Manuscript accepted on :28-07-2022
Published online on: 25-08-2022
Plagiarism Check: Yes
Reviewed by: Dr. Liudmila Spirina, Dr. Mohammed Abdalla Hussein
Second Review by: Dr. Francisco Solano
Final Approval by: Dr. Patorn Piromchai
Samar Kamel1 , Dalia W. Zeidan2
, Dalia W. Zeidan2 , Howayda E. Khaled3
, Howayda E. Khaled3  , Zinab Abd-Elhady Ali4 , Nadia G. Elrefaei4 and Mohamed S. El-Naggar5*
, Zinab Abd-Elhady Ali4 , Nadia G. Elrefaei4 and Mohamed S. El-Naggar5*
1Department of Physiology, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt
2Department of Home and Economics, Nutrition and Food Science Branch, Faculty of Education, Suez Canal University, Ismailia, Egypt
3Zoology Department, Faculty of Sciences, Suez University, Suez, Egypt
4Clinical Skills Lab Department, College of Medicine, Imam Abdulrahman Bin Faisal University, P.O. 1982, Dammam 31441, Saudi Arabia
5Zoology Department, Faculty of Science, Suez Canal University, Ismailia, 41522, Egypt.
Corresponding Author E-mail: msemaenr@science.suez.edu.eg
DOI : https://dx.doi.org/10.13005/bpj/2493
Abstract
A chronic low-grade inflammation is one of etiologic conditions closely associated with obesity. The study aims to examine the effect of lycopene on obesity inflammatory conditions in rats. 20 adult male albino rats were divided into four groups (n=5) and for 30-day treatment they were divided into the control group received corn oil as a vehicle, lycopene control group received lycopene extract (10 mg/kg) daily with gavage, obese control group subjected to high fat (HF) diet and received corn oil and obese/lycopene group subjected to HF diet and daily received lycopene extract by the same dose. Bodyweight, weight of thymus and spleen, cytokines [Resistin, Interleukin-1β (IL-1β), Tumor Necrosis Factor α (TNF-α) and Interleukin-6 (IL-6)], lipid profile, and immunohistochemical assay for the Nuclear Factor kappa-B receptors (NF-κB) expression were measured and analyzed. The results revealed that the induced obesity caused a remarkable increase in bodyweight, relative weight of thymus and spleen, levels of serum cytokines, total cholesterol and triglycerides and NF-κB receptors expression, but decreased high-density lipid (HDL) level significantly. Administration of lycopene to obese rats caused a significant depletion in the levels of serum cytokines, total cholesterol (TC) and triglycerides (TG) with a significant increase in HDL level and caused no change in bodyweight, while the relative weight of the spleen and thymus was improved. Also, lycopene caused a marked decline in NF-κB receptors expression in thymus and spleen. These results supported the importance of lycopene as a beneficial carotenoid in combating obesity and companied disturbed fat index and metabolism.
Keywords
Cytokines; Lycopene; NF-κB; Obesity
Download this article as:| Copy the following to cite this article: Kamel S, Zeidan D. W, Khaled H. E, Ali Z. A. E, Elrefaei N. G, El-Naggar M. S. In Vivo Assessment of Lycopene Effect on Obesity-Induced Inflammation. Biomed Pharmacol J 2022;15(3). |
| Copy the following to cite this URL: Kamel S, Zeidan D. W, Khaled H. E, Ali Z. A. E, Elrefaei N. G, El-Naggar M. S. In Vivo Assessment of Lycopene Effect on Obesity-Induced Inflammation. Biomed Pharmacol J 2022;15(3). Available from: https://bit.ly/3R5HaFG |
Introduction
Obesity is a medicinal disorder characterized by excess body fat and body mass index (BMI) greater than 30 kg/m2, leading to harmful health problems 1. There are more than 500 million people worldwide presently suffer from obesity and high pathological diseases such as disordered metabolic syndrome, diabetes, cardiovascular problems, chronic inflammatory diseases and sleep complaints cause the main health syndromes in developed and developing countries 2. Obesity as a lifestyle issue caused by an imbalance in energy expenditure and intake has previously been linked to more severe and complex pathogenic outcomes.
The adipose tissue is considered not only as body store of energy but also endocrine organ producing a variety of biologically active proteins and peptides known as adipokines 3. These released adipokines are a heterogeneous group including cytokines, hormones, growth factors, prostaglandins, acute-phase proteins, sex steroids and glucocorticoids, with complicated efficacy on the receptor organs such as kidneys, hypothalamus, immune system, skeletal muscle, pancreas and liver 4. In the obesity situation, the secretion of additional adipokines and numerous cytokines is supposed to cause a low-grade inflammation within the adipose tissue, and subsequently causes the progress of multiple secondary disorders like metabolic syndrome, diabetes, insulin resistance, asthma and arterial hypertension 5 , 6.
These alterations in cytokine secretion are associated with releasing some chemo-attractant mediators like free fatty acids and tumor-necrosis-factor alpha (TNF-α), which attract macrophages to penetrate the adipose tissue 7. In obesity situation, several cytokines enhance the alteration of stimulating process of macrophage from the main alternatively activated phase (M2) to the classic activated phase (M1) 8 , 9. Also, in obese persons, the release of pro-inflammatory adipokines by hypertrophied adipocytes of visceral adipose tissue, basically TNF-α and interleukin–6 (IL-6), was recorded to be increased 3 and the anti-inflammatory adipokines secretion appears to be inhibited 10.
Lycopene is considered a lipophilic non-provitamin A carotenoid, which gives rise to the red colour in different vegetables and fruits like tomato, guava, grapefruits and watermelon 11. This carotenoid is well-recognized for its antioxidant characteristics 12. It is beneficial for inhibiting of multiple inflammation-accompanied disorders such as cancer, cardiovascular diseases 13 , 14 and obesity 12 , 15. Lycopene is primarily stored in human adipose tissue due to its hydrophobicity 16. It was investigated that higher consumption of lycopene was accompanied by a lower waist circumference and lower fat mass 17. The efficacy of lycopene in adipose tissue can be accredited, at least, to its anti-inflammatory and anti-oxidative effects, depending on several studies. Primarily, lycopene reduced the mRNA expression of pro-inflammatory cytokines in adipose tissues of high-fat (HF) diet-fed mice 18 , 19. Moreover, lycopene decreased TNF-α release in lipopolysaccharide (LPS)-stimulated macrophages, which was accompanied by reduced NF-κB and mitogen-activated protein kinase (MAPK) signaling 20. So, lycopene appears to block the link between macrophages and adipocytes and thus inhibit obesity-accompanied adipose inflammation. On the other hand, it is still unclear whether and how lycopene modifies the inflammatory case of tissue macrophages in obese mice 21.
The current study aimed to examine the effect of lycopene on obesity inflammatory condition via assessment of bodyweight, the weight of the thymus and spleen, analysis of cytokines (Resistin, TNF-α, Interleukin-1β and Interleukin-6) and measurement of lipid profile. Also, immunohistochemical assay was used to evaluate their content of NF-κB receptors.
Materials and methods
Experimental animals and Ethics
Twenty adult male albino rats (94–100 g BW) were used in the current study. Animals were brought at the Faculty of Veterinary Medicine, Suez Canal University, Egypt, kept in polyethylene cages with free access for food and water, and left for a week for acclimatization to animal house conditions. Rats were maintained at a controlled 60 % humidity, 21 ± 1°C temperature and under a 12-hour light and dark program. The strategy of this experiment was approved by the Research Ethical Committee of the Faculty of Veterinary Medicine, Suez Canal University, Egypt (approval No. 2019030).
Study design and diet
The rats were divided into four groups (n=5), as the control group received corn oil as the vehicle, group 2 was the lycopene control, which received lycopene (Sigma-Aldrich, Egypt) extract (10 mg/kg BW) daily via gavage according to Kravchenko et al., 22, group 3 was the obese control and subjected to high fat (HF) diet and received corn oil. Group 4, the obese lycopene, was subjected to HF diet and received the same dose of lycopene extract. All treatments were sustained for 30 days. The control diet was designed to accomplish the nutritional supplies of rats according to National Research Council 23. According to Axen and Axen, 24, rats suffered from induced obesity after obtaining HF diet for 12 weeks (Table 1).
Table 1: The diet constituents for control and high fat diet groups.
| Ingredients (%) | Control diet (%) | High fat diet (%) |
| Yellow Corn | 70.24 | 29.50 |
| Corn Gluten | 5.00 | 2.1 |
| Soybean | 8.80 | 3.70 |
| Casein | 5.00 | 13.84 |
| Wheat Bran | 4.00 | 1.68 |
| Sucrose | —– | 5.14 |
| Vegetable Oil | 4.74 | 1.99 |
| Animal Fat | —– | 38.20 |
| Cellulose | —– | 1.50 |
| Methionine | 0.34 | 0.39 |
| Lysine | 0.07 | —- |
| Ground Limestone | 0.82 | 0.42 |
| Dicalcium Phosphate | 0.56 | 1.11 |
| Common Salt | 0.13 | 0.13 |
| Premix | 0.30 | 0.30 |
| Total | 100 | 100 |
Bodyweight, thymus and spleen relative weights
According to Abdel-Rahman et al., 25 method, rats were weighed on the first day of experiment and then weighed on the last experiment day, and the bodyweight gain (Bwt.G) was measured by subtracting the initial bodyweight from the final (F.Bwt). At the study period end, rats were anesthetized, to dissect the organs. For each rat, the thymus and spleen were extracted and weighed, and the relative organ weight was detected as follows: (Absolute organ weight / F.Bwt) × 100.
Blood sampling
Blood samples were drawn from retro-orbital venous plexus using non-heparinized microhematocrit tube and centrifuged at 3000 rpm for 20 min and serum is separated and kept at -20°C till analysis of cytokines and lipid profile.
Cytokines assessment
Resistin, Interleukin-1β, Tumor necrosis factor -α (TNF-α) and Interleukin-6 were assessed, using Elisa kits for murine (Bioassay Technology Laboratory Company, China).
Lipid profile
Total cholesterol (TC) , triglyceride (TG) and high-density lipoprotein (HDL) were measured by using a commercial enzymatic calorimetric method (Bio-diagnostic Company, Dokki, Giza, Egypt) for triglycerides (Cat. No. TR 20 30), total cholesterol (Cat. No. CH 12 20) and HDL- cholesterol (Cat. No. CH 12 30).
Immunohistochemistry of NF-KB receptors:
According to Liu et al., 26, NF-κB protein expression was analyzed by immunohistochemistry technique. In a standard method, sections were microwaved for antigen retrieval, pretreated with 0.3 % H2O2, then blocked with goat serum and incubated overnight at 4 °C in a primary antibody solution containing rabbit anti-NF-κB p65 antibody. The sections were washed and incubated for one hour at room temperature in a suitable secondary antibody solution. The next step is to incubate the sections for one hour at room temperature in HRP—streptavidin and then treated with diaminobenzidine to develop the color reaction. Finally, the sections were counterstained, dehydrated and investigated under a simple light microscope. The NF‐κB staining degree was measured in both the nucleus and cytoplasm of the thymus and spleen.
Statistical analysis
Statistical analyses were performed by one-way ANOVA by using the statistical software package (SPSS; ver. 20). Values were expressed as mean ± SE, and (P<0.05) was the statistical significant limit.
Results
Cytokines assessment
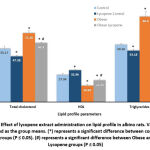
Figure 1 demonstrates serum levels of cytokines in control and treated groups. There was a marked (P<0.05) increase in serum Resistin, IL-1β, TNF-α and IL-6 in the obese group when compared with the control and lycopene control groups. Serum cytokines were significantly (P<0.05) decreased in the obese lycopene group as compared with the obese one.
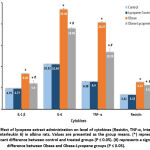 |
Figure 1: Effect of lycopene extract administration on level of cytokines (Resistin, TNF-α, Interleukin1-β and nterleukin 6) in albino rats. Values are presented as the group means. |
Lipid profile assay
There was a significant (P<0.05) increase in serum TG and TC, and a significant decrease (P<0.05) in HDL level in the obese group as compared with the control and lycopene control groups (Fig. 2).
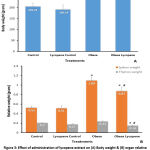
Body and organ relative weights
Concerning body & organ relative weights, the total body weight of the obese group was significantly (P<0.05) increased when compared with the control value, but lycopene administration didn’t show any effect in both lycopene control and lycopene obese groups (Fig. 3A). On the other hand, there was a significant elevation (P<0.05) in relative weight of the thymus and spleen in the obese group compared with the control one. After lycopene treatment, it showed a significant decrease (P<0.05) in relative weight of the thymus and spleen (Fig. 3B).
Immunohistochemistry of NF-κB
Both spleen and the thymus of the obese group showed a marked increase in NF-κB receptor expression, which is significantly decreased in the lycopene treated group (Fig. 4 and 5, respectively).
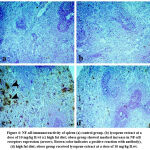 |
Figure 4: NF-κB immunoreactivity of spleen (a) control group. (b) lycopene extract at a dose of 10 mg/kg B.wt (c) high fat diet; obese group showed marked increase in NF-κB receptors expression |
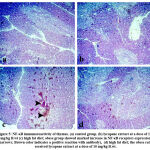 |
Figure 5: NF-κB immunoreactivity of thymus. (a) control group. (b) lycopene extract at a dose of 10 mg/kg B.wt (c) high fat diet; obese group showed marked increase in NF-κB receptors expression |
Discussion
Obesity is abnormal excess of fat accumulation caused by the imbalance between energy expenditure and intake 27 , 28. In the current study, we revealed that lycopene is an extremely valuable antioxidant carotenoid in combating obesity.
Concerning the cytokines measurement, it was revealed that there was a significant increase (P<0.05) in serum Resistin, IL-1β, TNF-α and IL-6 in obese animals compared with control animals. However, these serum cytokines were significantly decreased (P<0.05) in lycopene treated group compared with the obese one. These results indicated that lycopene has the ability to reduce these serum cytokines in obese individuals, and they are in general accord with a study of Riso et al., 29 that reported moderate efficacy on the released mediators of inflammation, like TNF-α, after the regular administration of lycopene by healthy volunteers. Also, Watzl et al., 30 explained that the highest TNF-α production was accompanied by a low lycopene intake. Also, Hazewindus et al., 31 in their investigation on lycopene recorded that lycopene significantly reduced inflammation by preventing the secretion of TNF-α and triggering production of IL-10. In vitro, lycopene reduced the release of IL-6 32. It has been also revealed that lycopene diminished pro-inflammatory cytokines (IL-6, IL-1β and TNF-α) expression in human macrophages 33.
Another study proved that lycopene declines the NF-κB translocation leads to reduction of inflammatory biomarkers expression, including TNF-α and IL-8 34. The redox feature of Lycopene is the reason for this anti-inflammatory influence 35. Furthermore, some studies have revealed that lycopene stimulates I-κB, the main inhibitory agent of NF-κB, leading to declining of its translocation 33 , 36. During the investigation of molecular mechanism, Gouranton et al., 18 observed that the existence of lycopene blocks the luciferase gene reporter under the control of the NF-κB-responsive element. Moreover, the lycopene/NF-κB interaction pathway was revealed to play a main role in the phosphorylation change of Iκ kinase α/β 37.
Regarding the lipid profile, there were a significant increase (P<0.05) in TC and TG contents and a significant decrease in HDL level the serum of the obese group compared with the control and lycopene control groups, while TC and TG were significantly decreased, and HDL was significantly increased after treatment of obese rats with lycopene, compared with the obese one. It was recorded that lycopene has the ability to decrease lipid content by diminishing TC, TGs as well as synthesizing of dysfunctional HDL-C 38. Moreover, lycopene had been reported to prevent the synthesis of harmful cholesterol by suppressing β-Hydroxy β-methylglutaryl-CoA (HMG-CoA) reductase that induces low density lipid-C (LDL-C) degradation 39 , 40. Furthermore, other studies have reported that lycopene could prevent the nitric oxide (NO) synthesis pathway 41 which would make more activation of lipolysis in adipose tissues and NEFA generation 39 , 42.
The total bodyweight was significantly increased in the obese rats compared with control, and the lycopene administration didn’t improve it in both the control and obese groups. On the other hand, the obese animals had a significant increase in spleen and thymus relative weights those were decreased significantly after lycopene treatment. The increase of weight gain was due to the high fat content of animal’s diet. These results were in agreement with the observations of Santos et al., 43; Santos et al., 44 and Kostrycki et al., 45. The possible cause is that higher energy intake in HF-diet rats caused increased fat mass and consequently the weight gain. Otherwise, studies by Wang et al., 46 and Jiang et al., 47 found that treatment of HF-diet rats with lycopene caused a significant decrease in bodyweight. Fenni et al., 19 found that lycopene modulated most of the phenotypical disorders except for the total bodyweight, which was not improved. Similar deficiency of lycopene influence on the bodyweight had previously been investigated in rats 48, contrary to the lately recorded data in mice 49. Tis conflict is currently unsolved due to possible interspecific variation and genetic differences between used animal models.
The present study showed also marked increase in NF-KB receptors expression in obese group, which is significantly decreased in the lycopene treated group. These results are parallel with that reported by Carlsen et al., 50 who found that the activity of NF-κB is enhanced in mice fed HF-diet. It was found that weight gain in HF-diet mice may be a powerful predictor of NF-κB action in the thoracic region of female mice. It is of great importance to observe that HF-diet and obesity typically stimulate the activity of NF-κB about twofold, which is much lower than the 10–100 fold stimulation in acute inflammatory reactions. This result typically explained the diagnosis of obesity as a chronic low-grade inflammatory disorder. The enhanced NF-κB activity in the thoracic region of HF-diet fed female mice is an indicator of enhanced activity of the thymic tissue. Former studies showed that mice fed HF-diet have larger thymus glands than mice fed a control diet 51. Accordingly, it is remarkable that the anti-inflammatory influence of lycopene in adipocytes, denoting that both direct and indirect effects are possibly responsible of the general anti-inflammatory efficacy 19.
Conclusion
The induced obesity is accompanied with metabolic disturbances associated with marked increase in serum Resistin, IL-1β, TNF-α and IL-6 with significant disturbance in lipid profile picture. Also obesity adversely affected thymus and spleen function through stimulation of NF-κB expression. Administration of lycopene to HF diet rats resulted in marked improvement of metabolic disturbances induced by obesity, thymus and spleen functional inefficiency. In addition, lycopene could diminish obesity-induced inflammatory cytokines as well as NF-κB expression.
Authors Contributions
Concept and design were done by Samar Kamel and Mohamed S. El-Naggar; Samar Kamel performed the data analyses; Samar Kamel and Mohamed S. El-Naggar interpreted the data and wrote the manuscript; critical revision of the manuscript for important intellectual content was done by Howayda Khaled and Mohamed S. El-Naggar; Dalia Zeidan, Howayda Khaled, Zinab Abd-Elhady Ali, Nadia Gouda Elrefaei performed the experiment, Statistical analysis was done by Dalia Zeidan and Zinab Abd-Elhady Ali, Nadia Gouda Elrefaei.
Ethical approval
Ethical approval was obtained from the Ethical research Committee of Faculty of Veterinary Medicine, Suez Canal University, Egypt.
Conflict of Interest
All authors declare that they have no relevant financial or non-financial interest to disclose.
Funding Sources
No external funding was received.
References
- Stein J., Luppa M., Ruzanska U., Sikorski, C., König, H.-H. and Riedel-Heller, S.G. (2014). Measuring negative attitudes towards overweight and obesity in the German population–psychometric properties and reference values for the German short version of the fat phobia scale (FPS). PloS one 9, e114641.
CrossRef - Finucane M. M., Stevens G. A., Cowan J., Danaei G., Lin J. K. Paciorek C. J., Singh G. M., Gutierrez H. R., Lu Y. and Bahalim, A.N. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9· 1 million participants. The lancet, 2011; 377: 557-567.
CrossRef - Trayhurn P. and Wood I.S. Adipokines: inflammation and the pleiotropic role of white adipose tissue. British journal of nutrition, 2004; 92: 347-355.
CrossRef - Fruhbeck G., Gómez-Ambrosi J., Muruzábal F.J. and Burrell M.A. The adipocyte: a model for integration of endocrine and metabolic signaling in energy metabolism regulation. American Journal of Physiology-Endocrinology And Metabolism, 2001; 280: E827-E847.
CrossRef - Engström G., Hedblad B., Stavenow L., Lind P., Janzon L. and Lindgärde F. Inflammation-sensitive plasma proteins are associated with future weight gain. Diabetes, 2003; 52: 2097-2101.
CrossRef - Newson R., Jones M., Forsberg B., Janson C., Bossios A., Dahlen S. E., Toskala E. M., Al‐Kalemji A., Kowalski M. and Rymarczyk B. The association of asthma, nasal allergies, and positive skin prick tests with obesity, leptin, and adiponectin. Clinical & Experimental Allergy, 2014; 44: 250-260.
CrossRef - Maury E. and Brichard S. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Molecular and Cellular Endocrinology, 2010; 314: 1-16.
CrossRef - Lumeng C. N., Bodzin J. L. and Saltiel A. R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. The Journal of clinical investigation, 2007; 117: 175-184.
CrossRef - Lacey D. C., Achuthan A., Fleetwood A. J., Dinh H., Roiniotis J., Scholz G. M., Chang M. W., Beckman S. K., Cook A. D. and Hamilton J. A. Defining GM-CSF–and macrophage-CSF–dependent macrophage responses by in vitro The Journal of Immunology, 2012; 188: 5752-5765.
CrossRef - Manigrasso M., Ferroni P., Santilli F., Taraborelli T., Guagnano M., Michetti N. and Davi G. Association between circulating adiponectin and interleukin-10 levels in android obesity: effects of weight loss. The Journal of Clinical Endocrinology & Metabolism, 2005; 90: 5876-5879.
CrossRef - Rao A.V., Ray M. and Rao L. Lycopene. Advances in food and nutrition research, 2006; 51: 99-164.
CrossRef - Palozza P., Parrone N., Catalano A. and Simone R. Tomato lycopene and inflammatory cascade: basic interactions and clinical implications. Current medicinal chemistry, 2010; 17: 2547-2563.
CrossRef - Krinsky N. I. and Johnson E. J. Carotenoid actions and their relation to health and disease. Molecular aspects of medicine, 2005; 26: 459-516.
CrossRef - Kilany O. E., Abdelrazek H. M., Aldayel T.S., Abdo S. and Mahmoud M. M. Anti-obesity potential of Moringa olifera seed extract and lycopene on high fat diet induced obesity in male Sprauge Dawely rats. Saudi Journal of Biological Sciences, 2020; 27: 2733-2746.
CrossRef - Senkus K. E., Tan L. and Crowe-White K. M. Lycopene and metabolic syndrome: a systematic review of the literature. Advances in Nutrition, 2019; 10: 19-29.
CrossRef - Chung H. Y., Ferreira A. L. A., Epstein S., Paiva S. A., Castaneda-Sceppa C. and Johnson, E. J. Site-specific concentrations of carotenoids in adipose tissue: relations with dietary and serum carotenoid concentrations in healthy adults. American Journal of Clinical Nutrition, 2009; 90: 533-539.
CrossRef - Sluijs I., Beulens J.W., Grobbee D.E. and Van Der Schouw Y.T. Dietary carotenoid intake is associated with lower prevalence of metabolic syndrome in middle-aged and elderly men. The Journal of nutrition, 2009; 139: 987-992.
CrossRef - Gouranton E., Thabuis C., Riollet C., Malezet-Desmoulins C., El Yazidi C., Amiot M., Borel P. and Landrier, J. Lycopene inhibits proinflammatory cytokine and chemokine expression in adipose tissue. The Journal of nutritional biochemistry, 2011; 22: 642-648.
CrossRef - Fenni S., Hammou H., Astier J., Bonnet L., Karkeni E., Couturier C., Tourniaire F. and Landrier, J.F. Lycopene and tomato powder supplementation similarly inhibit high‐fat diet induced obesity, inflammatory response, and associated metabolic disorders. Molecular nutrition & food research, 2017; 61:
CrossRef - Marcotorchino J., Romier B., Gouranton E., Riollet C., Gleize B., Malezet‐Desmoulins C. and Landrier J.F. Lycopene attenuates LPS‐induced TNF‐α secretion in macrophages and inflammatory markers in adipocytes exposed to macrophage‐conditioned media. Molecular nutrition & food research, 2012; 56: 725-732.
CrossRef - Chen G., Ni Y., Nagata N., Zhuge F., Xu L., Nagashimada M., Yamamoto S., Ushida Y., Fuke N. and Suganuma, H. Lycopene Alleviates Obesity‐Induced Inflammation and Insulin Resistance by Regulating M1/M2 Status of Macrophages. Molecular Nutrition & Food Research, 2019; 63:
CrossRef - Kravchenko L., Morozov S., Beketova N., Deryagina V., Avren’eva L. and Tutel’yan V. Antioxidant status of rats receiving lycopene in different doses. Bulletin of Experimental Biology and Medicine, 2003; 135: 353-357.
CrossRef - National Reseach Council. Nutrient requirements of laboratory animals: 1995.
- Axen K. V. and Axen K. Very Low‐Carbohydrate versus Isocaloric High‐Carbohydrate Diet in Dietary Obese Rats. Obesity, 2006; 14: 1344-1352.
CrossRef - Abdel-Rahman H. , Abdelrazek H., Zeidan D. W., Mohamed R. M. and Abdelazim A. M. Lycopene: hepatoprotective and antioxidant effects toward bisphenol A-induced toxicity in female Wistar rats. Oxidative medicine and cellular longevity, 2018.
CrossRef - Liu Z., Wang Y., Ning Q., Gong C., Zhang Y., Zhang L., Bu X. and Jing G. The role of spleen in the treatment of experimental lipopolysaccharide-induced sepsis with dexmedetomidine. springerplus, 2015; 4: 1-7.
CrossRef - Kopelman P.G. Obesity as a medical problem. Nature, 2000; 404: 635-643.
CrossRef - Spiegelman B. and Flier J. S. Obesity and the regulation of energy balance. Cell, 2001; 104: 531-543.
CrossRef - Riso P., Visioli F., Grande S., Guarnieri S., Gardana C., Simonetti P. and Porrini M. Effect of a tomato-based drink on markers of inflammation, immunomodulation, and oxidative stress. Journal of Agricultural and Food Chemistry, 2006; 54: 2563-2566.
CrossRef - Watzl B., Bub A., Briviba K. and Rechkemmer G. Supplementation of a low-carotenoid diet with tomato or carrot juice modulates immune functions in healthy men. Annals of nutrition and metabolism, 2003; 47: 255-261.
CrossRef - Hazewindus M., Haenen G.R., Weseler A.R. and Bast A. The anti-inflammatory effect of lycopene complements the antioxidant action of ascorbic acid and α-tocopherol. Food Chemistry, 2012; 132: 954-958.
CrossRef - Saedisomeolia A., Wood L. G., Garg M. L., Gibson P. G. and Wark P. A. Lycopene enrichment of cultured airway epithelial cells decreases the inflammation induced by rhinovirus infection and lipopolysaccharide. The Journal of nutritional biochemistry, 2009; 20: 577-585.
CrossRef - Palozza P., Simone R., Catalano A., Monego G., Barini A., Mele M. C., Parrone N., Trombino S., Picci N. and Ranelletti, F. O. Lycopene prevention of oxysterol-induced proinflammatory cytokine cascade in human macrophages: inhibition of NF-κB nuclear binding and increase in PPARγ expression. The Journal of nutritional biochemistry, 2011; 22: 259-268.
CrossRef - Blackwell T. S. and Christman J. W. The role of nuclear factor-κ B in cytokine gene regulation. American Journal of Respiratory Cell and Molecular Biology, 1997; 17, 3-9.
CrossRef - Kim G.Y., Kim J. H., Ahn S. C., Lee H. J., Moon D. O., Lee C. M. and Park Y. M. Lycopene suppresses the lipopolysaccharide‐induced phenotypic and functional maturation of murine dendritic cells through inhibition of mitogen‐activated protein kinases and nuclear factor‐κ Immunology, 2004; 113: 203-211.
CrossRef - Huang C. S., Fan Y. E., Lin C. Y. and Hu M. L. Lycopene inhibits matrix metalloproteinase-9 expression and down-regulates the binding activity of nuclear factor-kappa B and stimulatory protein-1. The Journal of nutritional biochemistry, 2007; 18: 449-456.
CrossRef - Ghavipour M., Saedisomeolia A., Djalali M., Sotoudeh G., Eshraghyan M.R., Moghadam A.M. and Wood, L.G. Tomato juice consumption reduces systemic inflammation in overweight and obese females. British Journal of Nutrition, 2013; 109: 2031-2035.
CrossRef - Mozos I., Stoian D., Caraba A., Malainer C., Horbańczuk J. O. and Atanasov A. G. Lycopene and vascular health. Frontiers in Pharmacology, 2018; 9: 521.
CrossRef - Jobgen W. S., Fried S. K., Fu W. J., Meininger C. J. and Wu, G. Regulatory role for the arginine–nitric oxide pathway in metabolism of energy substrates. The Journal of nutritional biochemistry, 2006; 17: 571-588.
CrossRef - Agarwal S. and Rao A. V. Tomato lycopene and low density lipoprotein oxidation: a human dietary intervention study. Lipids, 1998; 33: 981-984.
CrossRef - Li X. N., Lin J., Xia J., Qin L., Zhu S. Y. and Li J. L. Lycopene mitigates atrazine-induced cardiac inflammation via blocking the NF-κB pathway and NO production. Journal of Functional Foods, 2017; 29: 208-216.
CrossRef - Hong M.Y., Hartig N., Kaufman K., Hooshmand S., Figueroa A. and Kern M. Watermelon consumption improves inflammation and antioxidant capacity in rats fed an atherogenic diet. Nutrition Research, 2015; 35: 251-258.
CrossRef - Santos E. W., de Oliveira D.C., Hastreiter A., de Oliveira Beltran J.S., Rogero M.M., Fock R.A. and Borelli P. High-fat diet or low-protein diet changes peritoneal macrophages function in mice. Nutrire, 2016; 41: 1-9.
CrossRef - Santos E. W., Oliveira D. C., Hastreiter A., Silva G. B., Beltran J. S. d. O., Rogero M. M., Fock R. A. and Borelli P. Short-term high-fat diet affects macrophages inflammatory response, early signs of a long-term problem. Brazilian Journal of Pharmaceutical Sciences, 2019; 55.
CrossRef - Kostrycki I. M., Wildner G., Donato Y. H., Dos Santos A. B., Beber L. C. C., Frizzo M. N., Ludwig M. S., Keane K. N., Cruzat V. and Rhoden C. R. Effects of high-fat diet on eHSP72 and extra-to-intracellular HSP70 levels in mice submitted to exercise under exposure to fine particulate matter. Journal of diabetes research 2019.
CrossRef - Wang J. H., Bose S., Kim G. C., Hong S. U., Kim J. H., Kim J. E. and Kim H. Flos Lonicera ameliorates obesity and associated endotoxemia in rats through modulation of gut permeability and intestinal microbiota. PloS One, 2014; 9: e86117.
CrossRef - Jiang W., Guo M. H. and Hai X. Hepatoprotective and antioxidant effects of lycopene on non-alcoholic fatty liver disease in rat. World Journal of Gastroenterology, 2016; 22: 10180.
CrossRef - Luvizotto R. d. A. M., Nascimento A. F., Imaizumi E., Pierine D. T., Conde S. J., Correa C. R., Yeum K. J. and Ferreira, A.L.A. Lycopene supplementation modulates plasma concentrations and epididymal adipose tissue mRNA of leptin, resistin and IL-6 in diet-induced obese rats. British Journal of Nutrition, 2013; 110, 1803-1809.
CrossRef - Singh D., Khare P., Zhu J., Kondepudi K., Singh J., Baboota R., Boparai R., Khardori R., Chopra K. and Bishnoi M. A novel cobiotic-based preventive approach against high-fat diet-induced adiposity, nonalcoholic fatty liver and gut derangement in mice. International Journal of Obesity, 2016; 40: 487-496.
CrossRef - Carlsen H., Haugen F., Zadelaar S., Kleemann R., Kooistra T., Drevon C. A. and Blomhoff, R. Diet-induced obesity increases NF-κB signaling in reporter mice. Genes & Nutrition, 2009; 4: 215-222.
CrossRef - Mito N., Yoshino H., Hosoda T. and Sato K. Analysis of the effect of leptin on immune function in vivo using diet-induced obese mice. Journal of Endocrinology, 2004; 180: 167-174.
CrossRef