed that treatment the wounds with Aj-Af 10% demonstrated good healing findings in comparison with positive and negative control. Histopathological data observed better thickness of epidermal layer achieved by Aj-Af 10% treatment versus negative and positive control; greater epithelization degree (Fig. 4). From another side, in negative control MTS shows a minimal collagen deposit and moderate amount in the positive group, while abundant collagen deposit and granulation tissue have been shown in Aj-Af 10% treatment group (Fig. 4).
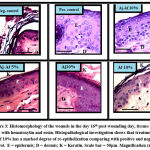 |
Figure 3: Histomorphology of the wounds in the day 16th post-wounding day, tissues were stained with hematoxylin and eosin. |
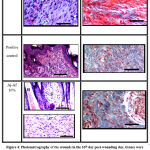 |
Figure 4: Photomicrography of the wounds in the 16th day post-wounding day, tissues were stained with H&E staining and MTS: |
Discussion
There is a remarkable increase in the use of medicinal plants worldwide, because their medicinal benefits and the potential therapeutic roles are still undergoing. Indeed, research conducted globally on plants to study their effectiveness has led to the production of medicines that are sourced from plants 17. The present study was performed to assess the combination capacity of both Aj and Af comparing with each herb alone in healing management, as there is no previous study. Wound models (incision, excision and burn) were performed in the dorsum back region of the rabbits, with suitable dimensions and procedure for each model. Animal were divided into many groups to study the potential healing activity of each herb alone as well as herb-herb combination with different concentration. Each treatment group were subdivided into two experimental groups, first group was specified to study tensile strength of the incision model, while the another group was specified for both burn and excision to study the contraction rates and histopathological findings 24,29.
Many studies showed potential activity of some herbal formulation in healing process 39. Both Aj and Af are widely used traditionally in rural people and traditional healers in the middle east for treatment of many health ailments including management of wound 21. Many studies strongly indicate the medical benefits of herb-herb combination therapy for treatment of many disease condition, due to the complementation between different constituents of all the herbs in such combinations 40. However, these combinations must be under scientific approach to avoid any undesirable combinations, as well as to study their synergistic therapeutic effects 41.
The present study showed that the synergy between the two herbs Aj and Af led to a significant decrease in time of healing (p < 0.05) compared with positive control, and the negative control. Wound healing is a complex inflammatory cascade that can be assessed by many criteria including: degree of epithelial remodeling, collagen and fibrous tissue deposition, infiltration rate of inflammatory cells, rate of wound contraction, tensile strength of incised wounds, and tissue granulation 42.
In the incision model, measurement of tensile strength of wound was performed as described by Lee (1968) and Nayak et al. (2011). Our data revealed that tensile strength was markedly significant (p < 0.05), by herb-herb combination compared with negative and positive control. Specifically, data revealed that maximum tensile strength was achieved with treatment of 10% Aj-Af combination (22.1 ± 1.34 g). This result was close to that achieved by povidone iodine group (21.8 ± 0.73 g). Previous studies demonstrated a high correlation between tensile strength of the wounds, collagen synthesis, neovascularization and fibroblast formation 43,44. In fact, these histological findings showed a marked potential collagination achieved by treatment with Aj-Af mixture than that obtained by negative and positive control (see Fig. 4 MTS). Moreover, efficiency of WCR of both excision and burn models, as well as tensile strength of incision model may due to the stabilization of the fibrous tissue and collage concentration 45. This result confirms the therapeutic potential activity of single-herb and herb-herb combination to treat wounds is consistent with what was previously published 46,47.
Present results showed a significant increase in closuring rates (p < 0.05) and healing in both excision and burn groups treated with herb-herb combination compared with positive and negative control. In fact, the herb combination showed a higher potential healing activity compared with single-herb use, however no statistically significant different between them. In excision model, it has been demonstrated that herb combination with 10 % concentration has markedly accelerated the wound closuring rate (17.4 ± 0.57 day) – better than with positive control (betadine) and negative control (18.5 ± 0.14 day; 24.5 ± 0.7 day, respectively). Histopathological findings showed that rate of re-epithelization of dermis layer is prominent in Aj-Af 10% treatment group with more contents of collagen and granulation tissues regarding positive and negative control. Such results are in agreement with many previous studies demonstrated potential activity of herb combination in collagenation and neovascularization during healing process rather than achieved by single herb treatment 46,48. Consequently, both single-herb and herbs combination exhibited significant potential healing activities compared with negative control (p < 0.05). Histological features of the wound tissues in both combined and single-herb groups characterized with efficient granulation, deposition of collagens, and increase in the dermal thickness and re-epithelization. Markedly, but non significant, herb-herb combination mainly (10%) exhibited partial healing potential activity than that by 5% concentration and positive control. Results just could be explained by the dose factor in the constituents of each plants, that is more effective for healing process in 10% 49,50.
For any herbal therapy to be efficient wound healer, herb(s) must contains phytoconstituents necessary for re-epithelization, collagenation and neovascularization process 51,52. Present study demonstrated an efficient potential capacity of aqueous extract of Aj-Af 10% combination as wound healer rather than singular herb. It was revealed that components of any two plants act synergistically and give out a remarkable potential activity during re-epithelialization, granulation, as well as collagen synthesis at the wound site 25. Moreover, using of Aj-Af mixture was superior to the using single extract besides to the betadine as control. Results make an evidence for preferentially using herb combination for therapeutic benefits rather than using one-herb therapy.
Conclusion
The current study showed an efficient activity of both plant (Aj and Af) for wound healing, singular and combination application. Plant combination (mainly 10%) induced significant healing markings; tensile strength, wound contraction rate, granulation and collagenation compared with negative and positive controls. In which, herb-combination exhibited better (non-significant) healing capacity regarding single-herb. Thus, two main conclusions could be achieved here; first: the potential therapeutic capacities of both plant (Aj and Af) as wound healer, second: results showed marked healing capacity of Aj-Af combination as wound healer rather than single-herb application, result need further work.
Acknowledgement
Author acknowledges the Deanship of scientific research – Mutah University for the financial support.
Conflict of Interest
The author declares no conflict of interests.
Funding Source
Deanship of Scientific Research – Mutah University (Grant No. 346/2020).
References
- Sidhu GS, Mani H, Gaddipati JP, et al. Curcumin enhances wound healing in streptozotocin induced diabetic rats and genetically diabetic mice. Wound Repair Regen. 1999;7(5):362-374.
- Kujath P, Michelsen A. Wounds–From physiology to wound dressing. Dtsch Arztebl Int. 2008;105(13):239.
- Wilkins RG, Unverdorben M. Wound cleaning and wound healing: a concise review. Adv Skin Wound Care. 2013;26(4):160-163.
- Albanna M, Holmes IV JH. Skin Tissue Engineering and Regenerative Medicine. Academic Press; 2016.
- Fisher‐Hubbard AO, Sung L, Hubbard SA, Hlavaty L. Hyperthermia, thermal injuries, and death from a forced convection heat source: a case report and experimental model. J Forensic Sci. 2017;62(3):686-690.
- Abboud MM, Saeed HA, Tarawneh KA, Khleifat KM, Al Tarawneh A. Copper uptake by Pseudomonas aeruginosa isolated from infected burn patients. Curr Microbiol. 2009;59(3):282-287.
- Ravishankar K, Kiranmayi GVN, Prasad YR, Devi L. Wound healing activity in rabbits and antimicrobial activity of Hibiscus hirtus ethanolic extract. Brazilian J Pharm Sci. 2018;54(4).
- Sen CK, Roy S, Gordillo G. Wound Healing (Neligan Plastic Surgery: Volume One). Published online 2017.
- Tarawneh KA, Al‐Tawarah N, Abdel‐Ghani AH, Al‐Majali AM, Khleifat KM. Characterization of verotoxigenic Escherichia coli (VTEC) isolates from faeces of small ruminants and environmental samples in Southern Jordan. J Basic Microbiol. 2009;49(3):310-317.
- Murphy PS, Evans GRD. Advances in wound healing: a review of current wound healing products. Plast Surg Int. 2012;2012.
- Tarawneh KA, Irshaid F, Jaran AS, Ezealarab M, Khleifat KM. Evaluation of antibacterial and antioxidant activities of methanolic extracts of some medicinal plants in northern part of Jordan. J Biol Sci. 2010;10(4):325-332.
- Althunibat OY, Qaralleh Q, Al-Dalin SYA, et al. Effect of Thymol and Carvacrol, the Major Components of Thymus capitatus on the Growth of Pseudomonas aeruginosa. J Pure Appl Microbiol. 2016;10(1):367-374. doi:10.1021/jf026203j
- Al-Asoufi A, Khlaifat A, Al Tarawneh A, Alsharafa K, Al-Limoun M, Khleifat K. Bacterial Quality of Urinary Tract Infections in Diabetic and Non Diabetics of the Population of Ma’an Province, Jordan. Pak J Biol Sci. 2017;20(4):179-188. doi:10.3923/pjbs.2017.179.188
- Khlaifat AM, Al-limoun MO, Khleifat KM, et al. Antibacterial synergy of Tritirachium oryzae-produced silver nanoparticles with different antibiotics and essential oils derived from Cupressus sempervirens and Asteriscus graveolens (Forssk). Trop J Pharm Res. 2019;18(12):2605-2616. doi:10.4314/tjpr.v18i12.21
- Khleifat KM, Matar SA, Jaafreh M, Qaralleh H, Al-limoun MO, Alsharafa KY. Essential Oil of Centaurea damascena Aerial Parts, Antibacterial and Synergistic Effect. J Essent Oil Bear Plants. 2019;22(2):356-367. doi:10.1080/0972060X.2019.1626292
- Qaralleh HA, Al-Limoun MO, Khlaifat A, et al. Antibacterial and antibiofilm activities of a traditional herbal formula against respiratory infection causing bacteria. arXiv Prepr arXiv210204301. Published online 2021.
- Patocka J, Navratilova Z. Achillea fragrantissima: pharmacology review. Clin Oncol. 2019;4:1601.
- Şahin A, Kiran Y, Arabacı T, Turkoğlu I. Karyological Notes on Eight Species of Achillea L. from Turkey (Asteraceae, Santolinoideae). Bot J Linn Soc. 2006;151(4):573-580.
- Danin A. Contributions to the flora of Jordan 3. A new species of Artemisia (Compositae, Anthemideae) from S Jordan. Willdenowia. 1999;29(1/2):147-153.
- Thakur R, Jain N, Pathak R, Sandhu SS. Practices in wound healing studies of plants. Evidence-based Complement Altern Med. 2011;2011.
- Abdelhalim A, Aburjai T, Hanrahan J, Abdel-Halim H. Medicinal plants used by traditional healers in Jordan, the Tafila region. Pharmacogn Mag. 2017;13(Suppl 1):S95.
- Lingaraju GM, Krishna V, Joy Hoskeri H, Pradeepa K, Venkatesh, Babu PS. Wound healing promoting activity of stem bark extract of Semecarpus anacardium using rats. Nat Prod Res. 2012;26(24):2344-2347.
- Dai T, Kharkwal GB, Tanaka M, Huang YY, Bil de Arce VJ, Hamblin MR. Animal models of external traumatic wound infections. Virulence. 2011;2(4):296-315.
- Masson-Meyers DS, Andrade TAM, Leite SN, Frade MAC. Cytotoxicity and wound healing properties of Copaifera langsdorffii oleoresin in rabbits. Int J Nat Prod Sci. 2013;3:10-20.
- Che CT, Wang ZJ, Chow MSS, Lam CWK. Herb-herb combination for therapeutic enhancement and advancement: theory, practice and future perspectives. Molecules. 2013;18(5):5125-5141.
- Kaur P, Mehta RG, Singh B, Arora S. Development of aqueous-based multi-herbal combination using principal component analysis and its functional significance in HepG2 cells. BMC Complement Altern Med. 2019;19(1):1-17.
- Monton C, Luprasong C. Effect of temperature and duration time of maceration on nitrate content of Vernonia cinerea (L.) Less.: Circumscribed central composite design and method validation. Int J food Sci. 2019;2019.
- Agyare C, Lechtenberg M, Deters A, Petereit F, Hensel A. Ellagitannins from Phyllanthus muellerianus (Kuntze) Exell.: Geraniin and furosin stimulate cellular activity, differentiation and collagen synthesis of human skin keratinocytes and dermal fibroblasts. Phytomedicine. 2011;18(7):617-624.
- Aderounmua AO, Omonisib AE, Akingbasotec JA, et al. Wound-healing and potential anti-keloidal properties of the latex of Calotropis procera (Aiton) Asclepiadaceae in rabbits. African J Tradit Complement Altern Med. 2013;10(3):574-579.
- Lipman NS, Marini RP, Erdman SE. A comparison of ketamine/xylazine and ketamine/xylazine/acepromazine anesthesia in the rabbit. Lab Anim Sci. 1990;40(4):395-398.
- Belo L, Serrano I, Cunha E, et al. Skin asepsis protocols as a preventive measure of surgical site infections in dogs: chlorhexidine–alcohol versus povidone–iodine. BMC Vet Res. 2018;14(1):1-6.
- Lee KH. Studies on the mechanism of action of salicylate II. Retardation of wound healing by aspirin. J Pharm Sci. 1968;57(6):1042-1043.
- Nayak BS, Kanhai J, Milne DM, Pereira LP, Swanston WH. Experimental evaluation of ethanolic extract of Carapa guianensis L. leaf for its wound healing activity using three wound models. Evidence-Based Complement Altern Med. 2011;2011.
- Mukherjee PK, Verpoorte R, Suresh B. Evaluation of in-vivo wound healing activity of Hypericum patulum (Family: Hypericaceae) leaf extract on different wound model in rats. J Ethnopharmacol. 2000;70(3):315-321.
- Shenoy C, Patil MB, Kumar R, Patil S. Preliminary phytochemical investigation and wound healing activity of Allium cepa Linn (Liliaceae). Int J Pharm Pharm Sci. 2009;2(2):167-175.
- Srivastava P, Durgaprasad S. Burn wound healing property of Cocos nucifera: An appraisal. Indian J Pharmacol. 2008;40(4):144.
- Bhat RS, Shankrappa J, Shivakumar HG. Formulation and evaluation of polyherbal wound treatments. Asian J Pharm Sci. 2007;2(1):11-17.
- Kahkeshani N, Farahanikia B, Mahdaviani P, et al. Antioxidant and burn healing potential of Galium odoratum extracts. Res Pharm Sci. 2013;8(3):197.
- Shetty S, Udupa S, Udupa L, Somayaji N. Wound healing activity of Ocimum sanctum Linn with supportive role of antioxidant enzymes. Indian J Physiol Pharmacol. 2006;50(2):163-168.
- Chen XW, B Sneed K, Pan SY, et al. Herb-drug interactions and mechanistic and clinical considerations. Curr Drug Metab. 2012;13(5):640-651.
- Colalto C. Herbal interactions on absorption of drugs: Mechanisms of action and clinical risk assessment. Pharmacol Res. 2010;62(3):207-227.
- Ghosh PK, Gaba A. Phyto-extracts in wound healing. J Pharm Pharm Sci. 2013;16(5):760-820.
- Habibipour S, Oswald TM, Zhang F, et al. Effect of sodium diphenylhydantoin on skin wound healing in rats. Plast Reconstr Surg. 2003;112(6):1620-1627.
- Rossi LF, Ramos RR, Ely JB, et al. Considerations that may influence the result of trials assessing tensile strength in experimental surgery. Acta Cir Bras. 2007;22(6):499-502.
- Udupa AL, Kulkarni DR, Udupa SL. Effect of Tridax procumbens extracts on wound healing. Int J Pharmacogn. 1995;33(1):37-40.
- Al-Henhena N, Mahmood AA, Al-Magrami A, et al. Histological study of wound healing potential by ethanol leaf extract of Strobilanthes crispus in rats. J Med Plants Res. 2011;5(16):3660-3666.
- Dhiyaaldeen SM, Alshawsh MA, Salama SM, et al. Potential activity of 3-(2-Chlorophenyl)-1-phenyl-propenonein accelerating wound healing in rats. Biomed Res Int. 2014;2014.
- Ganeshkumar M, Ponrasu T, Krithika R, Iyappan K, Gayathri VS, Suguna L. Topical application of Acalypha indica accelerates rat cutaneous wound healing by up-regulating the expression of Type I and III collagen. J Ethnopharmacol. 2012;142(1):14-22.
- Salimikia I, Aryanpour M, Bahramsoltani R, et al. Phytochemical and wound healing effects of methanolic extract of Salvia multicaulis Vahl. in rat. J Med Plants. 2016;1(57):38-46.
- Güzel S, Özay Y, Kumaş M, et al. Wound healing properties, antimicrobial and antioxidant activities of Salvia kronenburgii Rech. f. and Salvia euphratica Montbret, Aucher & Rech. f. var. euphratica on excision and incision wound models in diabetic rats. Biomed Pharmacother. 2019;111:1260-1276.
- Osunwoke Emeka A, Olotu Emamoke J, Allison Theodore A, Onyekwere Julius C. The wound healing effects of aqueous leave extracts of Azadirachta indica on wistar rats. J Nat Sci Res. 2013;3:181.
- Kiyohara H, Matsumoto T, Yamada H. Combination effects of herbs in a multi-herbal formula: expression of Juzen-taiho-to’s immuno-modulatory activity on the intestinal immune system. Evidence-Based Complement Altern Med. 2004;1(1):83-91.










