Siti Hajar Musa* , Nurul Huda Heri
, Nurul Huda Heri , Fatin Atirah Borhan
, Fatin Atirah Borhan , Nurul Fatin Inani Mustaffa, Nabilah Rosman and Faizan Naeem Razali
, Nurul Fatin Inani Mustaffa, Nabilah Rosman and Faizan Naeem Razali
Faculty of Pharmacy and Health Sciences, Universiti Kuala Lumpur, Royal College of Medicine Perak, Ipoh, Perak, Malaysia.
Corresponding Author E-mail: sitihajar.musa@unikl.edu.my
DOI : https://dx.doi.org/10.13005/bpj/2509
Abstract
Psoriasis is a widespread autoimmune inflammatory dermatological disease treated with oral cyclosporine to reduce the uneasiness of psoriasis. However, systemic therapy of cyclosporine is associated with high risk of side effect that limit the usage in psoriasis treatment. Topical delivery of cyclosporine is believed could overcome cyclosporine related toxicity issues. In this study, a new carrier for topical cyclosporine was developed, which cooperated with Moringa oleifera oil (MOO) that has been reported could enhance the moisture-retaining of the skin. Both high-shear homogenizer and overhead stirrer homogenizer were utilized in formulating a cyclosporine-loaded emulsion carrier. Two emulsions were prepared at different proportions of MOO, water and surfactant (Tween80:Span80) based on the constructed ternary phase diagram. Samples with different formulation (E1 and E2) were subjected to several tests including the stability, rheological, colony and in vitro release analysis. E1 and E2 possessed good stability against phase separation for 1 month at different storage temperatures (4, 25 and 40ºC), having pH values within the range of 4 to 5 as well as showing no mould and microbial growth after been incubated on nutrient agar plate at controlled conditions. Optimized formulations were found to be non-Newtonian and followed the pseudoplastic flow behaviour. Nonetheless, E2 exhibited highest permeation of cyclosporine (80.23%) through cellulose acetate membrane via Franz diffusion cell, which correspond to controlled release and best fitted to first order kinetic behaviour (R2=0.9819). This preliminary study suggested that the formulated emulsion has a promising potential as topical medicament for psoriasis.
Keywords
Cyclosporine; Emulsion; Formulation; Moringa oleifera oil; Psoriasis, Topical
Download this article as:| Copy the following to cite this article: Musa S. H, Heri N. H, Borhan F. A, Mustaffa N. F. I, Rosman N, Razali F. N. Fabrication of Emulsion Loaded with Cyclosporine and Moringa Oleifera Oil Potentially for Topical Psoriasis Treatment. Biomed Pharmacol J 2022;15(3). |
| Copy the following to cite this URL: Musa S. H, Heri N. H, Borhan F. A, Mustaffa N. F. I, Rosman N, Razali F. N. Fabrication of Emulsion Loaded with Cyclosporine and Moringa Oleifera Oil Potentially for Topical Psoriasis Treatment. Biomed Pharmacol J 2022;15(3). Available from: https://bit.ly/3KTio9W |
Introduction
Emulsions are widely used in the pharmaceutical and cosmetics industries, as the system provides excellent solubilizing capacities to hydrophilic and lipophilic components, which are acceptable for topical application on the skin surface. Emulsions are metastable systems, consisting of at least two immiscible phases, in which one liquid is dispersed as small globules in continuous phase and the dispersed phase is stabilized by surfactant (Goodarzi and Zendehboudi, 2019). It has been reported that emulsions have better bioavailability of the poorly water-soluble drug, excellent spreadability, increase rate of drug absorption and superior skin feel (Mohsin and Akhtar, 2017). The use of emulsion as a carrier definitely helps the stratum corneum in maintaining its integrity barrier due to close contact with the skin body, thus facilitating the drug’s penetration across the skin barrier (Musa et al., 2017). This could be attributed to the oil phase, which transports lipophilic molecules effectively as polar lipids. The oil phase component in an emulsion can form a protective layer that protects the epidermis layer from external insults. Therefore, incorporating cyclosporine in emulsion could be an intelligent approach to solve the problems related to cyclosporine.
Psoriasis is a chronic and intermittent autoimmune skin disorder often triggered by environmental insults and internal risk factors and in conjunction with genetic predisposition (Kamiya et al., 2019). Cyclosporine is one of the potent immunosuppressive agents typically employed to treat moderate to severe psoriasis. Initially, cyclosporine (C62H111N11O12) was purified from the filamentous fungus of Tolypocladium inflatum Gams for its antifungal activity and later it was approved by Food and Drug Administration (FDA) as an immunosuppressant agent (Yang et al., 2018). Cyclosporine is a highly lipophilic substance due to the presence of non-ribosomal cyclic peptide with eleven amino acids (Yang et al., 2018). Although cyclosporine has a positive effect on treating psoriasis, oral cyclosporine (5 mg/kg/day) induced renal toxicity, possibly leading to treatment discontinuation (Wu and Kuca, 2018). Moreover, cyclosporine caused lower bone mineral density, impetigo development, angioedema and herpetic infection (Dogra et al., 2016). Thus, it is believed that a viable alternative to avoid these adverse effects of cyclosporine is through topical administration.
Topical administration remains as first-line therapy in psoriasis treatment and is most preferred by patients, as this route improves patients’ satisfaction and adherence, less expensive and ease of application compared to systemic therapy. Several factors need to be considered during the selection of topical therapy, including body location, efficacy and patient preferences based on formulation texture and frequency of application. Topical corticosteroids are the most used drug for mild to moderate psoriasis treatment. However, psoriasis patients might suffer from local cutaneous like skin atrophy, telangiectasia and striae development, limiting the clinical usage of the drug (Torsekar and Gautam, 2017). According to the international guidelines, the corticosteroid is not suitable for pregnant women as it is associated with a high risk of intrauterine growth restriction and risk to the infant during breastfeeding (Kleyn et al., 2019). Application of cyclosporine through topical route would significantly enhance the treatment efficacy of psoriasis due to the directly targeted drug on mucous membrane and skin surface.
However, major limitations in delivering cyclosporine through the topical route are high molecular weight (MW=1200 Da) and low water solubility (Walunj et al., 2020). High molecular weight of cyclosporine hinders the potential of the drug’s permeation across the skin, while poor water solubility reduces the effectiveness of drug. Therefore, multiple strategies have been designed to increase the drug permeation rate through the lipid barrier, improving the effectiveness of the topical treatment. Recently, topical drug delivery systems such as nanoemulsion (Musa et al., 2017), microemulsion (Benigni et al., 2018), liposome (Walunj et al., 2020) and solid lipid nanoparticle (Essaghraoui et al., 2019) have been developed to incorporate cyclosporine into lipid carrier. Nevertheless, topical formulations of cyclosporine are not available in the market yet.
MOO is obtained from the dried seeds through cold mechanical pressed. The oil is available in the form of creams, lotions, ointment and gel which has many health benefits in relation to the skin. Thanks to its primary compound, unsaturated fatty acids represented by oleic acid (70.85%), MOO possessed antioxidant and anti-inflammatory activities (Fu et al., 2021). MOO has been reported could lessen skin erythema, improve skin hydration level and reduce epidermal hypertrophy when applied topically over the skin surface (Athikomkulchai et al., 2021). Moreover, Ventura et al. (2021) discovered the efficacy of topical MOO in treating chronic wounds of diabetic rat skin. Thus, MOO is believed to be suitable use as a main carrier in delivering drug for psoriasis treatment.
As a novel approach in this investigation, the cyclosporine-loaded emulsion will be formulated specifically for the skin treatment against psoriasis, where cyclosporine was utilized as an active substance while MOO acted as the oil phase. Incorporating cyclosporine in emulsion could be an intelligent approach to solve the problems related to cyclosporine and hence boost the treatment of dry and itchy skin.
Materials and methods
Solubility test
Solubility of cyclosporine was measured by adding 0.1% w/w of cyclosporine to the different concentration of MOO (5 and 10% w/w). The mixtures were mixed temperature of 45⁰C to dissolve the drug. Another concentration of cyclosporine (0.1% w/w) was added into the same mixture and this step was repeated until a saturated and non-transparent MOO-cyclosporine emulsion was observed. The total percentage of cyclosporine which was dissolved effectively in the clear transparent MOO with no precipitation sign after centrifugation were recorded as the highest amount of cyclosporine that can be dissolved in the oil.
Construction of ternary phase diagram
Ternary phase diagram of MOO, Tween 80:Span 80 mixture (1:1 ratio) and water was prepared at different weight ratios, ranging from 0:100 to 100:0. The mixture was homogenously mixed by vortex mixer for 5 min at ambient temperature before further proceed with centrifugation at 2,300 rpm for 15 min. The appearance and behaviour of samples were visually observed to confirm the isotropic nature of sample. The experiment was repeated with the addition of water according to different percentages from 0 to 100% range. The phase diagram was constructed using Chemix School 3.60 software based on the phase behaviours obtained from the observation.
Formulation of emulsion
Cyclosporine was dissolved in MOO together with beeswax. Surfactant mixture was added in the aqueous phase, followed by xanthan gum and phenonip. These two phases were prepared and heated separately in water bath at temperature of 45°C. An overhead stirrer was utilized at speed of 400 rpm for 1 hour to mix the two main phases. Lastly, the sample was further homogenized using high shear homogenizer for 30 min at speed of 13,000 rpm.
Over-time stability test
The formulated emulsions were stored at different storage temperatures; 4⁰C, 25⁰C, and 40⁰C. The physical appearance of the emulsions were observed and their individual pH reading was recorded weekly.
Freeze-thaw cycle stability test
The formulated emulsions were stored at two temperature storage for 24 hour alternately. The temperature were set at (i) 4 and 25⁰C, (ii) 25 and 40⁰C and (iii) 4 and 40⁰C. This storage cycle was repeated two times for each formulated emulsion. The physical observation and pH reading of the individual emulsion was evaluated to determine its stability.
Rheological test
Rheological behaviour of the formulated emulsions was measured using a dynamic shear rheometer equipped with a cone and plate geometry of 4⁰/40 mm and a plate gap of 0.15 mm. The experiment was carried out at controlled temperature (25⁰C), with a shear rate range between 0.01 to 50.00 sˉ¹. The measurement was recorded after the instrument equilibrated for 5 min.
Microbial count test
Two sets of eight micro centrifuge tubes were filled with 900 µL of sterile saline, where each set was numbered as 10ˉ¹ till 10ˉ⁷. Cyclosporine-loaded emulsion with 100 µL each was added into the respective first tube (10 ˉ¹ dilution) and serial dilution was carried out by transferring 100 µL of the suspension to the subsequent tube until the last numbered tube (10ˉ⁷ dilution). Then, 100 µL was taken out from each tube and was spread using the spreading technique onto the nutrient agar plate. The plate was further incubated at storage temperature of 37⁰C for 16 to 18 hours. Appearance of colonies (if any) were observed and the test were performed in triplicate.
In vitro permeation study
Franz diffusion cell was utilized to investigate the in vitro release of cyclosporine from emulsion formulation through a semi synthetic cellulose acetate membrane (13 mm). A 1 mL of the sample was added on the top of the membrane through donor-chamber compartment, while the prepared phosphate buffer solution (PBS) with pH 7.4 was placed in the receptor-chamber compartment. Analysis was carried out at time intervals of 0.5, 1, 2, 3, 4, 5, 6 hours. The collected sample was subjected to ultraviolet spectrophotometer at wavelength of 320 nm. The permeation analysis was carried out in triplicate.
Kinetic release of drug
The zero-order, first-order, Higuchi, Korsmeyer–Peppas and Hixson-Crowell kinetic models were applied to determine the release profiles of the formulations. A graph for each model was constructed using the model’s theoretical equation. Equation 1 represents the percentage of cumulative drug released vs time on the graph of the zero-order release kinetic. Equation 2 plots the log percentage of cumulative drug remaining vs time using the first-order model. Higuchi model represents the cumulative medication percentage released against the square root of time as shown in Equation 3. Korsmeyer–Peppas model translates the cumulative cyclosporine log percentage vs the log time determined by Equation 4. Hixson–Crowell release kinetic as stated in Equation 5 shows the cube root of the drug remaining vs time. The best-fitted model was chosen from the coefficient of determination (R2) value gained in each linear regression analysis. Following are the model equations used:
Q0 − Qt = k0t Equation 1
In (Q0/Qt) = −k1t Equation 2
Qt = kt1/2 Equation 3
Qt/Q∞ = ktn Equation 4
Q01/3 − Qt1/3 = kHCt Equation 5
Where, Q0 was represented as the initial amount of cyclosporine in emulsion and Qt represented as the amount of cyclosporine-loaded emulsion permeated. Meanwhile, k0 was referred as zero-order rate constant and k1 was referred to rate constant of first-order model. The constant reflecting the design variables of the system known as k, where Qt/Q∞ known as the fraction of drug released over time. The symbol of n was represented as the release exponent, kHC for the Hixson–Crowell rate constant, and t was referred as time.
Statistical analysis
All analyses were carried out in triplicate and all data are shown as mean ± standard deviation (n=3).
Results and Discussion
Solubility analysis of cyclosporine
Cyclosporine solubility was found to be maximum at 0.3% w/w in 5% of Moringa oleifera oil (MOO), while 10% of MOO exhibited excellent solubility up to 0.5% (w/w) of cyclosporine (Table 1). The solubility test was repeated until 0.7% of cyclosporine concentration to make a comparative assessment. Furthermore, it was found that both oil composition percentages could not solubilize cyclosporine with concentrations of 0.6% and 0.7%. This indicates that the solubilizing capability of cyclosporine in the emulsion system is greatly influenced by oil phase concentration.
Table 1: Limitation of cyclosporine concentration solubilized in Moringa Oleifera oil.
| Oil composition (%) | Cyclosporine content (%) | ||||||
| 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | 0.6 | 0.7 | |
| 5 | / | / | / | x | x | x | x |
| 10 | / | / | / | / | / | x | x |
/ = Cyclosporine solubilized; X = Saturated cyclosporine
Savjani et al. (2012) stated that the degree of solubility in a particular solvent (oil) is determined by a concentration of saturation, which does not increase its concentration with the addition of more solvents. As the oil concentration increased, the higher the amount of cyclosporine solubilized in the oil phase, the more space provided for the drug to be dissolved within the phase. In fact, the dispersed lipid in the emulsion contributed to drug solubility. However, drug supersaturation may occur under certain conditions if the equilibrium solubility exceeds the limit. Generally, oil-in-water emulsion is a good choice for the solubilization of cyclosporine as this drug exhibits lipophilic in nature. Sarheed et al. (2020) suggested that highly lipophilic drugs should be predominantly dissolved in the oil phase compared to the aqueous phase as it may affect the drug release permeation.
Ternary phase diagram analysis
The phase diagram was constructed to discover the existence range of stable emulsion composition by observing the layer formation after the centrifugation process. Two distinct of one-phase regions consisting of homogenous phase (yellow) and translucent emulsion (green) were detected from the phase diagram. In contrast, the remaining colours in the ternary phase diagram illustrated multi-layer emulsion (coral) and two-phase region (light blue). The figure clearly revealed that the emulsions’ stability was highly depended on the concentration of oil, surfactant and water.
The best emulsion was found to be at a high-water content (more than 60%), with oil and surfactant content not reaching 20% and 30%, respectively (Figure 1). Ramli et al. (2017) reported that the emulsion system with low surfactant content is suitable for skin delivery as it could avoid skin irritation (Ramli et al., 2017). The obtained emulsion systems (E1 and E2) seem to meet these criteria, since they involved a low surfactant concentration (Table 2).
 |
Figure 1: Constructed ternary phase diagram consisting of oil, water and surfactant (Tween80:Span 80). |
Table 2: Ingredients of emulsion formulation.
| Ingredients | Formulation composition (% w/w) | |
| E1 | E2 | |
| Cyclosporine | 0.3 | 0.3 |
| Moringa oleifera oil | 5 | 10 |
| Tween 80:Span 80 | 13 | 15 |
Over-time stability analysis
Physical observation
The metastable formulations were evaluated to identify the physical stability test of emulsions. For significant phase separation, the selected emulsions were subjected to centrifugation forces and storage stability for 4 weeks at different temperature conditions (4, 25 and 40°C) for significant phase separation. Based on the physical stability observation, the emulsions retained their milky color appearance with no phase separation after 1 month of storage. The reason is due to the sufficient amount of surfactant in the emulsion system, which could reduce the interfacial tension at the oil-water interface, preventing the instability phenomena such as Ostwald ripening, phase separation and creaming (Musa et al., 2017). Resistance to phase separation during over-time stability study gives a good sign in developing a stable formulation.
pH of emulsion
It is important to highlight that the optimum emulsion pH should be close or in the range of pH skin. After the samples were stored at temperatures of 4, 25, and 40⁰C the observed pH of the formulated emulsions were within the range from 4 to 5 (Figure 2). These pH values are acceptable for topical application as the skin’s pH is acidic, which exerts an antimicrobial barrier against bacterial and fungal proliferation at pH values between 4 and 6 (Proksch, 2018). Bigliardi (2018) stated that psoriatic skin is associated with a slightly higher in skin pH (alkaline condition), causing a dry, more scaling and itchy skin. Thus, an acidic emulsion formulation is a good option for the psoriasis treatment.
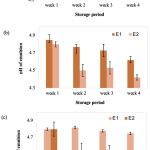 |
Figure 2: pH reading of E1 and E2 at different storage temperatures; (a) 4⁰C, (b) 25⁰C and (c) 40⁰C. |
Freeze-thaw stability analysis
Physical observation
E1 and E2 were stable to the freeze-thaw treatment as they did not show any phase separation and colour changes at the end of the experimental period. Pongsawatmanit and Srijunthongsiri (2008) reported that a freezing and thawing cycle may affect the stability of the emulsion by weakening the droplets interaction in which the aqueous phase tends to crystallize in freezing condition. However, the addition of a small volume of hydrocolloids could minimize the degradation of the emulsion system. A study conducted by Musa et al. (2017) demonstrated the capability of xanthan gum (hydrocolloid) to preserve the particle sizes as well as the consistency of the emulsions. Based on the obtained result, it was proven that the formulated emulsions has successfully eliminate deterioration of the system with the aids from hydrocolloid and thus able to withstand extreme temperature changes.
pH of emulsion
The pH value of E1 and E2 showed a decline in pH reading after the samples were subjected to freeze-thaw cycle (Figure 3). However, the pH value for both emulsions were maintained within the range of the human skin. This indicates that the kinetic energy and collision rate increased in the emulsion system as the oil droplets are more likely to coalescence each other at thawing stage, while the aqueous phase tends to solidify easily during freezing treatment, which may affect the pH on emulsion stability. The presence of non-ionic surfactants such as Tween 80 and Span 80 hampered the droplets collision due to an increased electrostatic repulsive interactions (Azhar et al., 2018). The capability of this network to withstand the temperatures would enhance the stability of the emulsions to gravitational separation.
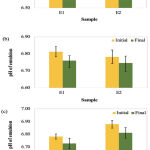 |
Figure 3: Comparison of pH value for E1 and E2 after freeze-thaw cycle at (a) 4 and 25⁰C, (b) 4 and 40⁰C and (c) 25 and 40⁰C. |
Rheology analysis
Rheological properties of the topical emulsions can be determined by the flow behavior, where it may influence the release and diffusion rates of a drug through the skin (Oliveira et al., 2018). The data obtained from the rheology analysis of the cyclosporine-loaded emulsion are presented in Figure 4. The plot of shear stress versus shear rate (Figure 3.4a) revealed that the shear stress increased gradually with the shear rate, representing both E1 and E2 having a non-Newtonian characteristic. This non-Newtonian behavior facilitates its application on the skin. In addition, Figure 3.4b shows the flow curves of viscosity against shear rate. The emulsion formulations were followed pseudoplastic flow behavior that is often referred to shear-thinning property because the viscosity decreases as the shear rate increases.
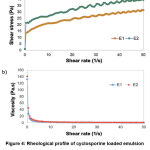 |
Figure 4: Rheological profile of cyclosporine loaded emulsion (a) non-Newtonian characteristic (b) pseudoplastic flow behavior. |
Shear-thinning property demonstrated the existence of weak attractive forces within the emulsion droplets caused by deformation and reorganization of oil droplet flocs (Azhar et al., 2018). The deformation may be attributed to the loss of internal droplets structure with respect to shear stress during the emulsification step, reaching a most stable deformed state of droplets. Moreover, shear-thinning formulations tested under high shear conditions and zero flow under gravity stress, indicate a low viscosity fluid behaviour (low resistance) to flow. This emulsion behaviour is considered ideal for topical formulations as the product optimizes its spreadability over the affected skin area, providing a good skin penetration of the cyclosporine (Kamal et al., 2020).
Colony count analysis
After several serial dilution processes, no colony growth was presented on the agar plate for E1 and E2 after being incubated for 18 hours at 37⁰C (Figure 5), showing the effectiveness of phenonip in the emulsion system. Phenonip is a natural preservative used to prevent from microbial spoilage and increase the shelf-life of topical formulations. Besides, plant oils are categorized as synthetic preservatives, wherein their antimicrobial action are based on the inhibition of proteins and polysaccharides synthesis in bacterial cells and denaturation of membrane proteins, leading to outer membrane disruption (Herman, 2018). It has been shown that Moringa oleifera oil has potent antimicrobial activity against different strains of bacteria and fungus (Amina et al., 2019). Therefore, these formulations are suitable for topical use as they effectively maintain the system from colony growth.
 |
Figure 5: Results of microbial growth on nutrient agar plate for (a) E1 and (b) E2. |
In vitro permeation analysis
A preliminary in vitro drug diffusion profile of optimized E1 and E2 was carried out by calculating the percentage of cumulative cyclosporine permeated across cellulose acetate membrane for 6 hours (Figure 6). It was found that E2 exhibited a high permeation rate, as it reached 80.23% release within 6 hours of analysis compared to E1 with the ability to permeate up to 66.71% only. The difference in cyclosporine permeation between E1 and E2 was the amount of the oil phase. Despite the thickening agent’s claimed effects on drug diffusion rate, the oil content proved to have a significant impact on drug diffusion rate (Musa et al., 2017). The higher the oil concentration, the quicker the oil permeates across the membrane (E2). This is because the quantity of oil droplets in the system has exceeded the available volume, leading to a more successful transfer of more cyclosporine to the membrane.
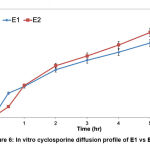 |
Figure 6: In vitro cyclosporine diffusion profile of E1 vs E2. |
Based on the graph, cyclosporine-loaded emulsion had a massive effect on the initial release, generally referred to as an initial burst with further sustained release up to 6 hours. A modest initial burst (25.6 and 26.5%) in the first 1 hour was observed from E1 and E2 due to superficial drug entrapment. For the following next hours, no burst release mechanism was reported for both formulations. With a sustained release pattern, E1 and E2 would be beneficial for psoriasis skin as the drug release is controlled by emulsion carrier. The rapid release of the drug is not suitable for topical treatment of psoriasis because it might cause toxicity due to faster absorption of the drug in inflamed psoriatic lesions (Rashid et al., 2021).
Kinetic release analysis
The process of drug diffusion during in vitro release studies can be determined by the application of mathematical models. Table 3 displays the coefficient of determination (R2) for each kinetic model tested on E1 and E2. All the graphs for the kinetic model revealed a linear relationship to different degrees of the model. The release kinetic for E1 and E2 were identified based on the kinetic models include Higuchi, First-order, Korsmeyer-Peppas, Zero-order and Hixson-Crowell. Cyclosporine release from emulsion for E1 and E2 were best fitted with Higuchi model (0.9985) and first order model (0.9819), respectively. Both formulations showed a difference in cyclosporine release due to the different water content. As the water concentration increased in the system, the diffusion rate decreased owing to lipophilic property of the membrane used.
Table 3: Correlation coefficients for different kinetic release models were obtained that used a plotted graph.
| Kinetic model | Coefficient of determination (R2) | |
| E1 | E2 | |
| Higuchi | 0.9985 | 0.9762 |
| First-order | 0.9971 | 0.9819 |
| Korsmeyer-Peppas | 0.9946 | 0.9452 |
| Zero-order | 0.9223 | 0.9554 |
| Hixson-Crowell | 0.0767 | 0.1898 |
Conclusion
In this study, the emulsion system containing cyclosporine and Moringa oleifera oil was successfully developed and optimized to improve the drug’s solubility and enhance drug permeation to obtain the therapeutic effect of cyclosporine to treat psoriasis. The two best emulsions (E1 and E2) were formulated using a high-energy emulsification method followed by a low-energy emulsification technique to form a stable emulsion. Both formulations were stable during the 1 month of storage stability test at different temperatures (4, 25 and 40⁰C) with no phase separation. The pH value of the emulsion formulations is within the range for skin application. The effectiveness of phenonip and Moringa oleifera oil were proved with no bacterial growth on the agar plate during processing and storage. The rheological properties of emulsions, showing non-Newtonian and pseudoplastic flow behaviour, which is suitable for topical administration. The release kinetic of cyclosporine through emulsion carrier for E1 and E2 has mimicked the Higuchi and first-order kinetic models, respectively. This formulation approach was found to be promising for the development of topical psoriasis.
Acknowledgement
The authors were grateful to Ministry of Higher Education (MOHE), Malaysia for funding this study under the Fundamental Research Grant Scheme (FRGS/1/2020/STG04/UNIKL/03/1). Authors also would like to thank Universiti Kuala Lumpur Royal College of Medicine Perak for providing research facilities.
Conflict of Interest
All authors declare there is no conflict of interest associated with this publication.
Funding sources
Ministry of Higher Education (MOHE), Malaysia for funding this study under the Fundamental Research Grant Scheme (FRGS/1/2020/STG04/UNIKL/03/1).
References
- Goodarzi, F. and Zendehboudi, S. A comprehensive review on emulsions and emulsion stability in chemical and energy industries. Can. J. Chem. Eng., 2019; 97(1): 281-309.
CrossRef - Mohsin, S. and Akhtar, N. Formulation and stability evaluation of bauhinia variegata extract topical emulsion. Acta Pol. Pharm., 2017; 74(3): 945-954.
- Musa, S., Basri, M., Fard Masoumi, H., Shamsudin, N. and Salim, N. Enhancement of physicochemical properties of nanocolloidal carrier loaded with cyclosporine for topical treatment of psoriasis: In vitro diffusion and in vivo hydrating action. J. Nanomedicine, 2017; 12: 2427-2441.
CrossRef - Kamiya, K., Kishimoto, M., Sugai, J., Komine, M., and Ohtsuki, M. Risk factors for the development of psoriasis. Int. J. Mol. Sci., 2019; 20(18): 4347-4361.
CrossRef - Yang, X., Feng, P., Yin, Y., Bushley, K., Spatafora, J. W. and Wang, C. Cyclosporine biosynthesis in Tolypocladium inflatum benefits fungal adaptation to the environment. M. Bio., 2018; 9(5): 1211-1218.
CrossRef - Wu, Q. and Kuca, K. Metabolic pathway of cyclosporine A and its correlation with nephrotoxicity. Curr. Drug Metab., 2019; 20(2): 84-90.
CrossRef - Dogra, S., Mahajan, R., Narang, T. and Handa, S. Systemic cyclosporine treatment in severe childhood psoriasis: a retrospective chart review. J. Dermatol. Treat., 2016; 28(1): 18-20.
CrossRef - Torsekar, R. and Gautam, M. M. Topical therapies in psoriasis. Indian Dermatol. Online J., 2017; 8(4): 235.
CrossRef - Kleyn, E. C., Morsman, E., Griffin, L., Wu, J. J., Cm van de Kerkhof, P., Gulliver, W., van der Walt, J. M., and Iversen, L. Review of international psoriasis guidelines for the treatment of psoriasis: recommendations for topical corticosteroid treatments. J. Dermatol. Treat., 2019; 30(4): 311-319.
CrossRef - Walunj, M., Doppalapudi, S., Bulbake, U. and Khan, W. Preparation, characterization, and in vivo evaluation of cyclosporine cationic liposomes for the treatment of psoriasis. J. Liposome Res., 2020; 30(1): 68-79.
CrossRef - Benigni, M., Pescina, S., Grimaudo, M. A., Padula, C., Santi, P. and Nicoli, S. Development of microemulsions of suitable viscosity for cyclosporine skin delivery. Int. J. Pharm., 2018; 545(1-2): 197-205.
CrossRef - Essaghraoui, A., Belfkira, A., Hamdaoui, B., Nunes, C., Lima, S. A. C. and Reis, S. Improved dermal delivery of cyclosporine a loaded in solid lipid nanoparticles. Nanomaterials, 2019; 9(9): 1204.
CrossRef - Fu, X., Su, J., Hou, L., Zhu, P., Hou, Y., Zhang, K., Li, H., Liu, X., Jia, C. and Xu, J. Physicochemical and thermal characteristics of Moringa oleifera seed oil. Adv. Compos. Hybrid Mater., 2021; 4(3): 685-695.
CrossRef - Athikomkulchai, S., Tunit, P., Tadtong, S., Jantrawut, P., Sommano, S. R. and Chittasupho, C. Moringa oleifera seed oil formulation physical stability and chemical constituents for enhancing skin hydration and antioxidant activity. Cosmetics, 2021; 8(1): 1-18.
CrossRef - Ventura, A. C. S. S. B., de Paula, T., Gonçalves, J. P., da Silva Soley, B., Cretella, A. B. M., Otuki, M. F. and Cabrini, D. A. The oil from Moringa oleifera seeds accelerates chronic skin wound healing. Phytomedicine Plus, 2021; 1(3): 100099-100109.
CrossRef - Savjani, K. T., Gajjar, A. K. and Savjani, J. K. Drug solubility: importance and enhancement techniques. Int. Sch. Res. Notices, 2012: 1-10.
CrossRef - Sarheed, O., Shouqair, D., Ramesh, K. V. R. N. S., Khaleel, T., Amin, M., Boateng, J., and Drechsler, M. Formation of stable nanoemulsions by ultrasound-assisted two-step emulsification process for topical drug delivery: Effect of oil phase composition and surfactant concentration and loratadine as ripening inhibitor. Int. J. Pharma., 2020; 575: 118952.
CrossRef - Ramli, S., Norhman, N., Zainuddin, N., Ja’afar, S. M. and Rahman, I. A. Nanoemulsion based palm olein as vitamin E carrier. Malaysian J. Anal. Sci., 2017; 21(6): 1399-1408.
CrossRef - Proksch, E. pH in nature, humans and skin. J. Dermat., 2018; 45(9): 1044-1052.
CrossRef - Bigliardi, P. L. Role of skin pH in psoriasis. pH of the Skin: Issues and Challenges, 2018; 54: 108-114.
CrossRef - Pongsawatmanit, R. and Srijunthongsiri, S. Influence of xanthan gum on rheological properties and freeze–thaw stability of tapioca starch. J. Food Eng., 2008; 88(1): 137-143.
CrossRef - Azhar, S. N. A. S., Ashari, S. E. and Salim, N. Development of a kojic monooleate-enriched oil-in-water nanoemulsion as a potential carrier for hyperpigmentation treatment. Int. J. Nanomedicine, 2018; 13: 6465.
CrossRef - Oliveira, C. A., Gouvea, M. M., Antunes, G. R., de Freitas, Z. M. F., de Carvalho Marques, F. F. and Ricci-Junior, E. (2018). Nanoemulsion containing 8-methoxypsoralen for topical treatment of dermatoses: development, characterization and ex vivo permeation in porcine skin. Int. J. Pharm., 2018; 547(1-2): 1-9.
CrossRef - Kamal, N. S., Krishnaiah, Y. S., Xu, X., Zidan, A. S., Raney, S., Cruz, C. N. and Ashraf, M. Identification of critical formulation parameters affecting the in vitro release, permeation, and rheological properties of the acyclovir topical cream. Int. J. Pharm., 2020; 590: 119914.
CrossRef - Herman, A. Antimicrobial ingredients as preservatives booster and components of self-preserving cosmetic products. Curr. Microbiol., 2018; 76(6): 744-754.
CrossRef - Amina, M., Al Musayeib, N. M., Alarfaj, N. A., El-Tohamy, M. F., Orabi, H. E., Bukhari, S. I. and Mahmoud, A. Z. Exploiting the potential of moringa oleifera oil/polyvinyl chloride polymeric bionanocomposite film enriched with silver nanoparticles for antimicrobial activity. Int. J. Polym. Sci., 2019: 1-11.
CrossRef - Rashid, S. A., Bashir, S., Ullah, H., ullah Shah, K., Khan, D. H., Shah, P. A., Danish, M. Z., Khan, M. H., Mahmood, S., Sohaib, M., Irfan, M. M. and Amin, A. Development, characterization and optimization of methotrexate-olive oil nano-emulsion for topical application. Pak. J. Pharm. Sci., 2021; 34(1): 205-215.








