Manuscript accepted on :18-08-2022
Published online on: 08-09-2022
Plagiarism Check: Yes
Reviewed by: Dr. Swati Vasisth
Second Review by: Dr. Vijay Rekulapally
Final Approval by: Dr. H Fai Poon
Oluwafunbi Christianah Adeleye* and Ida Masana Risenga1
and Ida Masana Risenga1
Department of Animal, Plants and Environmental Science, Faculty of Science, University of the Witwatersrand, Johannesburg, South Africa.
Corresponding Author Email: 2415662@students.wits.ac.za
DOI : https://dx.doi.org/10.13005/bpj/2494
Abstract
Portulacaria afra, is indigenous to South Africa and has been identified to have several medicinal properties according to traditional knowledge and few studies. The drive around this research is to evaluate the medicinal properties of the leaves, stems and for the first time the roots extracts of Portulacaria afra, using four solvents with different polarities. The aqueous (60°C), methanol, n-hexane and ethyl acetate whole plant extracts of P. afra were investigated for their phytochemical properties, antimicrobial and antioxidant activity. The phytochemical screening revealed that the methanolic and aqueous extracts of the leaves displayed high presence of secondary metabolites compared to n-hexane and ethyl acetate extracts. The methanolic leaves extracts showed strong presence of quinones, phenols, steroid and coumarins while the aqueous leaves extracts contained a moderate presence of saponins, terpenoids, quinones and coumarins. Ethyl acetate leaves extracts revealed a strong presence of tannins, moderate presence of phytosteroids and a low presence of volatile oil. Meanwhile, the leaves extracts with n-hexane showed a considerable amount of saponins with a moderate presence, and a low presence of tannins, volatile oils and terpenoids. The methanolic stems extracts displayed the most significant presence of secondary metabolites, showing a high presence of terpenoids, steroids, phenols and coumarins. The aqueous stems extracts showed a strong presence of glycosides with a moderate presence of saponins. However, ethyl acetate and n-hexane stems extracts displayed a few secondary metabolites with their concentration ranging from medium to low. The ethyl acetate roots extracts displayed a significant elevated amount of quinones with a strong presence. n-hexane roots extracts showed a moderate presence of volatile oil and a low presence of tannins and steroids. Methanolic roots extracts showed a moderate presence of coumarins and glycosides while aqueous roots extracts showed a low presence of glycosides. The overall highest total phenolics contents (TPCs) and total flavonoids contents (TFCs) in all the plant parts, were found to be in the methanol stems extracts and aqueous roots extracts respectively. Next to the methanol leaves and aqueous leaves extracts respectively. However, in the root’s extracts, the aqueous extracts showed the highest total phenolics content while the water extracts had the highest total flavonoids contents. The antimicrobial activities of P. afra whole plant extracts with the various four solvents were tested against three microorganisms Escherichia coli, Staphylococcus aureus and Streptomyces griseus using agar-well diffusion method. The Antimicrobial activity of the n-hexane extracts of the leaves, stems and roots of P. afra presented a wide range of inhibition against all the test microorganisms, ethyl acetate leaves extract showed a considerable effect against Staphylococcus aureus while the methanolic extracts were not active. Aqueous roots extracts demonstrated a strong antimicrobial activity against Staphylococcus aureus while the other extracts were not active. The zones of inhibition ranged from 13 to 24 mm for the plant extracts. The antioxidant activity potential of the aqueous, methanol, n-hexane and ethyl acetate extracts of P. afra leaves, stems and roots extracts were observed through a 2, 2 diphenylpicryhydrazyl (DPPH) free radical assay, hydrogen peroxide scavenging (H₂O₂) and metal chelating activity assay. Ethyl acetate roots extracts exhibited the strongest hydrogen peroxide scavenging activity compared to the other extracts. Meanwhile, aqueous stems extracts showed the highest antioxidant activity against DPPH radical. Aqueous and n-hexane roots extracts displayed the strongest metal chelating ability. These findings reveal the efficacy of the use of several solvents with different polarities for effective and more accurate extraction of various compounds and indicate that the antimicrobial and antioxidant activity of P. afra parts are dependent on the solvent extracts.
Keywords
Antimicrobial Activity; Antioxidant Activity assays; Extracts; Portulacaria afra; Phytochemical screening; Solvents; Whole plant
Download this article as:| Copy the following to cite this article: Adeleye O. C, Risenga I. M. Screening of Phytochemical Profile and Biological Activities in the Leaves, Stems and Roots of South African Portulacaria Afra using Four Extraction Solvents. Biomed Pharmacol J 2022;15(3). |
| Copy the following to cite this URL: Adeleye O. C, Risenga I. M. Screening of Phytochemical Profile and Biological Activities in the Leaves, Stems and Roots of South African Portulacaria Afra using Four Extraction Solvents. Biomed Pharmacol J 2022;15(3). Available from: https://bit.ly/3RrudXr |
Introduction
Medicinal plants from prehistoric times have long been employed in practicing traditional medicine. Hundreds of chemical compounds are synthesized by plants to perform various preventive roles like protection from insects, diseases, fungi as well as herbivores1. They are also used by humans as a form of medical antidote used for healing purposes and protection from diseases2-4. The World Health Organization (WHO) reported the use of traditional plants for therapeutic purposes by 80% of the world’s population5.
In Africa, particularly in certain tribes, plant medicine is not a novel practice, due to its existence over a long time2,6. South Africa has about 60 million people and largely, people in the Cape Province, Kwazulu-Natal, Mpumalanga and Limpopo Province continue to rely on those medicinal plants. These plants have proven their efficacy and potency by curing various malignant ailments and diseases like cancer, diabetes, tuberculosis (TB) and benign ones like common cold, arthritis, dysmenorrhoea, and stomach problems7,8.
Phytochemicals are plant chemicals that are brought about by primary/secondary metabolism. They are involved in biological activities and perform vital functions like plant protection from pathogens, competitors, and predators9. They are present in plant foods such as vegetables, fruits, beans, whole grains, nuts, and seeds. It is believed that the consumption of organic plant foods with high phytochemical properties are of great health benefits as they can be used in the avoidance of serious ailments and heart disease conditions9. These natural compounds synthesized by plants are categorized into two main groups namely the primary and secondary metabolites10. Secondary metabolites serve as mediators of antagonistic ecological interactions between the plants and their habitat but are not vital in their growth, development, and reproduction. They also help to defend plants against disease-causing agents with their medicinal properties (antibiotic, antifungal and antiviral)11.
Antioxidants are natural or manufactured compounds that slow down the oxidation process; they donate an electron to a free radical without making themselves unstable. This causes the free radical to stabilise and become less reactive11. Antioxidant constituents present in plant foods and materials are widely studied by researchers. They have received more attention in the food manufacturing companies and among consumers, due to their health benefits in terms of preventions from and treatment of chronic heart diseases and cancer caused by oxidative stress12. Natural antioxidants are the most beneficial for use or consumption because they do not have negative impacts like the synthetic ones and are present in food, fruits, vegetables, and other plant-based, whole foods. Several vitamins, such as vitamins E and C, selenium, and carotenoids, such as beta-carotene, lycopene, lutein, and zeaxanthin are effective antioxidants11.
According to Pietta13, phenolic components like flavonoids are responsible for the anti-oxidative effect, while their antioxidant activity is a resultant of the redox characteristics they possess. These redox characteristics absorb and neutralise free radicals, extinguishing singlet and triplet oxygen, or decomposing peroxides. Essentially, the presence of secondary metabolites in medicinal plants influence antioxidant activity by slowing down or preventing oxidative stress or damage14. However, to date there are limited scientific records of this.
Portulacaria afra belongs to the Family: Portulacaceae or more recently Didiereaceae. Portulacaria has 7 species in total, the other 6 are: P. armiana, P. carrissoana, P. fruticulose, P. longipedunculata, P. namaquensis, P. pygmaea while P. afra and P. namaquensis are the only 2 native tree species found in the South Africa. It is a succulent evergreen plant popularly used worldwide. It has various names such as elephant bush (English), spekboom, and porkbush (Afrikaans). The succulent garden plant is indigenous to South Africa, and it is found in several regions in South Africa15. It grows in warm weather conditions and can be found growing on rocky hillsides and it is useful for carbon sequestration. In addition, it is useful for reclamation programmes in dry and parched terrain15.
 |
Figure 1: Image showing P. afra leaves and stems. |
 |
Figure 2: Image showing P. afra roots. |
In P. afra, there is very limited scientific documentation on the full phytochemical profile in all the plant parts. Most studies have worked on the physiological aspects such as the leaves and leaf juice used for the treatment of skin conditions and mouth infections16. The overarching aim of this study is to establish the phytochemical profile, medicinal properties; phytopharmacological attributes of the leaves, stems and for the first time the roots extracts of Portulacaria afra, using four solvents with different polarities.
Materials and methods
Chemicals and solvents used for this study were of analytical grades. Gallic acid, Folin-Ciocalteau reagent, methanol, hexane, ethyl acetate, Mueller-Hinton agar and Baird Parker agar, dimethyl sulfoxide (DMSO), 2, 2 diphenylpicryhydrazyl (DPPH), iron (II) chloride, ferrozine, phosphate buffer, hydrogren peroxide were all purchased from Sigma-Aldrich, USA, order CAS N0. 1162-65-8.
Collection of Plant Material
afra plants were collected in July 2021, within the premises of the University of the Witwatersrand, Johannesburg and were propagated from cuttings.
Cutting/growing the plants
Cuttings with green, non-woody stems were selected from a healthy parent P. afra plant with absence of diseases and with good features like green leaves, lack of drooping and no dying foliage. Cuttings which were 40-45 cm long and 4cm thick were collected with a pair of sterilised secateurs and transferred into the greenhouse for planting17.
45 pots of 2 – 2.5 litres were filled to the brim with culterrean potting professional cutting mix soil to hold the stem cutting for rooting. This type of soil was used because it drains better, provides moist conditions and free from pathogens that could be harmful to the cuttings before they become rooted17.
Cuttings were carefully placed in the half-filled potting mix soil and were gently firmed and then filled to the brim with the potting mix soil17.
The plants were allowed to grow in the green house for a period of 3 months to acclimatise and develop new roots mass and until new leaves began to appear along the stems. The plants were monitored and watered with 500 ml of water in the greenhouse every two days since it does not require too frequent watering17.
Sample preparation and extraction
The leaves, stem and roots of P. afra were harvested, washed with deionized water and then allowed to dry in a hot air dryer under 40°C for four days. The dried plant material was ground into a fine crude powder using an electric grinder. The powdered plant material was then enclosed in foil and placed in an airtight container, which was stored in the dark under room temperature cupboard until further tests commenced18.
Preparation of Extracts
The crude plant extract was prepared using four solvents: 80% methanol, hexane, ethyl acetate and 100% distilled water at temperature (60°C). All crude plant extracts were prepared by placing 3g of the powdered plant into separate bottles and adding 30 ml of a solvent. The methanol, hexane, ethyl acetate was placed on a shaker, while the water extracts were prepared with a sonicator and was set at temperature (60°C) for 45 minutes18-20. All extracts were shaken for 48 hours, apart from the water extracts. The water extracts were centrifuged to decrease the viscosity of the extracts. All extracts were filtered through a filter paper into vials. The supernatant, which remained, were discarded whilst the vials were enclosed in foil and stored in the refrigerator until the tests were conducted18-20.
Qualitative phytochemical screening
The preliminary phytochemical analysis was done on all the plant extracts for secondary metabolites discovery and identification by using Harbone21 (1998), Roghini and Vijayalakshmi22 methodology with slight modification. The various tests for saponins, flavonoids, glycosides, quinones, phenols, terpenoids, steroids, phytosteroids, volatile oil, and coumarins were observed and the tests results were recorded.
Total Phenolic Contents and Total Flavonoids Contents Evaluation
The total phenolic and flavonoids content of the leaves, stems and roots extracts of P. afra with the different solvents were estimated by using the colorimetric Folin-Ciocalteu method. For the analysis, standard gallic acid solutions at different concentrations were used for the calibration curve. The concentrations of the total phenolic contents in the extracts were estimated from the standard curve (Figure 3) and the results were expressed as gallic acid equivalents per gram (mg GAE/g) dry weight of the extract. Total flavonoids contents of the different plant parts extracts were also calculated by making a standard curve of quercentine (Figure 4) and expressed as quercetin per gram (mg RE/g) of dry weight. All determinations were done in triplicates and data was expressed as mean ± standard error. P < 0.05 was considered statistically significant.
Antimicrobial activity assay
afra antimicrobial activity assay in the leaves, stems and root extracts with the different solvents were determined using the Agar-well diffusion assay described by Jain et al.23 The plant extracts were tested against gram-positive Staphylococcus aureus (ATCC 25923), gram-negative E. coli (ATCC 25922) and gram-positive streptomyces griseus.
Agar-well diffusion method
The Petri dishes were sterilised and put in the autoclave before pouring the nutrient agar medium -Mueller-Hinton agar and Baird Parker agar. Once the agar medium solidified, sterile cotton swabs were used to inoculate the whole surface of the agar medium with the test microorganisms. S. aureus was inoculated on the Baird Parker agar plates and E. coli was inoculated onto the Mueller-Hinton agar plates as well as the Streptomyces griseus plates. Six wells were made in the inoculated media using a 6mm sterile borer. The plant extracts (100μl) were impregnated only into five wells while the sixth well was impregnated with the negative control, dimethyl sulfoxide (DMSO). The plates were covered and placed in the fridge for 30 minutes to diffuse. The plates were put in the incubator for 24 hours at 37°C. After the incubation period, the zones of inhibition were measured in millimetres using a ruler. The presence of an inhibitory zone surrounding was indicative of a positive antibacterial activity result. The studies were performed in triplicate and data was expressed as mean ± standard deviation and was calculated by Microsoft excel. P < 0.05 was considered statistically significant while Tukey test HSD in RStudio was used to determine where the significance lies.
Evaluation of in vitro Antioxidant Activity
DPPH scavenging activity assay
A 2, 2 diphenylpicryhydrazyl (DPPH) free radical assay by Brand-Williams et al24 was used with a few alterations. 3 g of dried and powdered leaves, stems, and roots materials of P. afra was added to 80% methanol solvent and water respectively for the extract’s preparation. The scavenging potential of DPPH on the plant extracts were derived by adding 50 mg of DPPH to 100 ml of 80% methanol for preparation of the stock solution. A work solution was then prepared from the stock solution; the dilution mixture was of ratio 1:5 of the stock solution to 80% methanol. Each extract had 5 quantities (10-50 μL). A reaction mixture was made containing 10-50 μL of the extracts and 700 μL of the work solution which was topped with 80% methanol to make up a volume of 1 ml. A blank was made with the use of 80% methanol and the control solution was prepared using the work solution and 80% methanol. The mixtures were kept in the dark for 45 minutes, and then a spectrophotometer was used to measure the absorbance at a wavelength of 517 n. The inhibition (%) was expressed with the following equation:
DPHH Scavenging Effect (%) = [(Absorbance of sample – Absorbance of blank)/Absorbance of control] x 100
Metal chelating activity
To determine the metal chelating activity, the method used by Dinis et al.25 was employed. A volume of 2 ml of extracts was added to 0.25 ml of 250 mM iron (II) chloride, the reaction was observed when 0.25 ml of ferrozine was added. The mixture was firmly shaken together and allowed to sit at room temperature for 10 minutes. The absorbance of the mixture was measured at a wavelength 562 nm. Methanol served as the blank while the control was without the extracts. The metal chelation (%) was calculated using the following formula:
Metal chelation (%) = {(A0 − A1)/ A0} × 100
A0 is the absorbance of the control and A1is the absorbance with the test samples.
Hydrogen peroxide scavenging (H₂O₂) assay
The methodology used by Ruch et al.26 was used for this study. 1 ml of extracts was added to 3.4 ml of phosphate buffer (50 mM, pH 7.4). 600 μL of 40 mM hydrogen peroxide (30%) was added into the initial solution. The mixture was left at room temperature for 40 minutes; the absorbance was measured at a wavelength of 230 nm. The hydrogen peroxide scavenging activity was calculated with the following formula:
Scavenged H₂O₂ (%) = [(Absorbance of control – Absorbance of sample)/Absorbance of control] x 100
Statistical Analysis
The concentration values were then plotted against the % inhibition values using Microsoft excel sheet to generate a trendline equation used to calculate the IC50. A one-way analysis of variance (ANOVA) was used to determine any significant differences. Experiments were all performed in triplicate and data was expressed as mean ± standard error (SE) (when n= 3). P < 0.05 was considered statistically significant while Tukey test HSD in RStudio was used to determine where the significance lies.
Results and Discussion
Table 1 shows the preliminary phytochemical analysis done on the plant parts extracts of P. afra for secondary metabolites discovery and identification by using Harbone21, Roghini and Vijayalakshmi22 methodology with slight modification. Phytochemicals are plant chemicals that are brought about by primary/secondary metabolism and are involved in biological activities9. It is believed that the consumption of organic plant foods with high phytochemical properties are of great health benefits as they can be used in the avoidance of serious ailments and heart disease conditions27. Literature recommends the use of several solvents with different polarities for effective and more accurate extraction of various compounds28,29. The solvents used for the extraction were selected according to the degree of polarity of the solute of interest because a solvent of close polarity will be more effective and accurate in dissolution of the solute30. The polarity of some often-used solvents in the order of lowest polarities to highest polarities are Hexane < Chloroform < Ethyl acetate < Acetone < Methanol < Water30. For this study, the following four solvents were used: (water (60º), n-hexane, ethyl acetate, and methanol Table 1). The phytochemical screening as seen in (Table 1) revealed that the methanolic and aqueous extracts of the leaves displayed high presence of secondary metabolites compared to n-hexane and ethyl acetate extracts. Methanol and water commonly extracts chemical compounds like sugars, amino acids and glycosides during phytochemical screening. While ethyl acetate extracts alkaloids, aglycones and glycosides, n-hexane is targets waxes, fats, fixed oils and volatile oils for extraction31. Several studies have revealed that secondary metabolites like glycosides, phenols, steroids, saponins, alkaloids and terpenoids possess strong free radical scavenging and antioxidant potentials32. Glycosides, consisting of terpenoid or steroids and sugar chains, are of great health benefits and biological activities like anti-cancer and anti-inflammatory properties32. The methanolic leaves extracts showed strong presence of quinones, phenols, steroid and coumarins. These results were however not in complete accordance with previous work by Tabassum et al.33 on P. afra tissue extracts, as findings from their research showed strong presence of tannins, phenols, flavonoids, saponins and glycosides with methanolic extracts and a moderate presence with n-hexane fractions which can be linked to the variation in climatic conditions. Consequently, fluctuations in the abiotic factors affect how secondary metabolites are synthesized and gathered. Abiotic factors such as temperature and water deficit are a major hindrance to the reproduction of medicinal plants, which in turn affects the biosynthesis and causes irregularities in the secondary metabolites production and consequent phyto-phamacological activities34. The aqueous leaves extracts contained a moderate presence of saponins, terpenoids, quinones and coumarins. Ethyl acetate leaves extracts revealed a strong presence of tannins, moderate presence of phytosteroids and a low presence of volatile oil. Meanwhile, the n-hexane leaves extracts showed a considerable amount of saponins with a moderate presence, and a low presence of tannins, volatile oils and terpenoids. The methanolic stems extracts displayed the most significant presence of secondary metabolites, showing a high presence of terpenoids, steroids, phenols and coumarins. The aqueous stems extracts showed a strong presence of glycosides with a moderate presence of saponins. However, ethyl acetate and n-hexane stems extracts displayed a few secondary metabolites with their concentration ranging from medium to low. The ethyl acetate roots extracts displayed a significant elevated amount of quinones with a strong presence. n-hexane roots extracts showed a moderate presence of volatile oil, coumarins and a low presence of tannins and steroids. Methanolic roots extracts showed a moderate presence of coumarins and glycosides while aqueous roots extracts showed a low presence of glycosides. Saponins consist of triterpenoids or steroid, which is responsible for their therapeutic activities35, while coumarins is known to for its high phytopharmacological properties and particularly anti-cancer, anti-inflammatory and tranquilizer properties36.
Table 1: Phytochemical screening of P. afra samples.
| Sr No. |
|
Chemical tests | Leaves extracts
M H H2O EA |
Stems extracts
M H H2O EA |
Roots extracts
M H H2O EA |
|||||||||
| 1. | Tannins | FeCl3 | ++ | + | – | +++ | + | ++ | ++ | + | + | + | – | – |
| 2. | Saponins | Froth | + | ++ | ++ | + | + | ++ | ++ | – | + | – | – | – |
| 3. | Flavonoids | NaOH | – | – | – | – | – | – | – | – | – | – | – | – |
| 4. | Glycosides | Sulphuric acid | – | – | + | – | + | – | +++ | – | ++ | – | ++ | – |
| 5. | Quinones | Sulphiric acid | +++ | – | ++ | – | – | – | – | + | – | – | + | +++ |
| 6. | Phenols | FeCl3 | +++ | – | ++ | – | +++ | – | – | – | + | – | – | – |
| 7. | Terpenoids | Chloroform | ++ | + | – | – | +++ | – | – | – | + | – | – | – |
| 8. | Steroids | Reaction with Chloroform and sulphuric acid | +++ | – | – | – | ++ | – | – | – | + | + | – | – |
| 9. | Phytosteroids | Reaction with Chloroform and sulphuric acid | – | – | – | ++ | – | – | – | – | – | – | – | – |
| 10.
|
Volatile oil
|
Reaction with NaOH and dilute HCl | – | + | – | + | – | + | – | + | + | ++ | + | – |
| 11. | Coumarins | Reaction with NaOH | +++ | – | ++ | – | +++ | – | – | ++ | ++ | – | – | – |
M, methanolic extract; H, n-hexane extract; H2O, hot water extract (60ºC); EA, ethyl acetate extracts; +++, strongly present; ++, moderately present; +, present; -, not present or not detected.
Quantitative analysis of phytochemicals
Quantitative results of phytoconstituents in (Tables 2 and 3) showed the overall highest total phenolics contents (TPCs) and total flavonoids contents (TFCs) in all the plant parts, were found to be in the methanol stems extracts and aqueous roots extracts (9521.01±216.94 and 901.30±149.87 mg/g respectively). Next to the methanol leaves and aqueous leaves extracts (6073.19±4.09 and 150.47±1.17 mg/g respectively). However, in the root extracts, the aqueous extracts had the highest total phenolics and flavonoids contents (3331.31±58.32 and 901.30±149.87 mg/g respectively).
Table 2: Total phenolic content of P. afra samples extracts
| Plant parts
|
Total phenolic contents (mg/g)
Solvents |
|||
| M | H | H2O (60ºC) | EA | |
| Leaves | 6073.20±4.10a | 1237.04±13.47c | 1038.18±1.17d | 3008.08±81.65b |
| Stems | 9521.01±216.94b | 1755.55±69.98a | 1820.67±25.08a | 1472.73±11.66a |
| Roots | 1567.47±29.16c | 1351.52±11.66d | 3331.31±58.32a | 2361.62±11.66b |
M, methanolic extract; H, n-hexane extract; H2O, hot water extract (60ºC); EA, ethyl acetate extracts. All values are expressed as mean ± SE, n=3, and are equivalent to gallic acid (mg/g of GAE). a Values with different letters in the same column were significantly (p < 0.05) different.
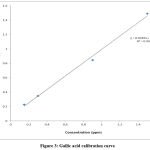 |
Figure 3: Gallic acid calibration curve |
Table 3: Total flavonoid content of P. afra samples extracts.
| Plant parts
|
Total flavonoid contents (mg/g)
Solvents |
|||
| M | H | H20 (60ºC) | EA | |
| Leaves | 150.47±1.17a | 141.34±0a | 592.70±49.99b | 108.60±5.13a |
| Stems | 107.42±2.04a | 134.51±1.18a | 585.63±48.29b | 114.49±2.04a |
| Roots | 359.01±8.16a | 104.59±8.16a | 901.30±149.87b | 154.53±2.36a |
M, methanolic extract; H, n-hexane extract; H2O, hot water extract (60ºC); EA, ethyl acetate extracts. All values are expressed as mean ± SE, n=3, and are equivalent to quercetin (mg/ g of quercetin). aValues with different letters in the same column were significantly (p < 0.05) different.
 |
Figure 4: Quercetin calibration curve |
Antimicrobial activity assay
The results of the antimicrobial properties of P. afra extracts showed that n-hexane extracts of the leaves, stems and roots of P. afra presented a wide range of inhibition against all the test microorganisms, ethyl acetate leaves extract showed a considerable effect against Staphylococcus aureus while the methanolic extracts were not active (Table 4). Aqueous roots extracts demonstrated a strong antimicrobial activity of 24mm against gram-positive Staphylococcus aureus while the other extracts were not active. The zones of inhibition ranged from 13 to 24 mm for the plant extracts. The results of this study are not in agreement with previous studies by Khanyile et al.18 where the leaves extract of P. afra was used to determine the inhibitory effect against positive Staphylococcus aureus and Pseudomonas aeruginosa. The results showed a slight inhibitory effect against the test microorganisms. It is observed that the examined plant extracts showed better activity against gram-positive microorganisms than gram-negative microorganisms. The variation observed in the ability of the extracts to inhibit the growth of these microorganisms could be because of the type of layers possessed by the cell wall of each microorganism. Several factors like chemical constituents in the different solvents, microbial growth and exposure of the test microorganisms may be responsible for the results from this study.
Table 4: Antimicrobial activity of different solvent extracts of all plant parts of P. afra.
| Plant parts | Zone of inhibition (mm) of extracts of different solvent | ||||
| Name of microorganism | M | H | H20 (60ºC) | EA | |
| Leaves
|
Escherichia coli | – | 16±0.6 | – | – |
| Staphylococcus aureus | – | 18±0.6 | – | 14±0.6 | |
| Streptomyces griseus | – | 18±0.6 | – | – | |
| Stems
|
Escherichia coli | – | 13±0.6 | – | – |
| Staphylococcus aureus | – | 13±0.6 | – | – | |
| Streptomyces griseus | – | 13±0.6 | – | – | |
| Roots
|
Escherichia coli | – | 15±0.6 | – | – |
| Staphylococcus aureus | – | 17±0.6 | 24±0.6 | – | |
| Streptomyces griseus | – | 18±0.6 | – | – | |
M, methanolic extract; H, n-hexane extract; H2O, hot water extract (60ºC); EA, ethyl acetate extracts. All values are expressed as mean ± SD, n=3.
Antioxidant Activity
Antioxidants are natural or synthetic compounds that slow down the oxidation process; they are molecules that donate an electron to a free radical without making themselves unstable. This causes the free radical to stabilize and become less reactive11. They neutralize free radicals and unstable molecules that can harm the cells. In this study, antioxidant activity potential of the aqueous, methanol, n-hexane and ethyl acetate extracts of P. afra leaves, stems and roots extracts were observed through a 2, 2 diphenylpicryhydrazyl (DPPH) free radical assay, hydrogen peroxide scavenging (H₂O₂) and metal chelating activity assay. The DPPH free radical is fast and mostly used for antioxidant activity determination against DPPH radical27. Meanwhile, hydrogen peroxide is mostly harmful as it transforms into hydroxyl and reactive radical metal complexes37. Studies have shown how metal chelating activity is used to determine the antioxidant potential of plant extracts as they interfere with the oxidation process by reacting with free radicals, chelating free catalytic metals and by acting as scavengers of oxygen by-products. Table 5 shows the various antioxidant activity assays used in this study. Aqueous stems extracts showed the overall highest antioxidant activity (0.480mg/ml) against DPPH radical. Ethyl acetate roots extracts exhibited the strongest hydrogen peroxide scavenging activity (0.89 mg/ml) as compared to the other plant part extracts. Aqueous and n-hexane roots extracts displayed the strongest metal chelating ability (3.19 and 4.51 mg/ml respectively). Antioxidant constituents present in plant foods and materials are widely studied by researchers. They have received more attention in the food manufacturing companies and among consumers, due to their health benefits in terms of preventions from and treatment of chronic heart diseases and cancer caused by oxidative stress12.
Table 5: Antioxidant activity assay of solvent extracts of P. afra plant parts
| Plant parts
|
IC50 (±SE, mg/ml)
(Extract fraction)
|
||||
| Antioxidant assay | M | H | H20(60ºC) | EA | |
| Leaves
|
DPPH | 32.23 ±0.040a | 8.49 ±0.081b | 3.21±0.064c | 1.70±0.162d |
| Hydrogen peroxide | 5.44±0.635b | 17.22±0.040a | 17.22±0.040a | 4.13±0.098b | |
| Metal chelating | 6.66±0.629a | 20.44±0.543c | 35.17±1.415b | 8.43±1.120a | |
| Stems
|
DPPH | 34.92 ±0.052b | 35.81±0.144a | 0.48±0.064d | 5.74±0.075c |
| Hydrogen peroxide | 4.44±0.214a | 2.94±0.017b | 4.70±0.064a | 4.41±0.075a | |
| Metal chelating | 63.25±0.664b | 6.56±0.600a | 4.23±1.085a | 5.38±1.085a | |
| Roots
|
DPPH | 29.63±0.267a | 10.23±0.156b | 1.37±0.058d | 3.0±0.092c |
| Hydrogen peroxide | 2.12±0.260c | 11.68±0.080a | 3.83±0.110b | 0.89±0.160d | |
| Metal chelating | 92.34±2.840b | 4.51±0.549a | 3.19±0.554a | 25.28±2.159c | |
DPPH, 2, 2 diphenylpicryhydrazyl; M, methanolic extract; H, n-hexane extract; H2O, hot water extract (60ºC); EA, ethyl acetate extracts. All values are expressed as mean ± SE, n=3. a Values with different letters in the same column were significantly (p < 0.05) different.
Conclusions
The results of the present study have shown a unique variation in the estimated phytochemical constituents and biological activities in the leaves, stems and roots extracts of the four different solvents of P. afra. It is worthy to note that P. afra parts have displayed high therapeutic properties; with a strong presence of quinones in the ethyl acetate roots extracts for the first time. P. afra aqueous roots extracts also had the highest TFCs content. Coumarins known for anticancer properties showed a strong presence in the methanolic leaves and stems extracts with a moderate presence in the roots extracts, this could potentially be of good use in the pharmaceutical industries for drug production and future role in public health.
Further research is needed, to determine how abiotic factors would influence therapeutic properties, values and activities of P. afra.
Acknowledgment
Authors are thankful to South Africa’s National Research Foundation (NRF) for supporting this work.
Conflict of interest
The authors declare that there are no conflicts of interest.
Funding source
South Africa’s National Research Foundation (NRF) TTK201129577193.
References
- Ahn K. The worldwide trend of using botanical drugs and strategies for developing global drugs. BMB reports. 2017 Mar; 50(3):111.
CrossRef - Mbuni Y. M, Wang S, Mwangi B. N, Mbari N. J, Musili P. M, Walter N. O, Hu G, Zhou Y, Wang Q. Medicinal plants and their traditional uses in local communities around Cherangani Hills, Western Kenya. Plants. 2020 Mar 5; 9(3):331.
CrossRef - Rasool Hassan B. A. Medicinal plants (importance and uses). Pharmaceutica Analytica Acta. 2012; 3(10):2153-435.
CrossRef - Thirumalai T, Kelumalai E, Senthilkumar B, David E. Ethnobotanical study of medicinal plants used by the local people in Vellore District, Tamilnadu, India. Ethnobotanical leaflets. 2009; 2009(10):10.
- Farnsworth N. R, Akerele O, Bingel A. S, Soejarto D. D, Guo Z. Medicinal plants in therapy. Bulletin of the world health organization. 1985; 63(6):965.
- Petrovska B. B. Historical review of medicinal plants’ usage. Pharmacognosy reviews. 2012 Jan; 6(11):1.
CrossRef - Abdullahi A. A. Trends and challenges of traditional medicine in Africa. African journal of traditional, complementary and alternative medicines. 2011; 8(5S).
CrossRef - Mander M, Ntuli L, Diederichs N, Mavundla K. Economics of the traditional medicine trade in South Africa care delivery. South African health review. 2007 Jan 1; 2007(1):189-96.
- Xiao Y. H, Huang Y, Burton-Freeman B. M, Edirisinghe I. Chemical changes of bioactive phytochemicals during thermal processing. Reference Module in Food Science. 2016: 9. doi: 10.1016. B978-0-08-100596-5.03055-9; 2016.
- Saxena M, Saxena J, Nema R, Singh D, Gupta A. Phytochemistry of medicinal plants. Journal of pharmacognosy and phytochemistry. 2013 Mar 1; 1(6).
- Javanmardi J, Stushnoff C, Locke E, Vivanco J. M. Antioxidant activity and total phenolic content of Iranian Ocimum accessions. Food chemistry. 2003 Dec 1; 83(4):547-550.
- Idamokoro E. M, Afolayan A. J. In vitro Evaluation of the Phytochemical and Antioxidant Properties of Bulbine abyssinica Extracts. International Journal of Agriculture and Biology. 2020 Jan 1; 24(6):1781-7.
- Pieta, P. Flavonoids in medicinal plants, in Flavonoids in Health and Disease, edited by C A Rice-Evans and L Packer, Marcel Dekker, New York, NY, USA, 1998; pp 343– 358.
- Pisoschi A. M, Negulescu G. P. Methods for total antioxidant activity determination: a review. Biochemistry and Analytical Biochemistry 2011; 1(1):106.
- Oakes A. J. Portulacaria afra Jacq.: a potential browse plant. Economic Botany. 1973 Oct 1:413-416.
CrossRef - De Wet H, Nciki S, van Vuuren S. F. Medicinal plants used for the treatment of various skin disorders by a rural community in northern Maputaland, South Africa. Journal of Ethnobiology and Ethnomedicine. 2013 Dec; 9(1):1-10.
- Poorter H, Fiorani F, Stitt M, Schurr U, Finck A, Gibon Y, Usadel B, Munns R, Atkin O. K, Tardieu F, Pons T. L. The art of growing plants for experimental purposes: a practical guide for the plant biologist. Functional Plant Biology. 2012 Jun 15; 39(11):821-38.
CrossRef - Khanyile, A., Maliehe, T. S., Shandu, J. S., and Khan, R. In vitro antibacterial, antioxidant, anti-quorum sensing and cytotoxic properties of Portulacaria afra leaves extract. Bioscience Research, 2021; 18(1), 455-463.
- Pakade V, Cukrowska E, Chimuka L. Comparison of antioxidant activity of Moringa oleifera and selected vegetables in South Africa. South African journal of science. 2013 Mar 1; 109(3):1-5.
CrossRef - Siddhuraju P, Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. Journal of agricultural and food chemistry. 2003 Apr 9; 51(8):2144-55.
CrossRef - Harborne A. J. Phytochemical methods a guide to modern techniques of plant analysis. springer science & business media; 1998 Apr 30.
- Roghini R, Vijayalakshmi K. Phytochemical screening, quantitative analysis of flavonoids and minerals in ethanolic extract of Citrus paradisi. International Journal of Pharmaceutical Sciences and Research. 2018 Nov 1; 9(11):4859-4864
- Jain I, Jain P, Bisht D, Sharma A, Srivastava B, Gupta N. Comparative evaluation of antibacterial efficacy of six Indian plant extracts against Streptococcus mutans. Journal of clinical and diagnostic research: JCDR. 2015 Feb; 9(2):ZC50.
CrossRef - Brand-Williams W, Cuvelier M. E, Berset C. L. Use of a free radical method to evaluate antioxidant activity. LWT-Food science and Technology. 1995 Jan 1; 28(1):25-30.
CrossRef - Dinis T. C, Madeira V. M, Almeida L. M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Archives of biochemistry and biophysics. 1994 Nov 1; 315(1):161-169.
CrossRef - Ruch R. J, Cheng S. J, Klaunig J. E. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989 Jun 1; 10(6):1003-1008.
CrossRef - Kim H, Seo K. S, Yun K. W. Antioxidant Activity and Flavonoid Estimation in Rosa multiflora and Rosa wichuraiana Fruits and Flowers. Biomedical and Pharmacology Journal. 2022 May 18; 15(2).
CrossRef - Altemimi A, Lakhssassi N, Baharlouei A, Watson D. G, Lightfoot D. A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants. 2017 Sep 22; 6(4):42.
CrossRef - Smetanska I. Sustainable production of polyphenols and antioxidants by plant in vitro cultures. InBioprocessing of Plant In Vitro Systems 2018 (pp. 225-269).
CrossRef - Koffi E, Sea T, Dodehe Y, Soro S. Effect of solvent type on extraction of polyphenols from twenty three Ivorian plants. Journal of Animal and Plant Sciences (JAPS). 2010; 5(3):550-558.
- Houghton, P. J and Raman, A. Methods for extraction and sample clean-up. In Laboratory Handbook for the Fractionation of Natural Extracts ., Springer, Boston , MA (1998); (pp. 22-53).
CrossRef - Shankar K. R, Gurjar C, Rajalakshmi V. G, Joshi N. H. Phytochemical Investigations of Plant Trichodesma Indicum. Biomedical & Pharmacology Journal. 2008; 1(2):453.
- Tabassum S, Ahmad S, Rehman Khan K. U, Tabassum F, Khursheed A, Zaman Q. U, Bukhari N. A, Alfagham A, Hatamleh A. A, Chen Y. Phytochemical Profiling, Antioxidant, Anti-Inflammatory, Thrombolytic, Hemolytic Activity In Vitro and In Silico Potential of Portulacaria afra. Molecules. 2022 Apr 7; 27(8):2377.
- Soni U, Brar S, Gauttam V. K. Effect of seasonal variation on secondary metabolites of medicinal plants. Int J Pharm Sci Res. 2015 Sep 1; 6(9):3654-3662.
- Jagtap K, Mulik A, Singh E. A, Jagtap S. Comparative Study to Evaluate Ethanol and Ethyl Acetate Extracts of Different ‘Vidanga’Species for Antioxidant Efficacy and Phyto-Constituents Screening. Biomedical and Pharmacology Journal. 2022 Mar 31; 15(1):165-177.
- Wu L, Wang X, Xu W, Farzaneh F, Xu R. The structure and pharmacological functions of coumarins and their derivatives. Current medicinal chemistry. 2009 Nov 1; 16(32):4236-4260.
CrossRef - Keser S, Celik S, Turkoglu S, Yilmaz O, Turkoglu I. Hydrogen peroxide radical scavenging and total antioxidant activity of hawthorn. Chem J. 2012; 2(1):9-12.







