A. S. Roshni1 , Arul Amutha Elizabeth
, Arul Amutha Elizabeth , S. Sneha Aishwarya*
, S. Sneha Aishwarya* and Brigida S
and Brigida S
Department of Pharmacology, Sree Balaji Medical College and Hospital, CLC works road, Chrompet, Chennai, Tamil Nadu, India.
Corresponding Author E-mail: snehaaishwarya.ph@bharathuniv.ac.in
DOI : https://dx.doi.org/10.13005/bpj/2482
Abstract
Cancer is not a modern disease but has been present since the very early dawn of life in both animals and humans”. Cancer is a disease that is caused due to alteration in the genetic configuration (mutation) that occurs due to either an inherent biological failure or an externally acquired factor leading to genomic instability and oncogenesis. The sudden upsurge in the incidence of cancer throughout the world in the last two decades has been attributed to the environmental and lifestyle changes which includes smoking, pollution, consumption of processed food etc…It is one among the prominent health problem responsible for morbidity and mortality. It is basically a cell proliferative disorder, initially specific to an organ and advances to invasion and metastasis. Chemotherapy with anticancer drugs remain the standard treatment modality. But those treatment drugs are associated with numerous serious side effects. On the other hand, phytomedicine is now attracting attention due to its potential anticancer properties. These medicines can be considered as an alternative form of therapy in cancer management due to their cost effectiveness, therapeutic efficacy and minimal side effects. Aesculus hippocastanum (AH)commonly known as European horse chestnut is found all over the world. The principal purpose of the study is to explore the anticancer property of ethanolic flower root out of Aesculus hippocastanum by performing the MTT assay (cytotoxic study) against human ovarian cell line. The study was evaluated by assessing the inhibition of percentage of cell viability and it showed minimum viability of cell (53.68 ± 0.8755 %) at the higher concentration of 200µl of Aesculus hippocastanum sample solution with Methotrexate as a standard comparison drug. This has proved the cytotoxic effect of Aesculus hippocastanum on PA1 human ovarian cancer cell line
Keywords
Anti Cytotoxic Action; Anticancer Drugs; Human ovarian cancer cell line; Methotrexate; Phytomedicine
Download this article as:| Copy the following to cite this article: Roshni A. S, Elizabeth A. A, Aishwarya S. S, Brigida S. In Vitro Cytotoxic Action of Aesculus hippocastanum Extract Against PA 1 Human Ovarian Cell-Line. Biomed Pharmacol J 2022;15(3). |
| Copy the following to cite this URL: Roshni A. S, Elizabeth A. A, Aishwarya S. S, Brigida S. In Vitro Cytotoxic Action of Aesculus hippocastanum Extract Against PA 1 Human Ovarian Cell-Line. Biomed Pharmacol J 2022;15(3). Available from: https://bit.ly/3RULSXj |
Introduction
Cancer turns to be a major killer disease of past few decades globally. The global statistics says 21% of males and 18% of females contribute to the worldwide burden of cancer. Like many cancers the incidence of ovarian cancer differs in distribution throughout the world. The occurrence of ovarian cancer ranks third among the gynecological malignancies, but it is associated with highest mortality rate. Among the cancer related deaths 4.4% mortality rate has been attributed to ovarian cancer 1. The standard treatment of cancer involves chemotherapy, radiotherapy and surgical intervention. Since the standard therapy is associated with numerous systemic, psychological and neurological side effects, phytomedicines plant based pharmaceuticals in cancer chemotherapy is the new research interest. The establishment of such drugs with equal or superior therapeutic efficacy as an alternative treatment will be a great breakthrough in the field of oncology. For example the discovery of Vincristine and Vinblastine obtained from Catharanthus roseus and campothecins Topotecan) from Campotheca accuminata has revolutionized the treatment of lymphoma , leukemia & ovarian cancer ,lung cancer respectively2 .
Aesculus hippocastanum is a famous medicinal tree with numerous health benefits. It belongs to the group called saponins. The medicinal value is found in almost all parts of the tree which includes leaves, flowers, seeds and bark. The biochemical evaluation of AH showed presence of saponins, hippocaesculin and barringotogenol collectively knowns as Escins. Along with escins they also contain flavenoids, carotenoids and fatty acids. All these constituents contribute to the anti-inflammatory, antioxidant and anticancer properties 3.
Aesculus hippocastanum oral formulation has been very commonly used in the treatment of varicose veins, hemorrhoids, rheumatism. The plant product is extensively used in the manufacture of numerous cosmetic products 4. Among all the biochemical constituent of AH, Escin is the most bioactive substance to exhibit effective anticancer property 3. Most of the anticancer studies on aesculus species were performed with bark and seed extracts. This is the first study on the evaluation of cytotoxic effect of ethanolic flower extract of Aesculus hippocastanum demonstrated on PA1 human ovarian cancer cell-line.
Methodology and Results
Flower Extract Preparation
Shadow dried, grounded, fine powdered fresh flowers of Aesculus hippocastanum was kept ready. The fine powder (50g) was then suspended in 100 ml of ethyl alcohol for one complete day (24 hours) at a standard room temperature of 25-28 degrees C. Following this, the obtained mixture was sieved using a cotton cloth, thereby keeping the bioactive constituents intact. The final product is the ethanolic flower extract of Aesculus hippocastanum which is the sample test solution.
Sample Preparation
1 ml of the ethanolic flower extract of Aesculus hippocastanum (sample) was taken and diluted with Dimethyl sulfoxide (DMSO) attain different concentrations of the sample such as10μl, 50μl, 100μl and 200μl after inoculation. With all the above-mentioned concentrations of the sample the cytotoxic property (anticancer) was evaluated on Human ovarian (PA-1) cancer cell culture and media using MTT {3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide} assay.
Methotrexate harnessed as the standard source drug to compare anticancer property of the sample test solution. Methotrexate 1mg/ml was taken and diluted using the agent Dimethyl sulfoxide (DMSO) to attain the concentration 10 µg/ml and the MTT assay was performed on the same above-mentioned cell line.
Human ovarian (PA-1) cancer cell culture and media
From NCCS, PA-1 cell lines were acquired and stock cells were cultivated in medium augmented with Dulbecco’s Modified Eagle Medium (DMEM), streptomycin (100 μg/ml), penicillin (100 IU/ml), in a dampened environment of 5% CO2 at 37oC up to coalescent. The cells were detached with Sterile filtered Trypsin Phosphate Versene Glucose (TPVG) Solution (0.1% Trypsin, 0.02% EDTA, 0.05% Glucose and Phenol red in Dulbecco’s Phosphate Buffered Saline). The sustainability of the cells were verified and centrifugated. Further 50,000 cells / well sown in a 96 well plate and incubated for 24 hours at 37oC, 5% CO2 incubator.
Source of reagents
Trypsin procured from Himedia, Dulbecco’s Modified Eagle Medium, Dulbecco’s Phosphate Buffered Saline, Fetal Bovine Serum (FBS), Pen strip.
Anti- proliferation assay
The trypsinized monolayer cell culture was adjusted to a cell count of 1.0 x 105 cells/ml using relevant media containing 10% Fetal Bovine Serum. To every single well of the 96 well microtiter plate, 100μl of the diluted cell suspension (50,000cells/well) was added. 24hrs later, when a partial monolayer had developed, it was separated and washed with medium after flicking off the supernatant. Then 100μl of various test concentrations of test drugs were supplemented on to the partial monolayer in microtiter plates for incubation at 37oC for 48hrs in 5% CO2 atmosphere. Once the incubation of the plates got over, the test solutions in the wells were tossed out and 100μl of MTT (5 mg/10 ml of MTT in PBS) was added to each well. Then again, the plates were incubated for 4h at 37oC in 5% CO2 atmosphere. Then 100μl of DMSO was added following the supernatant solution removal. The platters were slightly disconcerted to emulsify the formed formazan. The transmission density(absorbance) was computed using a microplate reader at a wavelength of 570 nm. The proportion of development reticence was computed by means of the following method and strength of the test formulation needed to restraint cell growth by 50% (IC50) values is spawned from the dose-response curves for each cell line.
IC50 Value
The ability and the efficiency of a mixture in hindering the biological and physiochemical function was measured as half maximal inhibitory concentration (IC50). This was a quantifiable measure, and it implies the quantity of the compound, or any ingredient (inhibitor) is needed to impede a given biological process (or element of a process, i.e. an enzyme, cell, cell receptor or microorganism) by half.
A dose response curve can be constructed to determine the IC50 of a drug. The reversal of agonistic action of the standard can be examined and hence can determine the antagonistic action of different concentration of the test drug. IC50 estimates can be assessed for a given antagonist by ascertaining the concentration needed to impede part of the highest biologic reaction of the agonist. On the basis of sigmoid dose response curve the IC50 values for cytotoxicity assessments were derived from a nonlinear regression analysis (curve fit).
MTT Assay
After staining with a fundamental dye, the viable cells were counted and hence the anti-proliferative effect of the given test formulations can be determined in vitro. This assay is a process of quantifying viable cells via mitochondrial dehydrogenase. The MTT method is straightforward, precise as well as it generates consistent results. The primary and the most important component
(3-4, 5-dimethylthiazol-2-yl]-2, 5-diphenyl tetrazolium bromide) or MTT, a water decipherable tetrazolium salt upon incubation , was converted to an unsolvable purple formazan by cleavage of the tetrazolium ring by mitochondrial dehydrogenase enzymes of viable cells. The colored solution obtained at the end of the process will be analyzed with the spectrophotometer. The amount of formazan that was formed will keep changing with respect to the increase and decrease in number of cells, demonstrating the intensity of cytotoxicity initiated by the sample material.
Table 1: Effect of various concentrations of Test drug AH on Cell viability of Human ovarian (PA-1) cell line with Methotrexate as the standard comparison drug .
|
S. No |
Concentration µl | Percentage of cell Viability |
| 1 | 10 µl | 95.65 ± 0.7615 |
| 2 | 50 µl | 86.59 ± 2.663 |
| 3 | 100 µl | 69.82 ± 0.6594 |
| 4 | 200 µl | 53.68 ± 0.8755 |
|
5 |
STD (METHOTREXATE 10 µg/ml) |
24.84 ± 3.366 |
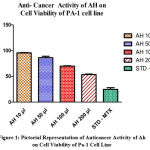 |
Figure 1: Pictorial Representation of Anticancer Activity of Ah on Cell Viability of Pa-1 Cell Line. |
Table 2: Shows the ability of various dilutions of Test drug AH to produce cell death on Human ovarian (PA-1) cell line with Methotrexate as the standard comparison drug .
|
S.No |
Test drug AH
Concentration in µg/ml |
Percentage of cell Death |
| 1 | 10 µl | 4.351 ± 0.76 |
| 2 | 50 µl | 13.41 ± 2.663 |
| 3 | 100 µl | 30.18 ± 0.6594 |
| 4 | 200 µl | 46.32 ± 0.8755 |
| 5 | STD (METHOTREXATE 10 µg/ml) | 75.16 ± 3.366 |
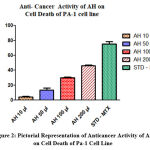 |
Figure 2: Pictorial Representation of Anticancer Activity of Ah on Cell Death of Pa-1 Cell Line. |
Result and Discussion
Varying doses of test drug AH (concentration ranges from 10 to 200 µl) aimed to reveal the cytotoxic action that is anti-cancer activity, In-vitro on the cell viability against Human ovarian (PA-1) cell line was executed. The result gained facts that the increased concentration of the study drug AH decrease the percentage of cell viability of PA-1 celline. Minimum viability of cell was reflected at the concentration of 200µl 53.68 ± 0.8755 %, followed by this at 100 µl and 50 µl shows 69.82 ± 0.6594%, 86.59 ± 2.663, similarly 10 µg/ml shows 95.65 ± 0.7615% cell viability in {MMT:3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide} assay. The resultant IC50 value was discovered to be 245.5 ± 84.78 µg/ml. It was determined from the result of the current study that the preparation AH possesses encouraging anti-cancer activity.
Note
The following series of images represent various degrees of cell death that occur when PA1 ovarian cancer cell line comes in contact with varying concentrations of the test drug Aesculus hippocastanum with Methotrexate as the standard comparison drug .
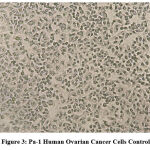 |
Figure 3: Pa-1 Human Ovarian Cancer Cells Control. |
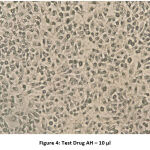 |
Figure 4: Test Drug AH – 10 µl. |
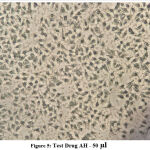 |
Figure 5: Test Drug AH – 50 µl. |
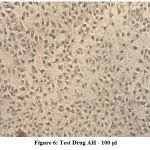 |
Figure 6: Test Drug AH – 100 μl. |
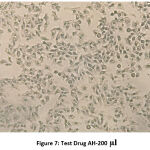 |
Figure 7: Test Drug AH-200 µl. |
 |
Figure 8: Methotrexate( STD Drug) – 10 μg/ml |
Discussion
The main objective of the study as mentioned above, was to establish the cytotoxic activity of ethanolic flower extract of Aesculus hippocastanum against PA-1 human ovarian cancer cell line with Methotrexate as the standard comparison drug. As discussed in the earlier part of the study, Escin is the most vital biochemical component of AH with good anti-inflammatory, antiedematous properties. In the recent times it has been discovered that escin has potential cytotoxic properties which can be used in the treatment of cancer. The major antiproliferative effect of the plant is attributed to the cell cycle arrest 5.
The anticancer property of the plant was demonstrated with numerous cell lines predominantly with breast, hepatic,colorectal cancer cell lines. Apart from the cell cycle arrest mechanism involved, the escin is also found to interfere with molecular signaling pathway which is similar to pathway targeted cancer therapy. A study performed by Rimmon et al on pancreatic cell line showed escin induced cell apoptosis, decreased pancreatic cancer cell viability, interference of NF-κB signaling pathway. It’s synergistic action with chemotherapeutic drugs enhances sensitivity of cancer cells to anticancer drugs 6.
Jan zhu et al performed a similar study on human osteosarcoma cell line displayed escin induced cell death through inhibition of ROS/P38 MAPK signaling pathway7. Another study done on A549 (alveolar cell line in lung)showed Beta escin inhibiting tyrosine kinase thereby knocking down JAK-STAT pathway induced protein synthesis8.
As far as ovarian carcinoma is concerned, a research performed with AH seed extract lead to a milestone finding that the extract is capable of preventing the ovarian cancer metastasis. The biochemical substances beta escin and cardiac glycosides displayed an effective inhibition of ovarian cancer adhesion / invasion into the peritoneal cavity by breaking the HIF 1 α stability of the ovarian cancer cells and suppress the production of extracellular matrix which prohibits malignant dissemination into the body cavities 9,10.
Therefore, from numerous studies performed on the anticancer property of Aesculus hippocastanum and other saponins it is very evident that it is a very potent anticarcinogenic agent. These saponins displayed a very effective cytotoxic nature and carry outgrowth signals inhibitory responses in both invitro and in vivo evaluations 11. The MTT assay we performed proves that the ethanolic flower extract of Aesculus hippocastanum has significant cytotoxic property. But since the phyto extracts are associated with poor bioavailability and shorter half-life it is not found to be as potent as the current allopathic anticancer drugs. Hence to make it an effective anticancer drug, the extracts need to be treated chemically for semisynthetic formulations or to be given in combination with other chemotherapeutic agents for attaining successful therapeutic efficacy 2.
Conclusion
Hence the MTT assay performed with Methotrexate as the standard drug and Ethanolic flower extract of Aesculus hippocastanum as sample, proves the significant anticancer property of Aesculus. The effective cytotoxic effect of flower extract was observed at higher concentrations of the test sample. Further animal studies and clinical trials are required to confirm the therapeutic application of the above test sample.
Acknowledgement
I wish to thank my professors and my seniors who always kindle my eagerness to do research on topics which helps further progression in my career and a best feed for my inquisitive enthusiastic nature.
Conflict of Interest
There is no conflict of Interest.
Funding Sources
There is no funding sources.
References
- Epidemiology and risk factors. International journal of women’s health. 2019;11:287.
CrossRef - Alfonzetti T, Yasmin-Karim S, Ngwa W, Avery S. Phytoradiotherapy: An Integrative Approach to Cancer Treatment by Combining Radiotherapy With Phytomedicines. Frontiers in Oncology. 2021:3333.
CrossRef - Thomas PA, Alhamd O, Iszkuło G, Dering M, Mukassabi TA. Biological Flora of the British Isles: Aesculus hippocastanum. Journal of Ecology. 2019 Mar;107(2):992-1030.
CrossRef - Drăghici LR, Hădărugă DI, Hădărugă NG. Aesculus species: a review on biologically active compounds and their possible applications. Journal of Agroalimentary Processes and Technologies. 2020;26(4):422-8.
- Cheong DH, Arfuso F, Sethi G, Wang L, Hui KM, Kumar AP, Tran T. Molecular targets and anti-cancer potential of escin. Cancer letters. 2018 May 28;422:1-8.
CrossRef - Rimmon A, Vexler A, Berkovich L, Earon G, Ron I, Lev-Ari S. Escin chemosensitizes human pancreatic cancer cells and inhibits the nuclear factor-kappaB signaling pathway. Biochemistry research international. 2013 Jan 1;2013.
CrossRef - Zhu J, Yu W, Liu B, Wang Y, Wang J, Xia K, Liang C, Fang W, Zhou C, Tao H. Escin induces caspase-dependent apoptosis and autophagy through the ROS/p38 MAPK signalling pathway in human osteosarcoma cells in vitro and in vivo. Cell death & disease. 2017 Oct;8(10):e3113-.
CrossRef - Ji DB, Xu B, Liu JT, Ran FX, Cui JR. β‐Escin sodium inhibits inducible nitric oxide synthase expression via downregulation of the JAK/STAT pathway in A549 cells. Molecular carcinogenesis. 2011 Dec;50(12):945-60.
CrossRef - Kenny HA, Hart PC, Kordylewicz K, Lal M, Shen M, Kara B, Chen YJ, Grassl N, Alharbi Y, Pattnaik BR, Watters KM. The Natural Product β-Escin Targets Cancer and Stromal Cells of the Tumor Microenvironment to Inhibit Ovarian Cancer Metastasis. Cancers. 2021 Jan;13(16):3931.
CrossRef - Beaufort, Corine M et al. “Ovarian cancer cell line panel (OCCP): clinical importance of in vitro morphological subtypes.” PloS one vol. 9,9 e103988. 17 Sep. 2014, doi:10.1371/journal.pone.0103988.
CrossRef - Khan AA, Naqvi TS, Naqvi MS. Identification of phytosaponins as novel biodynamic agents: an updated overview. Asian J Exp Biol Sci. 2012;3(3):459-67.