Ni Wayan Armerinayanti , Samuel Widodo*
, Samuel Widodo* and Desak Putu Oki Lestari1
and Desak Putu Oki Lestari1
Department of Anatomic Pathology, Faculty of Medicine and Health Sciences Warmadewa University, Denpasar, Indonesia.
Corresponding Author E-mail: drsamuelwidodo@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2505
Abstract
Papillary thyroid carcinoma (PTC) comprises 80% of well-differentiated thyroid carcinomas. PTC progression is determined by a variety of biological markers, some of which are connected to the activities of Tumor-Associated Macrophage (TAM). TAM activity is difficult to observe using simply traditional histology techniques. Matrix Metalloproteinase 9 (MMP-9) is an important marker for identifying the development of PTC. However, no studies have linked the expression of MMP-9 to intratumoral macrophages in PTC patients, demonstrating that these macrophages are TAMs implicated in the development of PTC. Through MMP-9 expression on intratumor macrophages, the objective of this work was to evaluate TAM's involvement as a progression determinant of PTC. This cross-sectional study analyzed 40 samples, which included 21 PTC patients with intratumor macrophages and 19 PTC instances without intratumor macrophages. Medical records and paraffin blocks of the Biomedical Laboratory, Faculty of Medicine and Healthcare, Warmadewa University were used to collect samples. The proportion of MMP-9 expression in both groups' macrophages was then determined by immunohistochemical labeling and evaluated using Chi-Square with a significance level (a) of . The results revealed a statistically significant difference in MMP-9 expression between the PTC groups with and without intratumor macrophages, with p-value = 0.001 and OR = 11.9.
Keywords
Histopathology; Immunohistochemistry Staining; Intratumor Macrophages; Matrix Metalloproteinase 9; Papillary Thyroid Carcinoma; Tumor-Associated Macrophage
Download this article as:| Copy the following to cite this article: Armerinayanti N. W, Widodo S, Lestari. Intratumor Macrophages as Tumor-Associated Macrophage Marker of Papillary Thyroid Carcinoma. Biomed Pharmacol J 2022;15(3). |
| Copy the following to cite this URL: Armerinayanti N. W, Widodo S, Lestari. Intratumor Macrophages as Tumor-Associated Macrophage Marker of Papillary Thyroid Carcinoma. Biomed Pharmacol J 2022;15(3). Available from: https://bit.ly/3QZbIIS |
Introduction
Thyroid carcinoma is a malignant endocrine tumor that originates in thyroid tissue. The incidence of thyroid cancer has grown by 3.8% annually across the globe. In 2008, the incidence of thyroid cancer by age in the United States was 6.47 per 100,000 males and 19.39 per 100,000 women.(1) Using data from Indonesian cancer registrations in 2015, thyroid carcinoma placed fifth behind breast, cervical, skin, and rectal carcinomas. In contrast, in the same year in Bali, thyroid carcinoma was rated third, behind breast and cervical cancer, with a relative incidence of 27 per 100.000 people.1,2
In general, thyroid carcinomas are classed as well-differentiated carcinomas, since 80% of cases are papillary thyroid carcinomas (PTC) and the remainder are follicular carcinomas (FC) (Gupta et al., 2012). Despite being categorized as well-differentiated, PTC can develop, resulting in organ growth and metastasis. Typically, lymph nodes are the site of metastases in patients with PTC. 5 3
Many determinants factors of thyroid carcinomas progression have been identified in recent years, including PTC, as for clinical features of the lesions (solitary/multiple, solid/cystic consistency, thyroid function test results), histological features (capsule and cell structure, cell morphology, invasion to blood vessel) and, in particular, immunohistochemistry examinations, such as the expression of Matrix Metalloproteinase-9 (MMP-9).2
Current cancer research is beginning to establish a connection between the involvement of cells in the body’s defense system (immune/anti-inflammatory cells) and the creation of a tumoral microenvironment by cancer cells. These immune cells include macrophages. On the basis of their phenotype, macrophages/monocytes can be divided into M1 and M2 subtypes.4 Classically activated macrophages by pro-inflammatory cytokines such as interferon-g, interleukin [IL]-12 and [IL]-23, react to both tumor cells and inflammation. Tumor-Associated Macrophages (TAM) are a component of the tumoral microenvironment with leukocytes, fibroblasts, and endothelium that influence the genesis, progression, and metastasis of cancer.5 TAM’s significance in the progression of thyroid cancer, particularly PTC, has not been well researched yet. Due to the inability of standard histopathological evaluation to detect the phenotype of M1 or M2 macrophages, it cannot be determined whether macrophages observed in patients with PTC are anticancer macrophages or TAM.3,6 Previous research has revealed the association between TAM and the advancement of breast cancer.7
According to various previous research, matrix metalloproteinase (MMP) is one of the extracellular components whose proteolytic impact contributes to the destruction of the extracellular matrix (ECM).8 MMP-9, a gelatinase group that plays a significant role in the breakdown of collagen IV, the primary component of the epithelial, interstitial, and vascular basement membranes, is the subject of special interest.9 In contrast to the expression level in cancer situations, MMP-9 has a modest degree of basal expression.10 In addition, MMP-9 influences neoplastic transformation by initiating genetic instability, stimulating angiogenesis, and promoting tumor growth.11 This demonstrates the significance of MMP-9 in the invasion and metastasis processes, allowing it to serve as an indicator of tumor aggressiveness.12 MMP-9 expression is reportedly high in PTC since its expression is substantially connected with stage, tumor size, lymph node metastases, and PTC aggressiveness.2,13,14
However, no studies have linked the expression of MMP-9 to intratumoral macrophages in PTC patients, demonstrating that these macrophages are TAMs implicated in the development of PTC. This study was conducted to demonstrate the function of intratumor macrophages in PTC, since TAM is engaged in the development of PTC via the production of MMP-9 in macrophage cytoplasm and cytosol.
Material and methods
This study was done in the Biomedical Laboratory of the Faculty of Medicine and Health Sciences at Warmadewa University using a cut-out observational analytical approach from April through November 2019. The research sample consists of all paraffin block archives of patients with an ID card diagnosis stored in the Biomedical Laboratory of the Faculty of Medicine and Health Sciences at Warmadewa University that meets the researcher’s inclusion and exclusion criteria, as calculated by the following formula:
| Za | = | Z value for a value (a = 0.05, Za = 1.96 ) |
| Zb | = | Z value for power (1-b) (b = 0.10, Zb = 1.28) |
| n
P1 P2 |
=
= = |
Sample size
PTC with positive MMP-9 expression (0,6) (Marecko, 2014) PTC with negative MMP-9 espression (0,2) (marecko, 2014) |
The minimal sample size based on this calculation is 38.95, rounded to 39. In this investigation, forty samples were split into 21 groups of PTC with intratumor macrophages and 19 groups of PTC without intratumor macrophages.
In addition, a Chi-Square test was used to determine the proportion of MMP-9 expression in each sample. The significance test was judged to be significant at p<0.05. The accuracy of the data is assessed by a 95% Confident Interval (CI).
Results and Discussion
In this study, patients ranged in age from 25 to 84 years old, with a mean age of 44.501.64 years. The majority of patients were between the ages of 40 and 49 (25%), with the largest concentration of cases occurring between the ages of 30 and 59 (more than 50% of cases).2 This conclusion differs slightly from the WHO report, which claims that PTC often develops between the ages of 20 and 50.14 The higher frequency of PTC in individuals aged 30-59 years in this research may be attributable to goiter as a risk factor for the disease in Bali, which differs from radiation risks seen in the West. In the case of PTC, which is triggered by benign goiter lesions, malignant transformation will take longer to occur. Additionally, the accumulation of somatic mutations throughout the aging process enhances malignant transformation at an advanced age.15 In addition, in Indonesia, especially Bali, early diagnosis of thyroid nodules/enlargement is relatively uncommon, therefore patients often get the test when the nodule is large and at an advanced age.
Table 1: Research Sample Characteristic (n=40).
| Characteristic | Mean ±Standard Deviation | n(%) |
| Age (years) | 44.50±1.64 | |
| 20-29 | 7 (17.5%) | |
| 30-39 | 9 (22.5%) | |
| 40-49 | 10 (25.0%) | |
| 50-59 | 7 (17.5%) | |
| 60-69 | 3 (7.5%) | |
| 70-79 | 2 (5.0%) | |
| 80-89 | 2 (5.0%) | |
| Sex | ||
| Female | 24 (60%) | |
| Male | 16 (40%) | |
| PTC Groups | ||
| 1. PTC with Intratumor Machrophage | 21 (52.5%) | |
| 2. PTC without Intratumor Machrophage | 19 (47.5%) |
In 21 instances of TPC with intratumor macrophages, the mean age was 54.51.71, whereas in 19 cases of PTC without intratumor macrophages, the mean age was 39.51.44. Thus, the PTC group with intratumor macrophages had a higher mean age than the PTC group without intratumor macrophages. According to prior research, the aging process is intimately linked to the formation of tumor microenvironments, including tumor-induced inflammatory cells such as intratumor macrophage. During the aging process, there was an accumulation of genetic and epigenetic changes, accumulation of free radicals due to oxidative stress, and progressive damage of DNA repair mechanisms, cell cycle control, and stem cell renewal, all of which have had an impact on the formation of inflammatory cells, including the M2 phenotype macrophages, which have more pro- than anti-tumor activities. In elderly, some study also found there was an increase in IL-10 macrophage that reveals higher polarization M2 activity 15,16
Chi-square analysis revealed that the expression of MMP-9 macrophages was substantially different in the PTC group with intratumor macrophages compared to the PTC group without intratumor macrophages, p=0.001 (p<0.05). In the PTC group with intratumor macrophages, the proportion of MMP-9-positive macrophages was 81%, compared to 26.3% in the PTC group without intratumor macrophages, or a ratio of 4: 1.5.
Table 2: Difference in MMP-9 Expression Proportion between
PTC with Intratumor Macrophages and PTC Without Intratumor Macrophages
| Group | Number (n) | Percentage (%) | p Value |
| Intratumor Macrophage PTC (+)
Intratumor Macrophage PTC (-) |
17
5 |
81%
26.3% |
0,001 |
This study showed that PTC intratumor macrophages have similar activity to M2 macrophages, which are engaged in PTC growth and invading capability via MMP-9 production. Other investigations have also demonstrated that macrophages in the tumor’s stroma, capsule, and perivascular regions express MMP-9.14,17 This relates to the hypothesis that in the early and late stages of cancer, the tumor is able to transform proinflammatory agents, such as macrophages, into agents that promote the tumor microenvironment.5,18,19 This work has demonstrated that the considerable presence of MMP-9 expression in intratumor macrophages of PTC may enhance angiogenesis and neovascularization as well as stromal activation and remolding, consequently influencing the development and metastasis of PTC.18-20
Fisher’s exact test revealed a strong correlation (p = 0.001) between the presence of intratumor macrophages and the presence of positive MMP-9 staining on macrophages. Macrophages within the tumor were 12 times more likely to have positive MMP-9 staining in the cytoplasm than macrophages outside the tumor. This demonstrates that the presence of intratumor macrophages correlates with tumor invasion and development.
Table 3: Fisher’s Analysis
| Positive MMP-9 Expression in Intratumoral Macrophage | ||||
| PTC | P | OR | 95% CI | P |
| Intratumor Macrophage (+) | 0,001 | 11.9 | 2.674-52.959 | 92% |
Through the observation of significant MMP-9 expression in PTC intratumor macrophages, it has been demonstrated that intratumor macrophages in PTC have a 92% probability/tendency of expressing MMP-9, which suggests that intratumor macrophages will exhibit the M2 phenotype, which is a Tumor Associated Macrophage (TAM). This conclusion corroborates the findings of a prior study that implicated TAM in the development of PTC, particularly in terms of its potential to invade and metastasis via the lymph node route.16
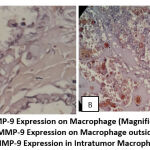 |
Figure 1: MMP-9 Expression on Macrophage (Magnification 400x). |
Numerous variables may affect the propensity of intratumor PTC macrophages to have a TAM phenotype, including as the presence of tumor cells and tumor microenvironments that stimulate activation and reprogramming/change of macrophage phenotype to M2 direction or become TAM. PTC is characterized by many genetic molecular alterations that serve as initial and additional initiators for oncogenic activation, hence enhancing invasion and metastasis, which are hallmarks of carcinogenesis. These genetic molecular alterations may influence the reprogramming and polarization of intratumor macrophages toward TAM, including the PAX8-PPARg and the activation of the JUN Activated Kinase (JAK) pathway, which activates Signal Transducer and Activator of Transcription (STAT). 2,3.
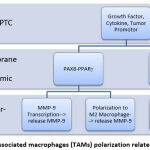 |
Figure 2: Tumor-associated macrophages (TAMs) polarization related PTC Progression |
This mechanism induces macrophage recruitment via MMP-9, wherein these macrophages are first drawn to the surface of endothelial cells and subsequently activated, allowing them to manufacture TIMP-independent MMP-9. In addition to releasing angiogenic components held in the extracellular matrix, the activation of MMP-9 generated by these macrophages facilitates the intravasation and dissemination of tumor cells.2,3,6
Conclusion
Expression of MMP-9 differed significantly between PTC with intratumor macrophages and PTC without intratumor macrophages. Positive expression of MMP-9 in intratumor macrophages indicates that intratumor macrophages of PTC exhibit activities consistent with the M2 phenotype (TAM), which unquestionably influences the course of PTC, particularly by enhancing its propensity to penetrate and metastasis. Therefore, in the histopathological reporting of PTC cases, it can be considered to include the findings of intratumor macrophages, as they have clinical implications as predictive biologic markers for the progression of PTC, which will influence decisions regarding the most appropriate therapy for patients.
Acknowledgement
We would like to thank all parties who have contributed to the implementation of this research, including WARC Warmadewa University, Community Unit and Service and The Biomedical Laboratory Unit of Medical and Health Sciences, Warmadewa University. Thanks are also expressed to the Dean of the Faculty of Medicine and Health Sciences at Warmadewa University who has given permission to conduct the research.
Conflict of Interest
The Authors have no conflict of Interest.
Funding Sources
There is no funding Source.
References
- Nikiforov YE. Thyroid Tumors: Classification, Staging, and General Considerations. Diagnostic Pathology and Molecular Genetics of the Thyroid. 1997;
- Haddad RI, Nasr C, Bischoff L, Busaidy NL, Byrd D, Callender G, et al. Thyroid carcinoma, version 2.2018 featured updates to the NCCN guidelines. JNCCN Journal of the National Comprehensive Cancer Network. 2018;16(12).
CrossRef - Chen H, Izevbaye I, 2# ;, Chen F, Weinstein B. Recent Advances in Follicular Variant of Papillary Thyroid Carcinoma. Vol. 5, North American Journal of Medicine and Science. 2012.
CrossRef - Yang L, Zhang Y. Tumor-associated macrophages: from basic research to clinical application. Vol. 10, Journal of hematology & oncology. 2017.
CrossRef - Segovia-Mendoza M, Morales-Montor J. Immune tumor microenvironment in breast cancer and the participation of estrogens and its receptors into cancer physiopathology. Vol. 10, Frontiers in Immunology. 2019.
CrossRef - Bouchet S, Bauvois B. Neutrophil gelatinase-associated lipocalin (NGAL), pro-matrix metalloproteinase-9 (pro-MMP-9) and their complex pro-MMP-9/NGAL in leukaemias. Vol. 6, Cancers. 2014.
CrossRef - Chrisoulidou A, Boudina M, Tzemailas A, Doumala E, Iliadou PK, Patakiouta F, et al. Histological subtype is the most important determinant of survival in metastatic papillary thyroid cancer. Vol. 4, Thyroid Research. 2011.
CrossRef - Penyakit Kanker S. InfoDATIN 4 Februari-Hari Kanker Sedunia.
- Roderfeld M, Rath T, Lammert F, Dierkes C, Graf J, Roeb E. Innovative immunohistochemistry identifies MMP-9 expressing macrophages at the invasive front of murine HCC. World J Hepatol. 2010;2(5).
CrossRef - Loffek S, Schilling O, Franzke CW. Biological role of matrix metalloproteinases: a critical balance. European Respiratory Journal. 2011;38(1).
CrossRef - Meng X. Expression and clinical significance of matrix metalloproteinase 9 (MMP9) papillary thyroid carcinomas. Afr J Pharm Pharmacol. 2012;6(44).
CrossRef - Farina AR, Mackay AR. Gelatinase B/MMP-9 in tumour pathogenesis and progression. Vol. 6, Cancers. 2014.
CrossRef - Patologi M, Wayan Armerinayanti N, Ketut Mulyadi I, Putu Iin Indrayani Maker L. PENELITIAN Ekspresi Matriks Metaloproteinase-9 pada Infoltrasi Karsinoma Ekspresi Matriks Metaloproteinase 9 pada Infiltrasi Karsinoma Tiroid Papiler Klasik dan Varian Folikuler. Vol. 31, Luh Putu Iin Indrayani Maker. 2017.
- Livolsi VA. Papillary thyroid carcinoma: An update. Modern Pathology. 2011;24.
CrossRef - Gündüz G, Fişkin K. Aging and cancer: Molecular facts and awareness for Turkey. Vol. 38, Turkish Journal of Biology. 2014.
CrossRef - Jackaman C, Tomay F, Duong L, Abdol Razak NB, Pixley FJ, Metharom P, et al. Aging and cancer: The role of macrophages and neutrophils. Vol. 36, Ageing Research Reviews. 2017.
CrossRef - Marečko I, Cvejić D, Šelemetjev S, Paskaš S, Tatić S, Paunović I, et al. Enhanced activation of matrix metalloproteinase-9 correlates with the degree of papillary thyroid carcinoma infiltration. Croat Med J. 2014;55(2).
CrossRef - Jeong H, Hwang I, Kang SH, Shin HC, Kwon SY. Tumor-associated macrophages as potential prognostic biomarkers of invasive breast cancer. J Breast Cancer. 2019;22(1).
CrossRef - Huang H. Matrix metalloproteinase-9 (MMP-9) as a cancer biomarker and MMP-9 biosensors: Recent advances. Vol. 18, Sensors (Switzerland). 2018.
CrossRef - Lin J der, Hsueh C, Huang BY. Papillary thyroid carcinoma with different histological patterns. Vol. 34, Chang Gung Medical Journal. 2011.
- Gupta S, Ajise O, Dultz L, Wang B, Nonaka D, Ogilvie J, et al. Follicular variant of papillary thyroid cancer – Encapsulated, nonencapsulated, and diffuse: Distinct biologic and clinical entities. Archives of Otolaryngology – Head and Neck Surgery. 2012;138(3).
CrossRef - Haque ASMR, Moriyama M, Kubota K, Ishiguro N, Sakamoto M, Chinju A, et al. CD206+ tumor-associated macrophages promote proliferation and invasion in oral squamous cell carcinoma via EGF production. Sci Rep. 2019;9(1).
CrossRef - Kim S, Cho SW, Min HS, Kim KM, Yeom GJ, Kim EY, et al. The Expression of Tumor-Associated Macrophages in Papillary Thyroid Carcinoma. Endocrinology and Metabolism. 2013;28(3).
CrossRef - Pelekanou V, Villarroel-Espindola F, Schalper KA, Pusztai L, Rimm DL. CD68, CD163, and matrix metalloproteinase 9 (MMP-9) co-localization in breast tumor microenvironment predicts survival differently in ER-positive and -negative cancers 11 Medical and Health Sciences 1112 Oncology and Carcinogenesis. Breast Cancer Research. 2018;20(1).
CrossRef







