Mohamed G Hamed1 , Abdelmonem Awad Hegazy2*
, Abdelmonem Awad Hegazy2* , Ahmed Embaby1
, Ahmed Embaby1 , Shimaa Abdelmoneem1
, Shimaa Abdelmoneem1 , Amany Abd Al Badea3
, Amany Abd Al Badea3 , Ali Awad3
, Ali Awad3 , Mohammad Walaa4
, Mohammad Walaa4 , Mai Ahmed Gobran5
, Mai Ahmed Gobran5 , Omnia Awwad6
, Omnia Awwad6 , Doaa AbdElmonem7
, Doaa AbdElmonem7 , Nahla A Zaitoun8
, Nahla A Zaitoun8 , Mona Ahmed Abdelmaksoud9
, Mona Ahmed Abdelmaksoud9 , Alhoussein Alsayed AbdelAal1
, Alhoussein Alsayed AbdelAal1
1Internal Medicine Department, Faculty of Medicine, Zagazig University, Egypt,
2Medical Lab Department, Faculty of Allied Medical Sciences, Zarqa University, Jordan,
3Otorhinolaryngology Department, Faculty of medicine, Zagazig University, Egypt,
4Chest Medicine Department. Faculty of Medicine Zagazig University, Egypt,
5Pathology Department, Faculty of Medicine, Zagazig University, Egypt,
6Family Medicine Fellowship in the Egyptian MOHP, Egypt,
7Clinical Pathology Department, Faculty of Medicine, Zagazig University, Egypt,
8Family Medicine Department, Faculty of Medicine, Zagazig University, Egypt,
9Tropical Medicine Department, Faculty of Medicine, Zagazig University, Egypt
Corresponding Author E-mail: dr.abdelmonemhegazy@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2483
Abstract
Background: Coronavirus disease 2019 (COVID-19) may lead to immunosuppression, leaving patients vulnerable to secondary invasive fungal infection like mucormycosis. The present study aimed to determine whether there are any risk factors associated with mortality in mucormycosis among COVID-19 patients. Patients and Methods: Patients with COVID-19 diagnosed with mucormycosis who received treatment at University Hospitals were included in the study. Complete blood count (CBC), glycated hemoglobin (HBA1c), C-reactive protein (CRP), serum albumin level, creatinine, ferritin levels, lactate dehydrogenase (LDH), D-dimer and histopathological observations were performed for all participants’ specimens. Results: The number (N) of patients included in the study was 46. About 85 % (39/46) of patients had post-COVID-19 syndrome and the other 7 cases were in the active phase of the disease. CRP, serum ferritin, D-dimer, CRP/albumin ratio and CRP/absolute lymphocyte counts were statistically significant (P<0.05) within non-survivors as compared to survivors. After analysis of multivariate analysis that patients had oxygen support, while elevated CRP/albumin ratios were independent predictors of mortality in COVID-19 patients associated with mucormycosis. Conclusions: Mucormycosis can be caused by immunosuppression conditions associated with COVID-19 infection. Oxygen levels and C-reactive protein/albumin are independent predictors of mortality and morbidity in post COVID-19 patients.
Keywords
Mucormycosis; Outcome predictors; Prognostic markers; SARS-CoV-2
Download this article as:| Copy the following to cite this article: Hamed M. G, Hegazy A. A, Embaby A, Abdelmoneem S, Al-Badea A. A, Awad A, Walaa M, Gobran M. A, Awwad O, AbdElmonem D, Zaitoun N. A, Abdelmaksoud M. A, AbdelAal A. A. Identifying Independent Predictors of Mortality in COVID-19 Patients with Mucormycosis. Biomed Pharmacol J 2022;15(3). |
| Copy the following to cite this URL: Hamed M. G, Hegazy A. A, Embaby A, Abdelmoneem S, Al-Badea A. A, Awad A, Walaa M, Gobran M. A, Awwad O, AbdElmonem D, Zaitoun N. A, Abdelmaksoud M. A, AbdelAal A. A. Identifying Independent Predictors of Mortality in COVID-19 Patients with Mucormycosis. Biomed Pharmacol J 2022;15(3). Available from: https://bit.ly/3SaOFM9 |
Introduction
The new form of the coronavirus called SARS-CoV-2 has been accused of causing COVID-19, which the World Health Organization declared a global pandemic in March 2020 1,2. While the majority of COVID-19 patients develop a mild to moderate respiratory illness who recover without requiring specific medications, the severe variant of COVID-19 is likely to have serious impacts both in the elderly as well as in people with comorbidities 3. In these individuals, the infection proceeds rapidly, causing respiratory impairment and the possibility of acute respiratory distress syndrome (ARDS) 4.
COVID-19 patients with ARDS who require mechanical ventilation and are treated with high doses of corticosteroids, immunomodulators, interleukin antagonists, and broad-spectrum antibiotics are at a greater risk to developing fungal infections i.e., aspergillosis, mucormycosis, mucosal candidiasis, candidemia, and pneumocystis jiroveci pneumonia (PJP) 5.
Mucormycosis is an acute angioinvasive illness caused by Mucor including Rhizopus, Absidia, Rhizomucor, and Cunninghamella fungi that belonging the Mucorales order 6,7. Mucor is a saprophytic fungus that may be found in soil decomposing organic debris (7). The airborne hyphae are inhaled and then settle in the upper or lower respiratory tract. However, mucormycosis is more common amongst people with impaired immune system 8.
This study aimed to predict mucormycosis associated with COVID-19 infection in hospitalized patients.
Patients and Methods
In this retrospective cohort study, COVID-19 patients diagnosed with mucormycosis were included. The study was including both active and recovered COVID-19 patients from the period of May 2021 to December 2021. All patients were ≥ 18 years and received treatment in the Departments of Intensive Care Unit (ICU) of Internal Medicine and Otorhinolaryngology and Isolation at Zagazig University Hospitals, Egypt.
Eligible COVID-19 patients who were tested positive for mucormycosis underwent a full examination including previous medical history and chronic diseases such as diabetes mellitus (DM), hypertension (HTN) and chronic obstructive pulmonary disease (COPD), renal insufficiency, chronic liver disease and malignancy in addition to clinical examination along with blood pressure and oxygen saturation. The following laboratory tests were performed: CBC, HbA1c, CRP, serum creatinine, serum albumin level, serum ferritin, LDH and de-dimer.
The diagnosis of mucormycosis was based on Magnetic Resonance Imaging (MRI); T1 MRI with post gadolinium enhancement to evaluate inferior turbinate and T2 with fat suppression to evaluate orbits, intracranial extension and pterygopalatine fossa; preoperative endoscopy for evaluation of the vascularity of the nose and paranasal sinuses and histopathological illustration of fungal spores and non-septate hyphae surrounded by areas of necrosis and aggregates of inflammatory cells. Infection sites were classified as rhinosinusitis, nasopalatine, naso-orbital, rhinocerebral and rhino-orbito-cerebral mucormycosis. Histopathological specimens were stained with hematoxylin and eosin (H&E) and Periodic acid-Schiff (PAS) to detect fungal spores and non-septate hyphae surrounded by areas of necrosis and aggregates of inflammatory cells. The degree of necrosis, inflammation (as percentage of total area of tissue sampled and graded as mild and moderate to severe) and vascular invasion (present/absent) were assessed 9.
Treatment Regimen
Medical treatment
All patients received amphotericin derivatives; liposomal form (AmBisome) at a dose of 5 mg/kg or B-deoxycholate at a dose of 1:1.5mg/kg (10,11). This was concurrent with other important considerations in medical management including strict blood glucose control in patients with diabetes and weaning off glucocorticoids.
Surgical intervention
Surgical debridement was performed under general anesthesia after good topical nasal preparation for 80.4% patients (N=37). Endoscopic debridement was performed for necrotic tissues of the nose and paranasal sinuses including inferior turbinate, medial maxillectomy and pterygopalatine fossa. Gel foam pledgets impregnated with amphotericin B solution were then applied to the nasal cavity after completion of the procedure and achievement of haemostasias. The patients (N=33) had orbital affection so they subjected to endoscopic orbital decompression; and of these, patients (N=5) underwent orbital exenteration. The patients (N=8) were also developed palatal affection and underwent resection of the palate. Patients were clinically evaluated after surgery and endoscopy.
Outcome Assessment
Clinical progression of all patients with any sign or symptom, severity of previous symptoms, and exacerbation of symptoms, needed mechanical ventilation and development of shock, sepsis or disseminated intravascular coagulation (DIC) were assessed. Clinical improvement was demonstrated through resolution as well as a general regression in severity of previously reported signs or symptoms. Global response to the main efficacy endpoint was rated as success when the patient was alive and improving according to clinical and radiographic assessment or failing if the patient was dead or progressing according to clinical and radiographic assessment.
Statistical analysis
The collected data were analyzed using the SPSS (Statistical Package for Social Science) version 20 and NCSS 12, LLC, United States. The Shapiro Walk test was used to determine data distribution. Frequencies and relative percentages were used to describe qualitative data. The difference between qualitative variables was calculated using the Chi square test (χ2) and Fisher exact. The median and range were used to express quantitative data. For non-normally distributed data, the Mann Whitney test was employed to quantify the difference between quantitative variables in two groups. The receiver operating characteristic (ROC) curve was created to allow for the selection of test result threshold values as well as the comparison of various testing methods. Univariate and multivariate logistic regression analysis models were done. All statistical comparisons were two-tailed (software version), with a P: ≤ 0.05 reflecting a significant difference 12.
Ethical approval
Official permission was obtained from the Institutional Review Board (IRB) of the Zagazig Faculty of Medicine (Letter N: ZU-IRB #7055/25-7-2021); and a written informed consent was obtained from all enrolled patients after describing the aim of the study and ensuring privacy and confidentiality. The research was carried out in accordance to the World Medical Association’s Code of Ethics for Human Studies (Declaration of Helsinki).
Results
Patients (N=46) were included in the study with mean age of 60 years (range: 45-73); and 19 patients (41.3%) were male. Past medical history showed that 27 cases (58.7%) were known with DM, 30.4% recently discovered DM, one case (2.2%) with COPD, 5 (10.9%) with cerebrovascular disease, 3 cases (6.5%) with renal insufficiency and two cases (4.3%) with chronic hepatic disease. No patient had a past history of mucormycosis prior to infection with SARS-CoV-2. Meanwhile, 39 cases of the total 46 of patients (84.8%) had post-COVID-19 syndrome; and the other 7 patients were in the active phase of the disease. Not all of the seven patients in the active phase survived (Table 1).
Comparison between survivors and non-survivors with respect to clinical features as well as pre-existing conditions for patients revealed that several clinical manifestations (Figure 1) including the following: Disturbed consciousness, nasal congestion, black lesions on nose or mouth, dyspnea and cutaneous ulcers or blisters appeared in higher percentage in non-survivors with statistically significant differences (P: 0.001, 0.023, 0.013, <0.001, and 0.005, respectively). Renal impairment was also significantly higher in non-survivors (P: 0.033). Patients who were shocked upon admission to hospital and who required oxygen support were more likely to die (P: 0.033 and 0.001, respectively). Regarding the patients’ COVID-19 status at the time of mucormycosis diagnosis, cases with active COVID-19 infection were more likely to have a poor outcome compared to those who recovered (Table 1).
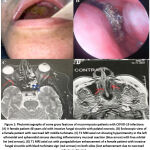 |
Figure 1: Photomicrographs of some gross features of mucormycosis-patients with COVID-19 infections: |
Table 1: Comparison of clinical characteristics between patients with invasive mucormycosis according to their clinical outcome after diagnosis.
| Outcome | Total N=46 |
P-value | ||||||
| Survivors N=27 |
Non-Survivors N=19 |
|||||||
| N | % | N | % | N | % | |||
| Age, years | 56 (45-71) | 62 (54-73) | 60 (45-73) |
0.072 |
||||
| Sex | Male | 14 | 51.9% | 5 | 26.3% | 19 | 41.3% | 0.083 |
| Female | 13 | 48.1% | 14 | 73.7% | 27 | 58.7% | ||
| Known Diabetic | 17 | 63.0% | 10 | 52.6% | 27 | 58.7% | 0.483 | |
| Recently discovered | 6 | 22.2% | 8 | 42.1% | 14 | 30.4% | 0.149 | |
| DM | Known Diabetic | 17 | 63.0% | 10 | 52.6% | 27 | 58.7% | 0.274 |
| Recently discovered | 6 | 22.2% | 8 | 42.1% | 14 | 30.4% | ||
| No DM | 4 | 14.8% | 1 | 5.3% | 5 | 10.9% | ||
| COPD | 0 | 0.0% | 1 | 5.3% | 1 | 2.2% | 0.228 | |
| Cerebrovascular disease | 2 | 7.4% | 3 | 15.8% | 5 | 10.9% | 0.368 | |
| Renal insufficiency | 0 | 0.0% | 3 | 15.8% | 3 | 6.5% | 0.033 | |
| Chronic hepatic disease | 1 | 3.7% | 1 | 5.3% | 2 | 4.3% | 0.798 | |
| COVID | Active | 0 | 0.0% | 7 | 36.8% | 7 | 15.2% | 0.001 |
| Recovered | 27 | 100.0% | 12 | 63.2% | 39 | 84.8% | ||
| Fever | 0 | 0.0% | 2 | 10.5% | 2 | 4.3% | 0.085 | |
| Headache | 21 | 77.8% | 17 | 89.5% | 38 | 82.6% | 0.303 | |
| Confusion or coma | 0 | 0.0% | 7 | 36.8% | 7 | 15.2% | 0.001 | |
| Unilateral facial swelling | 19 | 70.4% | 16 | 84.2% | 35 | 76.1% | 0.279 | |
| Nasal or sinus congestion | 18 | 66.7% | 18 | 94.7% | 36 | 78.3% | 0.023 | |
| Black lesion on nose or mouth | 0 | 0.0% | 4 | 21.1% | 4 | 8.7% | 0.013 | |
| Cough | 0 | 0.0% | 2 | 10.5% | 2 | 4.3% | 0.085 | |
| Dyspnea | 1 | 3.7% | 9 | 47.4% | 10 | 21.7% | <0.001 | |
| Chest pain | 0 | 0.0% | 1 | 5.3% | 1 | 2.2% | 0.228 | |
| GI symptoms | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | ||
| Cutaneous ulcers or blisters | 0 | 0.0% | 5 | 26.3% | 5 | 10.9% | 0.005 | |
| Shock at hospital admission | 0 | 0.0% | 3 | 15.8% | 3 | 6.5% | 0.033 | |
| O2 Sat% RA
|
Not Needed | 26 | 96.3% | 9 | 47.4% | 35 | 76.1% | 0.001
|
| Nasal Canula/Mask | 1 | 3.7% | 4 | 21.1% | 5 | 10.9% | ||
| biBAP/MV | 0 | 0.0% | 6 | 31.6% | 6 | 13.0% | ||
| Use Of Steroid | 27 | 100.0% | 18 | 94.7% | 45 | 97.8% | 0.228 | |
| Use Of Actemra (Tocilizumab) | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | ||
| Use Of Ivermectin | 27 | 100.0% | 18 | 94.7% | 45 | 97.8% | 0.228 | |
Quantitative data were expressed as Median (range) and compared using Mann Whitney test, while qualitative data were expressed as numbers and percentages and compared using Chi-square X2 test.
The most common site of infection was the rhino-orbital form followed by the nasal and then the orbital types with no significant difference between the groups. On the other hand, a significant difference between survivors and non-survivors regarding treatment received for mucormycosis with the best outcome was found in patients who underwent surgical debridement in addition to treatment with amphotericin B rather than receiving amphotericin B alone (P: 0.001). The most commonly recognized organism was mucor species. Moreover, necrosis, inflammation and vascular invasion were significantly more abundant in non-survivors than in survivors with P value of 0.001, <0.001 and <0.001, respectively (Table 2; Figures 2,3).
Table 2: Comparison of invasive mucormycosis data according to patient outcome after diagnosis.
| Outcome | Total N=46 |
P-value | ||||||
| Survivors N=27 |
Non-Survivors N=19 |
|||||||
| N | % | N | % | N | % | |||
| Location of Mucor mycosis | Nasal/sinus | 7 | 25.9% | 2 | 10.5% | 9 | 19.6% |
0.155 |
| Nasal/sinus, Orbit | 9 | 33.3% | 8 | 42.1% | 17 | 37.0% | ||
| Nasal/sinus, Orbit, Cerebral | 0 | 0.0% | 1 | 5.3% | 1 | 2.2% | ||
| Nasal/sinus, Orbit, Cerebral, Cutaneous | 0 | 0.0% | 4 | 21.1% | 4 | 8.7% | ||
| Nasal/sinus, Orbit, Plate | 1 | 3.7% | 0 | 0.0% | 1 | 2.2% | ||
| Nasal/sinus, Orbit, Cutaneous | 0 | 0.0% | 1 | 5.3% | 1 | 2.2% | ||
| Nasal/sinus, Plate | 2 | 7.4% | 1 | 5.3% | 3 | 6.5% | ||
| Orbit | 5 | 18.5% | 1 | 5.3% | 6 | 13.0% | ||
| Orbit, Plate | 2 | 7.4% | 1 | 5.3% | 3 | 6.5% | ||
| Plate | 1 | 3.7% | 0 | 0.0% | 1 | 2.2% | ||
| Treatment received for mucormycosis | Amphotericin B | 1 | 3.7% | 8 | 42.1% | 9 | 19.6% | 0.001
|
| Surgical debridement with Amphotericin B | 26 | 96.3% | 11 | 57.9% | 37 | 80.4% | ||
| Isolated
organism |
N/A | 13 | 48.1% | 15 | 78.9% | 28 | 60.9% | 0.081 |
| Mucormycosis | 7 | 25.9% | 2 | 10.5% | 9 | 19.6% | ||
| Rhizopus | 2 | 7.4% | 2 | 10.5% | 4 | 8.7% | ||
| Others | 5 | 18.5% | 0 | 0.0% | 5 | 10.9% | ||
| Necrosis
|
Mild
Moderate Severe |
20
1 6 |
74.1%
3.7% 22.2% |
4
5 10 |
21.1%
26.3% 52.6% |
24
6 16 |
52.2%
13% 34.8% |
0.001 |
| Inflammatory cells
|
Mild
Moderate Severe |
20
2 5 |
74.1%
7.40% 18.50% |
1
8 10 |
5.3%
42.1%| 52.6% |
21
10 15 |
45.7%
21.7% 32.6% |
< 0.001 |
| Angioinvasion
|
Present
Absent |
4
23 |
14.8%
85.2% |
12
7 |
63.2%
36.8% |
16
30 |
34.78%
65.22% |
< 0.001 |
Qualitative data were expressed as numbers and percentages and compared using Chi-square X2 test.
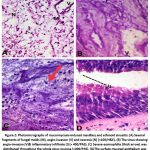 |
Figure 2: Photomicrographs of mucormycosis-induced maxillary and ethmoid sinusitis: |
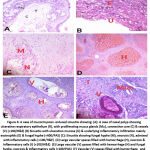 |
Figure 3: A case of mucormycosis -induced sinusitis showing: |
With respect to laboratory investigations, the ratios of CRP, serum ferritin, D-dimer, CRP-to-albumin (CRP/Alb) and CRP to absolute lymphocyte count (CRP/ALC) were significantly higher in non-survivors than in survivors with P: value: 0.012, 0.013, 0.012, 0.015 and 0.007, respectively (Table 3; Figures 4,5).
Table 3: Comparison of baseline laboratory values between patients with invasive mucormycosis according to their clinical outcome after diagnosis.
|
|
Outcome | Total N=46 |
P-value | |
| Survivors N=27 |
Non-Survivors N=19 |
|||
| Median (Range) | Median (Range) | Median (Range) | ||
| WBCs count | 12.0 (4.4-30.0) | 16.6 (3.2-28.7) | 13.0 (3.2-30) | 0.237 |
| Lymphocytes | 1.7 (0.2-3.4) | 1.6 (0.1-3.0) | 1.6 (0.1-3.4) | 0.377 |
| Hemoglobin | 10.9 (8.0-15.4) | 11.2 (6.6-14.4) | 11.2 (6.6-15.4) | 0.489 |
| Platelet count | 250 (108-430) | 218 (57-646) | 243 (57-646) | 0.409 |
| Creatinine | 1.20 (0.30-3.20) | 1.10 (0.50-5.70) | 1.15 (0.30-5.7) | 0.592 |
| Albumin | 3.10 (2.10-4.00) | 3.03 (2.08-3.80) | 3.07 (2.08-4) | 0.695 |
| CRP | 118 (5-294) | 181 (38-438) | 138 (5-438) | 0.012 |
| Ferritin | 600 (380-1107) | 786 (27-1637) | 655 (27-1637) | 0.013 |
| D-dimer | 0.6 (0.5-0.6) | 0.8 (0.7-1.5) | 0.8 (0.5-1.5) | 0.012 |
| CK | 39.05 (6-124) | 85.75 (9.5-162) | 39.05 (6-162) | 0.667 |
| ESR | 58 (22-105) | 88 (29-127) | 70 (22-127) | 0.055 |
| PLR | 147.7 (53.3-540) | 167.5 (69.5-2010) | 156.8 (53.3-2010) | 0.455 |
| CRP/Alb | 38.60 (1.50-110.4) | 71.6 (10.9-166.3) | 43.85 (1.5-166.3) | 0.015 |
| CRP/ALC | 72 (1.9-1115) | 176.7 (12.7-930) | 94.55 (1.9-1115) | 0.007 |
| HbA1C | 10 (7-13) | 9 (7-14) | 10 (7-14) | 0.961 |
Quantitative data were expressed as Median (range) and compared using Mann Whitney test
 |
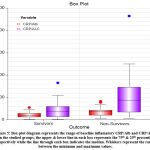
Figure 4: Box-plot diagram represents the range of baseline inflamatory CRP and ferritin in the studied groups; the upper & lower line in each box represents the 75th & 25th percentile respectively. |
ROC curve analysis revealed that CRP/ALC and CRP yielded the best accuracy for the prediction of mortality (cutoff >129.1 had an AUC of 0.735, cutoff >139 had an AUC of 0.72 respectively) with a sensitivity of 63.16, 73.68 respectively and a specificity of (85.19, 70.37 respectively), P: 0.002, 0.005, respectively. On the other hand, serum ferritin, CRP/Alb, ALC and PLR had lower AUC (0.717, 0.712, 0.577 and 0.565, respectively (Table 4; Figure 6).
Table 4: The validity of baseline markers with area under the ROC curve (AUC) as a marker for poor outcome in patients with invasive mucormycosis.
| Marker | Criterion | Sensitivity | Specificity | PPV | NPV | AUC | P Value |
| 95% CI | 95% CI | 95% CI | 95% CI | 95% CI | |||
| Ferritin | >778 | 68.42 | 92.59 | 86.7 | 80.6 | 0.717 | 0.021 |
| 43.4 – 87.4 | 75.7 – 99.1 | 62.3 – 96.2 | 68.1 – 89.1 | 0.565 to 0.840 | |||
| CRP | >139 | 73.68 | 70.37 | 63.6 | 79.2 | 0.72 | 0.005 |
| 48.8 – 90.9 | 49.8 – 86.2 | 48.0 – 76.9 | 63.3 – 89.3 | 0.568 to 0.842 | |||
| ALC | ≤2.2 | 84.21 | 33.33 | 47.1 | 75 | 0.577 | 0.37 |
| 60.4 – 96.6 | 16.5 – 54.0 | 39.0 – 55.3 | 48.3 – 90.6 | 0.423 to 0.721 | |||
| CRP/Alb | >44.2 | 73.68 | 70.37 | 63.6 | 79.2 | 0.712 | 0.008 |
| 48.8 – 90.9 | 49.8 – 86.2 | 48.0 – 76.9 | 63.3 – 89.3 | 0.559 to 0.835 | |||
| CRP/ALC | >129.1 | 63.16 | 85.19 | 75 | 76.7 | 0.735 | 0.002 |
| 38.4 – 83.7 | 66.3 – 95.8 | 53.3 – 88.8 | 64.1 – 85.8 | 0.584 to 0.854 | |||
| PLR | >156.7 | 63.16 | 59.26 | 52.2 | 69.6 | 0.565 | 0.465 |
| 38.4 – 83.7 | 38.8 – 77.6 | 38.2 – 65.9 | 54.0 – 81.7 | 0.411 to 0.711 |
The 95%CI: 95% confidence interval, Positive predictive value (PPV) and negative predictive value (NPV), Area under the ROC curve (AUC).
 |
Figure 6: The validity of baseline markers with area under the ROC curve (AUC) as a marker for poor outcome (mortality) in patients with invasive mucormycosis. |
The univariate analysis of predictors for outcome in COVID-19 patients infected with mucormycosis showed that the significant predictors were the need for oxygen support, treatment received for mucormycosis and CRP/Alb while age, sex and serum ferritin were marginally statistically insignificant. In multivariate regression analysis, the need for oxygen support along with CRP/Alb was spotted as independent risk factor for poor outcome in our patients (Table 5).
Table 5: Univariate and multivariate logistic regression of potential predictors of poor outcome (mortality) in patients with invasive mucormycosis.
|
Covariates |
Univariate | Multivariate | ||
| Sig. | RR (95% CI) | Sig. | RR (95% CI) | |
| O2 sat% indicating support | 0.003 | 5.37 (1.89-16.07) | 0.046 | 8.1 (2.25-29.12) |
| CRP/Alb | 0.019 | 1.02 (1.00-1.05) | 0.045 | 1.1 (1.11-1.23) |
| Treatment received for mucormycosis | 0.009 | 0.23 (0.08-0.69) | 0.755 | |
| Age | 0.057 | 1.10 (1.00-1.20) | 0.090 | |
| Sex | 0.088 | 0.33 (0.09-1.18) | 0.072 | |
| Ferritin | 0.074 | 1.00 (1.00-1.00) | 0.381 | |
| Recently discovered DM | 0.154 | |||
| Known Diabetic | 0.484 | |||
| COPD | >0.999 | |||
| Cerebrovascular disease | 0.379 | |||
| Renal insufficiency | 0.999 | |||
| Chronic hepatic disease | 0.799 | |||
| COVID- | 0.999 | |||
| Fever | 0.999 | |||
| Shock at admission | 0.999 | |||
| Use of steroid | >0.999 | |||
| Use of ivermectin | >0.999 | |||
| Location of mucormycosis | 0.95 | |||
| Histopathology | 0.647 | |||
| WBCs count | 0.218 | |||
| Lymphocytes | 0.213 | |||
| Hemoglobin | 0.432 | |||
| Platelet count | 0.97 | |||
| Creatinine | 0.407 | |||
| Albumin | 0.831 | |||
| PLR | 0.352 | |||
| CRP/ALC | 0.165 | |||
All variable with P-value <0.1 in univariate analysis were entered in multivariate regression model O2 sat% indicating support and CRP/Alb were found to be independent risk factor for poor outcome in patients with invasive mucormycosis.
Discussion
Mucormycosis, initially identified by Paltauf in 1885, is a rare and fatal fungal infection that severely affects people with weakened immune systems 13. Despite its low incidence ranging from 0.005 to 1.7 per million people, many cases have been reported recently during the coronavirus pandemic 14. COVID-19 infection causes severe lymphocytopenia in 85 percent of patients; since lymphocytes play an essential role in immune homeostasis, this leaves patients vulnerable to opportunistic co-infections, such as fungal infections (15). Identification of factors associated with survival in patients with mucormycosis is essential in clinical practice and development of protocols for the upcoming management of patients with such diseases 16.
84.8 percent (39/46) of patients in this study had post-COVID-19 syndrome. Similarly, Mishra et al 17 evaluated COVID-19 associated mucormycosis (CAM) in a tertiary medical institution in India and found that 65.6 percent of CAM patients had post-COVID-19 syndrome after they completely recovered from COVID-19 in terms of clinical assessment. The rhino-orbital variant of mucormycosis was the most prevalent in this study. This could be attributed to getting mucormycosis through contact with fungal spores in the environment e.g., inhalation of the spore from air. Furthermore, it was noted during surgical intervention that pterygopalatine fossa containing orbital vascular supply was the focus of infection. The rhino-orbital-cerebral type is the most prevalent in India, followed by the pulmonary and cutaneous variants 18. However, in developed nations, the pulmonary type is the most common presentation 19.
As Egypt ranked the tenth globally in the number of type 2 diabetic patients by the International Diabetes Federation (IDF) in 2021, and suspected to be the ninth by 2045 20, it has been shown to be the most common co-morbidity in CAM, appearing in 89.1% in this study. The infection rate was 73.5% of cases in India 21 and 17% in Western nations 19; also, in other literature review it was the most common risk factor for infection of mucormycosis 22. In the study by Mishra et al 17, it was shown that 28 out of 32 patients (87.5%) who were infected with CAM had diabetes with poor glycemic control, as measured by a mean HbA1c of 9.06 percent upon admission. However, HbA1c was not as a dependent factor for estimation of glycemic control in this study, as most of patients were anemic and HbA1c was not documented in all of them.
COVID-19 is usually linked with lymphopenia, which may be related to its severity 23. In this study, 19.6% of the patients developed lymphopenia; also, Roushdy and Hamid 24 reported four cases with mucormycosis post-COVID-19 with relative lymphopenia. However, the relationship between lymphopenia and CAM needed further investigation.
Non-survivors had considerably higher serum ferritin levels than survivors. This is similar with results of Spellberg et al., 16 who reported that greater serum ferritin levels were accompanied with significantly higher death rates. Kell et al., 25 added that ferritin arises from damaged cells and can be raised significantly in response to inflammation and or variety of disease which may explain correlation of mortality to high serum ferritin level in this study. The mortality rate for patients with mucormycosis varies according to the site of infection, the cause, and the age at diagnosis of the disease 26. The authors added that overall mortality rate is higher in malignancies and at old ages. It ranges from 35% in cases with no underlying pathology to 66% in diabetic patients and 66% in malignant tumors. The overall mortality in the current study was 19 out of 46 cases (41.3%).
Regarding histopathology results, Goel et al., 9 and Luo et al., 27 agreed with the results that angioinvasion was higher in immunocompromised patient. However, Castillo et al., 28 disagreed with this and stated that patients presented with florid inflammation had a better prognosis.
Previous studies showed that rhino-orbital cases had a 24% death rate and rhino-cerebral cases had a 62% mortality rate 29. The mortality rate in pulmonary mucormycosis was reported to be 80% by Tedder et al 30 and to be 29% by Lin et al 31.
In our study, patients undergoing combined medical-surgical therapy had a better prognosis when compared to those received medical therapy alone. Piromchai and Thanaviratananich emphasized the necessity of surgical debridement. They added that the appropriate time for treatment to achieve better results in these patients is within two weeks of the onset of symptoms 32. In comparison to AmBisome monotherapy, Prakash and Chakrabarti 33, who studied the epidemiology of mucormycosis in India, also found that patients treated with a combination of AmBisome with surgical debridement of diseased tissue had a lower death rate. These results are consistent with worldwide statistics 34. Muthu et al., 35 found that combination medical-surgical treatment was associated with a substantially decreased risk of death in a meta-analysis of 79 trials that included 1544 participants with pulmonary and disseminated mucormycosis.
Lin et al 31 observed no statistically significant association between mortality and age, sex, DM, CKD, or WBCs of pulmonary mucormycosis. This discovery is in line with the findings of this study. Spellberg et al., 16 also reported that age and DM were not associated with mortality; however, neutropenia at enrollment was associated with increased mortality and DM was not associated with a greater mortality risk, which supports these previous findings that DM is not a predictor of outcome in mucormycosis.
In this study, multivariate analysis revealed that only the requirement for O2 support and CRP/Alb were the two independent predictors of death in CAM; however, Lin et al., 31 found no link between mortality and the need for mechanical ventilation in 35 patients with pulmonary mucormycosis. This discrepancy may be attributed to the difference in sample size, timing of diagnosis, risk factors and site of mucormycosis. CRP/Alb has been used as an indicator of prognosis in various diseases. A study conducted by Karakoyun et al., 36 CRP/Alb was a useful prognostic marker of hospital stay and mortality in hospitalized patients infected with COVID-19. Also, CRP/Alb was an independent risk factor for 30-day mortality rate in patients with COVID-19 in a study conducted by El-Shabrawy et al 37.
Conclusions
Present study showed that need of oxygen and CRP/Albumin ratios were independent prognostic factors of survival in COVID-19 patients infected with mucormycosis. Therefore, it is convenient to use these parameters, i.e., oxygen and CRP/Albumin ratio in clinical practice to predict the outcome of patients.
Acknowledgment
We would like to thank Dr. Ahmed Refat, AG Professor of Community Medicine, Faculty of Medicine, Zagazig University for his assistance in conducting the statistical analysis of the study data. We would also like to thank the patients who participated in the research and the clinical staff in the Zagazig Mucor Care Unit for their contribution to the care of our patients.
Contributions
Mohamed G Hamed, Ahmed Embaby, Shimaa Abdelmoneem, Alhoussein Alsayed AbdelAal, Amany Abd Al Badea, Mohammad Walaa, Ali Awad & Mai Ahmed Gobran: Conceptualization, methodology, statistical analysis, interpreting the results, designed the figures, investigation, performed patients’ clinical assessment and follow-up as well as drafted this manuscript with help from Mohamad Walaa, Omnia Awwad, Doaa AbdElmonem & Abdelmonem A Hegazy. Mohamed G Hamed, Ahmed Embaby, Shimaa Abdelmoneem & Abdelmonem A Hegazy: Writing, Review & Editing, Nahla A Zaitoun, Mona Ahmed Abdelmaksoud & Alhoussein Alsayed AbdelAal were involved in planning, organization, supervision and reviewing of the work, and the final manuscript. Mai Ahmed Gobran, & Doaa AbdElmonem Data Curation, Measurements, Lab. and histopathologic analysis like CBC, blood smears, biopsy. All authors have reviewed and approved the manuscript.
Funding Sources
There is no funding source.
Conflict of Interest
The authors declare that they have no competing interests.
References
- Hegazy AA, Hegazy RA. COVID-19: Virology, pathogenesis and potential therapeutics. Afro-Egypt J Infect Endem Dis 2020;10(2):93-9. doi: 10.21608/AEJI.2020.93432.
CrossRef - Khan A, Soni A, Kumar A. Mucormycosis: Current Update in Pandemic COVID-19. Acta Sci Microbiol 2021;4.8 (2021): 01-2.
CrossRef - Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708-20. doi: 10.1056/NEJMoa2002032.
CrossRef - Ahmadikia K, Hashemi SJ, Khodavaisy S, Getso MI, Alijani N, Badali H, Mirhendi H, Salehi M, Tabari A, Mohammadi Ardehali M, Kord M, Roilides E, Rezaie S. The double-edged sword of systemic corticosteroid therapy in viral pneumonia: A case report and comparative review of influenza-associated mucormycosis versus COVID-19 associated mucormycosis. Mycoses. 2021;64(8):798-808. doi: 10.1111/myc.13256.
CrossRef - Pakdel F, Ahmadikia K, Salehi M, Tabari A, Jafari R, Mehrparvar G, Rezaie Y, Rajaeih S, Alijani N, Barac A, Abdollahi A, Khodavaisy S. Mucormycosis in patients with COVID-19: A cross-sectional descriptive multicentre study from Iran. Mycoses. 2021;64(10):1238-52. doi: 10.1111/myc.13334.
CrossRef - Skiada A, Pavleas I, Drogari-Apiranthitou M. Epidemiology and Diagnosis of Mucormycosis: An Update. J Fungi (Basel). 2020;6(4):265. doi: 10.3390/jof6040265.
CrossRef - Vasudevan B, Hazra N, Shijith KP, Neema S, Vendhan S. Mucormycosis: The Scathing Invader. Indian J Dermatol. 2021;66(4):393-400. doi: 10.4103/ijd.ijd_477_21.
- Bhogireddy R, Krishnamurthy V, Jabaris S SL, Pullaiah CP, Manohar S. Is Mucormycosis an inevitable complication of Covid-19 in India? Braz J Infect Dis. 2021;25(3):101597. doi: 10.1016/j.bjid.2021.101597.
CrossRef - Goel A, Kini U, Shetty S. Role of histopathology as an aid to prognosis in rhino-orbito-cerebral zygomycosis. Indian J Pathol Microbiol. 2010;53(2):253-7. doi: 10.4103/0377-4929.64342.
CrossRef - Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen SCA, Dannaoui E, Hochhegger B, Hoenigl M, Jensen HE, Lagrou K, Lewis RE, Mellinghoff SC, Mer M, Pana ZD, Seidel D, Sheppard DC, Wahba R, Akova M, Alanio A, Al-Hatmi AMS, Arikan-Akdagli S, Badali H, Ben-Ami R, Bonifaz A, Bretagne S, Castagnola E, Chayakulkeeree M, Colombo AL, Corzo-León DE, Drgona L, Groll AH, Guinea J, Heussel CP, Ibrahim AS, Kanj SS, Klimko N, Lackner M, Lamoth F, Lanternier F, Lass-Floerl C, Lee DG, Lehrnbecher T, Lmimouni BE, Mares M, Maschmeyer G, Meis JF, Meletiadis J, Morrissey CO, Nucci M, Oladele R, Pagano L, Pasqualotto A, Patel A, Racil Z, Richardson M, Roilides E, Ruhnke M, Seyedmousavi S, Sidharthan N, Singh N, Sinko J, Skiada A, Slavin M, Soman R, Spellberg B, Steinbach W, Tan BH, Ullmann AJ, Vehreschild JJ, Vehreschild MJGT, Walsh TJ, White PL, Wiederhold NP, Zaoutis T, Chakrabarti A; Mucormycosis ECMM MSG Global Guideline Writing Group. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19(12):e405-21. doi: 10.1016/S1473-3099(19)30312-3.
CrossRef - Spellberg B, Walsh TJ, Kontoyiannis DP, Edwards J Jr, Ibrahim AS. Recent advances in the management of mucormycosis: from bench to bedside. Clin Infect Dis. 2009;48(12):1743-51. doi: 10.1086/599105.
CrossRef - Khan A, Singh R, Sharma S, Singh V, Sheoran A, Soni A, Dhull V, Gill PS, Yadav A, Chaudhary D, Gupta MC, Mehta PK. Diagnosis of osteoarticular tuberculosis by immuno-PCR assay based on mycobacterial antigen 85 complex detection. Lett Appl Microbiol. 2022;74(1):17-26. doi: 10.1111/lam.13567.
CrossRef - Paltauf A. Mycosis mucorina: Ein Beitrag zur Kenntnis der menschilchen Fadenpiltzer-krankungen. Virchows Arch. Pathol. Anat. 1885;102:543–64. doi: 10.1007/BF01932420.
CrossRef - Sharma S, Grover M, Bhargava S, Samdani S, Kataria T. Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. J Laryngol Otol. 2021;135(5):442-7. doi: 10.1017/S0022215121000992.
CrossRef - Bhatt K, Agolli A, Patel MH, Garimella R, Devi M, Garcia E, Amin H, Domingue C, Guerra Del Castillo R, Sanchez-Gonzalez M. High mortality co-infections of COVID-19 patients: mucormycosis and other fungal infections. Discoveries (Craiova). 2021;9(1):e126. doi: 10.15190/d.2021.5.
CrossRef - Spellberg B, Kontoyiannis DP, Fredricks D, Morris MI, Perfect JR, Chin-Hong PV, Ibrahim AS, Brass EP. Risk factors for mortality in patients with mucormycosis. Med Mycol. 2012;50(6):611-8. doi: 10.3109/13693786.2012.669502.
CrossRef - Mishra Y, Prashar M, Sharma D, Akash, Kumar VP, Tilak TVSVGK. Diabetes, COVID 19 and mucormycosis: Clinical spectrum and outcome in a tertiary care medical center in Western India. Diabetes Metab Syndr. 2021;15(4):102196. doi: 10.1016/j.dsx.2021.102196.
CrossRef - Prakash H, Ghosh AK, Rudramurthy SM, Singh P, Xess I, Savio J, Pamidimukkala U, Jillwin J, Varma S, Das A, Panda NK, Singh S, Bal A, Chakrabarti A. A prospective multicenter study on mucormycosis in India: Epidemiology, diagnosis, and treatment. Med Mycol. 2019;57(4):395-402. doi: 10.1093/mmy/myy060.
CrossRef - Skiada A, Pagano L, Groll A, Zimmerli S, Dupont B, Lagrou K, Lass-Florl C, Bouza E, Klimko N, Gaustad P, Richardson M, Hamal P, Akova M, Meis JF, Rodriguez-Tudela JL, Roilides E, Mitrousia-Ziouva A, Petrikkos G; European Confederation of Medical Mycology Working Group on Zygomycosis. Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin Microbiol Infect. 2011;17(12):1859-67. doi: 10.1111/j.1469-0691.2010.03456.x.
CrossRef - Ogurtsova K, Guariguata L, Barengo NC, Ruiz PL, Sacre JW, Karuranga S, Sun H, Boyko EJ, Magliano DJ. IDF diabetes Atlas: Global estimates of undiagnosed diabetes in adults for 2021. Diabetes Res Clin Pract. 2022;183:109118. doi: 10.1016/j.diabres.2021.109118.
CrossRef - Patel A, Kaur H, Xess I, Michael JS, Savio J, Rudramurthy S, Singh R, Shastri P, Umabala P, Sardana R, Kindo A, Capoor MR, Mohan S, Muthu V, Agarwal R, Chakrabarti A. A multicentre observational study on the epidemiology, risk factors, management and outcomes of mucormycosis in India. Clin Microbiol Infect. 2020;26(7):944.e9-944.e15. doi: 10.1016/j.cmi.2019.11.021.
CrossRef - Garg D, Muthu V, Sehgal IS, Ramachandran R, Kaur H, Bhalla A, Puri GD, Chakrabarti A, Agarwal R. Coronavirus Disease (Covid-19) Associated Mucormycosis (CAM): Case Report and Systematic Review of Literature. Mycopathologia. 2021;186(2):289-298. doi: 10.1007/s11046-021-00528-2.
CrossRef - Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 Apr 30;382(18):1708-1720. doi: 10.1056/NEJMoa2002032.
CrossRef - Roushdy T, Hamid E. A case series of post COVID-19 mucormycosis-a neurological prospective. Egypt J Neurol Psychiatr Neurosurg. 2021;57(1):100. doi: 10.1186/s41983-021-00355-8.
CrossRef - Kell DB, Pretorius E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics. 2014;6(4):748-73. doi: 10.1039/c3mt00347g.
CrossRef - Wali U, Balkhair A, Al-Mujaini A. Cerebro-rhino orbital mucormycosis: an update. J Infect Public Health. 2012;5(2):116-26. doi: 10.1016/j.jiph.2012.01.003.
CrossRef - Luo LC, Cheng DY, Zhu H, Shu X, Chen WB. Inflammatory pseudotumoural endotracheal mucormycosis with cartilage damage. Eur Respir Rev. 2009;18(113):186-9. doi: 10.1183/09059180.00000709.
CrossRef - Castillo L, Hofman V, Bétis F, Piche M, Roger PM, Santini J, Hofman P. Longterm survival in acute rhinocerebral mucormycosis with giant cell arteritis and foreign body granulomas. Pathol Res Pract. 2001;197(3):199-203. doi: 10.1078/0344-0338-00034.
CrossRef - Mohamed MS, Abdel-Motaleb HY, Mobarak FA. Management of rhino-orbital mucormycosis. Saudi Med J. 2015;36(7):865-8. doi: 10.15537/smj.2015.7.11859.
CrossRef - Tedder M, Spratt JA, Anstadt MP, Hegde SS, Tedder SD, Lowe JE. Pulmonary mucormycosis: results of medical and surgical therapy. Ann Thorac Surg. 1994;57(4):1044-50. doi: 10.1016/0003-4975(94)90243-7.
CrossRef - Lin E, Moua T, Limper AH. Pulmonary mucormycosis: clinical features and outcomes. Infection. 2017;45(4):443-8. doi: 10.1007/s15010-017-0991-6.
CrossRef - Piromchai P, Thanaviratananich S. Impact of treatment time on the survival of patients suffering from invasive fungal rhinosinusitis. Clin Med Insights Ear Nose Throat. 2014;7:31-4. doi: 10.4137/CMENT.S18875. Prakash H, Chakrabarti A. Epidemiology of Mucormycosis in India. Microorganisms. 2021;9(3):523. doi: 10.3390/microorganisms9030523.
CrossRef - Jeong W, Keighley C, Wolfe R, Lee WL, Slavin MA, Chen SC, Kong DCM. Contemporary management and clinical outcomes of mucormycosis: A systematic review and meta-analysis of case reports. Int J Antimicrob Agents. 2019;53(5):589-97. doi: 10.1016/j.ijantimicag.2019.01.002.
CrossRef - Muthu V, Agarwal R, Dhooria S, Sehgal IS, Prasad KT, Aggarwal AN, Chakrabarti A. Has the mortality from pulmonary mucormycosis changed over time? A systematic review and meta-analysis. Clin Microbiol Infect. 2021;27(4):538-49. doi: 10.1016/j.cmi.2020.12.035.
CrossRef - Karakoyun I, Colak A, Turken M, Altin Z, Arslan FD, Iyilikci V, Yilmaz N, Kose S. Diagnostic utility of C-reactive protein to albumin ratio as an early warning sign in hospitalized severe COVID-19 patients. Int Immunopharmacol. 2021;91:107285. doi: 10.1016/j.intimp.2020.107285.
CrossRef - El-Shabrawy M, Alsadik ME, El-Shafei M, Abdelmoaty AA, Alazzouni AS, Esawy MM, Shabana MA. Interleukin-6 and C-reactive protein/albumin ratio as predictors of COVID-19 severity and mortality. Egypt J Bronchol. 2021;15(1):5. doi: 10.1186/s43168-021-00054-1.
CrossRef
List of abbreviations
ALC: Absolute lymphocyte count;
ARDS: Acute respiratory distress syndrome;
CAM: COVID-19 associated mucormycosis;
CBC: Complete blood count;
COVID-19: Coronavirus disease 2019;
CDC: Centers for Disease Control and Prevention;
CKD: Chronic kidney disease;
COPD: Chronic obstructive pulmonary disease;
CRP: C-reactive protein;
DIC: Disseminated intravascular coagulopathy;
DM: Diabetes mellitus;
H&E: Hematoxylin and eosin stain;
HBA1C: Glycated hemoglobin;
HTN: Hypertension;
ICU: Intensive Care Unit;
IDF: International Diabetes Federation;
LDH: Serum lactate dehydrogenase;
MRI: Magnetic Resonance Imaging;
N: Number;
O2: Oxygen;
PAS: Periodic acid-Schiff stain;
PJP: Pneumocystis jiroveci pneumonia;
PLR: Platelet to lymphocyte ratio;
ROC: Receiver operating characteristic;
SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus-2;
WBCs: White blood cells.