Manuscript accepted on :21-07-2022
Published online on: 20-09-2022
Plagiarism Check: Yes
Reviewed by: Dr. Huang Wei Ling
Second Review by: Dr. Hanefi ÖZBEK
Final Approval by: Dr. Patorn Promchai
Devasrita Dash1* , Laxminarayana Bairy Kurady2
, Laxminarayana Bairy Kurady2 and Bharti Chogtu3
and Bharti Chogtu3
1Department of Pharmacology, Melaka Manipal Medical College (Manipal Campus), Manipal Academy of Higher Education (MAHE), Manipal-576104, Karnataka, India.
2Department of Pharmacology, Ras Al Khaimah College of Medical Sciences, Ras Al Khaimah-11172, UAE
3Department of Pharmacology, Kasturba Medical College, Manipal Academy of Higher Education (MAHE), Manipal-576104, Karnataka, India.
Corresponding Author E-mail: devasrita@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2462
Abstract
Introduction: Type 2 diabetes is considered a pivotal risk factor for Alzheimer’s disease (AD). Aluminium chloride induces hippocampal structural & functional abnormality and causes neurodegeneration. Our study evaluated the effects of vildagliptin on spatial memory, cholinergic activity, and neuronal survival in cornu ammonis 3 (CA3) region of hippocampus in an aluminium chloride-induced AD in male Wistar rats. Materials and method: Male Wistar rats were randomly divided into five groups. All animals except normal control were exposed to aluminium chloride (17 mg/kg/day) and group 3, 4 and 5 were simultaneously received rivastigmine (6 mg/kg/day), vildagliptin (5 mg/kg/day and 10 mg/kg/day) treatment respectively for 30 days. Assessment of spatial memory was followed by estimation of acetylcholinesterase (AChE) activity and quantification of neuronal cell count in CA3 region of hippocampus. Results: Vildagliptin improved spatial memory, decreased acetylcholinesterase levels, and improved neuronal count in CA3 region of hippocampus through multimodal approach. Conclusion: Vildagliptin treatment significantly attenuated aluminium chloride-induced cognitive deficits. It may serve as a promising candidate in the management of concomitant AD and type 2 diabetes mellitus (T2DM).
Keywords
Alzheimer’s disease; Aluminium chloride; CA3 region; Cognition; Type 2 diabetes; Vildagliptin
Download this article as:| Copy the following to cite this article: Dash D, Kurady L. N, Chogtu B. Effect of Vildagliptin on Cognitive Deficits in an Experimental Model of Alzheimer’s Disease. Biomed Pharmacol J 2022;15(3). |
| Copy the following to cite this URL: Dash D, Kurady L. N, Chogtu B. Effect of Vildagliptin on Cognitive Deficits in an Experimental Model of Alzheimer’s Disease. Biomed Pharmacol J 2022;15(3). Available from: https://bit.ly/3DDdbBj |
Introduction
Alzheimer’s disease (AD) is a slowly progressing brain disorder with deleterious effects on memory, cognition, and behavior. Neuropathological hallmarks of AD include senile plaques1,2, neurofibrillary tangles1,2, brain atrophy, cholinergic dysfunction1,2, and neurodegeneration1,2. AD is the most common form of dementia3 in the older population and poses a significant economical and social burden to society. The predominance of AD doubles every 5 years4, after the age of 654. By the year 2050, the number of people aged 65 and above with Alzheimer’s dementia, is projected to reach 12.7 million5.
Current drug therapy for AD improves cognitive impairment, only through symptomatic relief. Hence, there has been a constant need for drugs, that will delay, prevent and/or reverse cognitive and behavioral changes seen in AD.
Evidence suggests that AD is multifactorial in origin. Over the years, T2DM has been considered an important risk factor for AD. Insulin in the brain is essential for the regulation of memory and cognitive functions. Commonalities shared by T2DM and AD are as follows: insulin resistance, neuronal and synaptic damage, amyloid β (Aβ) deposition, tau hyperphosphorylation, brain atrophy, oxidative stress, apoptosis, inflammation, ApoE4, cardiovascular disease, and higher cholesterol levels6–9. Chronic hyperglycemia also induces similar neurodegenerative changes. The bidirectional link between the two diseases and the lack of disease-modifying drugs for AD makes it imperative to find new drugs that will target comorbidities and improve cognitive impairment at a cellular level.
Dipeptidyl peptidase-4 (DPP-4) inhibitors have dramatically changed the treatment aspect of diabetes mellitus 10. Vildagliptin, a dipeptidyl peptidase-4 inhibitor affords benefit in diabetic patients by increasing insulin secretion through GLP-1 mediated mechanism. GLP-1 in the brain acts via GLP-1 R (receptors) located in the cortex11, hippocampus11, and cerebellum 11. As reported, GLP-1 can cross the blood-brain barrier12, and prevent neuronal damage through central anti-inflammatory and antiapoptotic effects13. It decreases amyloid protein precursors (APP), Aβ levels, hyperphosphorylated tau protein levels, brain inflammation and ameliorates brain mitochondrial dysfunction11,14.
Aluminium chloride on administration, crosses BBB, accumulates in the hippocampus, frontal cortex and induces neurodegenerative changes (AD). It can induce oxidative stress, neuroinflammation, insulin resistance, synaptic & neuronal damage, cholinergic dysfunction, protein self-aggregation, misfolding, forming senile plaques, neurofibrillary tangles, which are classical hallmarks of Alzheimer’s disease (AD)15–28. With this background, our study was planned to explore the effects of vildagliptin in attenuating neurodegenerative changes in AD alone and with concomitant T2DM on an experimental model of aluminium chloride-induced AD.
Materials and Methods
Animals
Three months aged, male Wistar rats, weighing 140–200 g, were procured from Central Animal Research Facility (CARF) of Manipal University, Manipal. Animals were maintained under 23 ± 2∘C and 50 ± 65% humidity. A 12 h light/dark cycle was maintained. Rats were maintained on normal food and water ad libitum. The experimental protocol was approved by the Institutional Animal Ethics Committee, Kasturba Medical College, Manipal, and was completed as per the guidelines stated by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India.
Drugs and chemicals
Drugs used were obtained as follows: Aluminium chloride (AlCl3) from Merck life sciences private limited, metformin from Franco-Indian Pharmaceuticals Pvt Ltd., rivastigmine from Sun Pharmaceutical Industries Ltd., and vildagliptin from Novartis Healthcare Pvt Ltd. Analytical grade chemicals and reagents were used for the study.
Experimental design
Thirty animals were divided into 5 groups with 6 animals each (Table 1). They were subjected to drug administration as follows:
Table 1: Study design.
| Groups | Drugs, dose, and route of administration (for 30 days) |
| 1. Control | Distilled water orally
|
| 2. Disease model | Aluminium chloride (17 mg/kg) orally
|
| 3. Disease model treated with rivastigmine | Aluminium chloride (17 mg/kg) + rivastigmine (6 mg/ kg) orally
|
| 4. Disease model treated with vildagliptin (Dose 1) | Aluminium chloride (17 mg/kg) + vildagliptin (5 mg/ kg) orally |
| 5. Disease model treated with vildagliptin (Dose 2) | Aluminium chloride (17 mg/kg) + vildagliptin (10 mg/ kg) orally |
Aluminium chloride model of Alzheimer’s disease
Induction of disease was done through oral administration of aluminium chloride (17 mg/kg), once daily for 30 days 29,30.
Spatial memory assessment through Morris water maze (MWM)
Spatial learning and memory were assessed using water maze. The apparatus consisted of a round tank of 1.8 m in diameter placed in a room illuminated by poorly lit white fluorescent lamps with distant visual stimulus. The tank was half-filled with water and made cloudy by adding milk. Four points were assigned N, E, S, W along the circumference of the pool dividing the tank into 4 equal quadrants. A plexiglass escape platform was immersed 2 cm underneath the water surface and paced at mid-point of any one quadrant. The positioning of the escape platform was maintained through the acquisition trials. Experiment was conducted for 5 days, with 4 days of acquisition trials followed by retention test (probe trial) on 5th day of the test. All animals underwent 1 session of 4 trials/day for 4 consecutive days31. Starting points for all trials were randomized. Animals incapable of discovering the platform within 60 s were directed to locate it by us. Animals remained on it for 30 s. The interval between trials was 60 s. On the 5th day, the platform was taken out of the tank, and each animal was allowed to swim for a 60 s31,32. Data were expressed as escape latencies and time spent in the target quadrant.
Sampling and Preparation of Brain Tissue
After MWM was conducted, animals were sacrificed by cervical decapitation. The brain was dissected and washed with ice-cold phosphate buffer (PBS- pH 7.4) thoroughly. Hippocampus was identified and homogenized in PBS (10% w/v, pH 7.4). Centrifugation of the homogenate was done at 4°C for 10 min. at 3000 rpm. Supernatant was carefully separated and was utilized for estimating the levels of acetylcholinesterase.
Acetylcholinesterase assay
Acetylcholinesterase activity was determined as per Ellman et al. 33 method with necessary modifications. Enzyme activity was read spectrophotometrically at 420 nm and calculated based on the changes in absorbance/min32.
Histopathological study
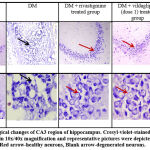
Brain extraction was done after perfusion with ice-cold phosphate-buffered saline and stored in 10% formalin. Brain tissue was dehydrated in varying grades of alcohol and xylene. Tissue was embedded with paraffin wax and coronal sections of 5 µm thickness were sliced from dorsal hippocampus with a rotatory micrometer (Leica RM2245, Leica microsystems, Germany). Each section was mounted on air-dried gelatinized slides34. Every 20th section was selected and 25-30 sections from each slide were stained and mounted on air-dried gelatinized slides. Cresyl violet stain was prepared using a standard protocol34. Each slide was stained with cresyl violet (0.1%) and observed under a light microscope (10X and 40X magnification).
Neuronal quantification in CA3 region of hippocampus
Quantification of healthy neurons in CA3 region of hippocampus was done in a light microscope under 10X magnification (Olympus BX43 microscope with attached DP21 digital camera, Germany). Counting was done by using ImageJ software v1.53.o (National Institute of Health, available free online). Six sections from each rat were used for the counting. The cell counts were expressed as the number of cells per unit length of the cell (cells/200 µm) as described by 35. Well-rounded cells with distinct nuclei and no pyknosis were counted as surviving cells.
Statistical analysis
For analyzing the data, one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. Data analysis was done in SPSS software (version 22). Results were expressed as mean ± SEM. P-value less than 0.05 was set as the level of significance.
Results
Effect of vildagliptin on AlCl3-induced spatial memory deficits
Effect of aluminium chloride and drug treatments on spatial learning memory was assessed by recording escape latencies and time spent in target quadrant through water maze test (figure 1 and 2).
During acquisition trial, control groups and drug treated groups showed improvement in escape latencies. On day 4 of acquisition, control groups and drugs treated groups showed a significant decrease in escape latency to locate the platform in the target quadrant in comparison with aluminium chloride group (p<0.05).
Probe trial demonstrated aluminium chloride group exhibited an escape latency of 45.3 seconds in comparison with control group 2.35 seconds indicative of reduced retention of spatial memory(p<0.05). Rivastigmine treated group showed improved escape latency at 1.49 seconds (in comparison with aluminium chloride group on day 5; however, the spatial memory was better restored in vildagliptin dose 2 treated groups with an escape latency of 1.47 seconds (p<0.05).
Throughout the acquisition trials, a significant increase in time spent in the target quadrant was observed in control and drug treatment groups on day 4 in comparison with aluminium chloride group (p<0.05). A significant decline in time spent in the target quadrant was observed in aluminium chloride group throughout the acquisition trials (p<0.05).
Time spent by aluminium chloride group in target quadrant on the retention trial day was 0.82 seconds indicative of severe decline in spatial learning and memory retention (p<0.05) Rivastigmine with 48.2 seconds, vildagliptin treated groups dose 1 with 43.8 seconds, and dose 2 with 48.7 seconds showed a significant increase in time spent in the target quadrant compared to aluminium chloride group indicating better spatial learning and memory retention (p<0.05).
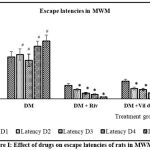 |
Figure 1: Effect of drugs on escape latencies of rats in MWM. |
One way ANOVA followed by Tukey’s post-hoc test showed *vs DM (*p<0.05), #vs Control (#p<0.05), n= 6
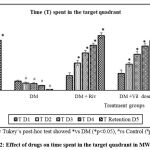 |
Figure 2: Effect of drugs on time spent in the target quadrant in MWM. |
One way ANOVA followed by Tukey’s post-hoc test showed *vs DM (*p<0.05), #vs Control (#p<0.05), n=6
Reversal of AlCl3-induced elevation of acetylcholinesterase activity by vildagliptin
Four weeks of aluminium chloride administration significantly increased AChE activity in comparison with control (p<0.001). Rivastigmine and vildagliptin reduced the AChE activity in comparison with aluminium chloride group (p<0.05) as shown in table 2.
Table 2: Reversal of AlCl3-induced elevation of acetylcholinesterase activity by vildagliptin.
| Groups | Acetylcholinesterase activity(μmol/mg/min)
|
| Control | 0.03078 ± 0.0021* |
| Disease model (DM) | 0.08091 ± 0.0140# |
| DM + Rivastigmine | 0.03568 ± 0.0015* |
| DM + Vildagliptin dose 1 | 0.05120 ± 0.0030* |
| DM + Vildagliptin dose 2 | 0.03043 ± 0.0013* |
One way ANOVA followed by Tukey’s post-hoc test showed *vs DM (*p<0.05), #vs Control (#p<0.05).; n=6
Effect of vildagliptin on histopathological changes and neuronal cell count in CA3 region of the hippocampus
Photomicrographs of CA3 region observed in different groups under light microscope at 10x and 40x magnification are depicted in figure IV. Pyramidal cells in control group of animals presented with well-rounded cells with clear cytoplasm and distinct nucleus. However, aluminium chloride group of animals presented with irregularly shaped hyper-dense stained cells, pyknotic nucleus, lack of well-defined boundary between cytoplasm and nucleus, cellular dispersions with formation of pockets in between cells representing necrotic cells (dead cells). Pyramidal cell count showed a significant decrease compared to control group (p<0.05). Rivastigmine and vildagliptin treated groups, showed a significant improvement in cellular morphology with visibility of clear cytoplasm and distinct nucleus. Drug treatments increased healthy cell count compared to aluminium chloride group (p<0.05). Vildagliptin dose 2 showed a significant increase in healthy cell count compared to vildagliptin dose 1 (p<0.05) as shown in figure III.
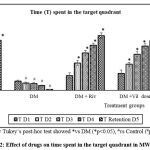 |
Figure 3: Change in the number of CA3 hippocampal neurons in male Wistar rats post aluminium chloride, rivastigmine, and vildagliptin administration. |
One way ANOVA followed by Tukey’s post-hoc test showed *vs DM (*p<0.05), #vs Control (#p<0.05), Vil2 vs Vil1 (α p value < 0.05) respectively; n=6
Discussion
Alzheimer’s disease is multifactorial in origin and current drugs focus on restoring the cholinergic dysfunction. T2DM is a risk factor for AD and both chronic conditions share a bidirectional link between their pathophysiology. Few studies have labelled AD as type 3 diabetes/brain diabetes36,37. We hypothesized that antihyperglycemic drug like DPP-4 inhibitors can have a role in amelioration of the neurodegenerative changes seen in AD.
The cholinergic neurons from the nucleus basalis of Meynert undergo degeneration in Alzheimer’s disease and contribute to the memory loss 38,39. Hence, AChE activity is a key determinant of AD. Our study showed an increase in acetylcholinesterase activity in aluminium chloride group in comparison with control (p<0.001). Vildagliptin and rivastigmine groups showed a significant decline in acetylcholinesterase activity in comparison with aluminium chloride group suggesting that there is an improvement in cognition. Rivastigmine interacts with the esteratic site of brain AchE and increases the availability of brain acetylcholine thereby restoring cholinergic dysfunction 40. It also inhibits AChE in plaques and tangles with the same potency as those in neurons and axons and thus compensates the cholinergic deficit caused by aluminium 41–43. In a transgenic mice model of AD, rivastigmine has modified the levels of several shedding proteins and directed APP processing towards the α-secretase pathway 43. Overall, through these actions, rivastigmine enhances cholinergic functions in the brain and restores functions of neural cells thereby mitigating cognitive deficits. GLP-1 reduces AChE activity, possibly through activation of its central receptor44. Combination of vildagliptin and galantamine has shown a marked decrease in AChE activity45. Although, there is limited evidence to point a finger at the exact mechanism behind decrease in AChE activity with vildagliptin in our study.
Hippocampal degeneration, impaired neuronal network, reduced neurogenesis46, and cognitive46 deficits are seen in AD. CA3 is the largest area in the hippocampus which regulates major cognitive functions and is more prone to oxidative stress and injury. Further, oxidative stress can initiate neuroendocrine alterations within the amygdala, including amygdalar hyperactivity and dendritic shrinking 47. Histopathological examination of cresyl violet-stained hippocampal slices of aluminium chloride group in present study have shown presence of pyknotic cells, densely stained pyramidal cells with irregular morphology with cellular dispersions and loss of cell bodies with their Nissl’s substance connoting neuronal degeneration. Earlier studies have reported similar morphological irregularities in pyramidal cells with aluminium chloride exposure35,48–50. A significant decline in pyramidal cell count in CA3 region of the hippocampus has been observed in aluminium chloride group in this study. The extent of neuronal degeneration seen in CA3 region is high compared to other areas of hippocampus. Aluminium chloride induced hippocampal damage has been reported in other studies as well 49.
Vildagliptin and rivastigmine treatment dramatically mitigated cellular death and damage and improved pyramidal cell count in CA3 region. Treatment with vildagliptin dose 1 and dose 2 effectively caused a significant increase in neuronal cell count and ameliorated neurodegenerative changes seen in AD. Dose 2 proved to be more effective against AD compared to dose 1. Pyramidal neurons in CA3 region of the hippocampus express GLP-1R 51. GLP-1 influences brain metabolism52 and stimulates neuritic growth in CNS neurons52. GLP-1 exerts a protective effect against excitotoxic cell death and toxic Aβ 1-42 53. i.v. administration of GLP-1 has reduced nerve cell damage and improved learning and memory in a mice model 54. Several studies support our findings that vildagliptin improves pyramidal cell count and reverses cognitive deficits induced by aluminium chloride through its neuroprotective effect and probably this also compliments its antiapoptotic effect. Decreased levels of AChE as reported in our study has a potential to improve cholinergic function and ameliorate neuronal death in CA3 of the hippocampus. A study on total and phosphorylated tau in a combined model of AD and T2DM also supports our findings55.
Aluminium chloride group has shown a significant increase in escape latency and a significant decrease in the time spent in the target quadrant. Research findings in MWM are suggestive of impairment of hippocampal functioning and cognition due to aluminium chloride exposure. Vildagliptin and rivastigmine treatment have improved hippocampal functions as evident through decreased escape latency and significantly more time spent by these animals in the target quadrant.
Chronic aluminium chloride exposure interferes with insulin signaling and induces brain insulin resistance. Studies mention that insulin can alter AChE activity and influence cholinergic functions 56. Brain insulin regulates cellular apoptosis, neuronal proliferation, glial cell activity, amyloid-beta protein clearance, tau protein phosphorylation, synaptic plasticity, and memory formation. Impairment in signaling will interfere with these metabolic processes and induce the formation of senile plaque, neurofibrillary tangles, atrophic changes, neuronal damage, and death in the brain leading to irreversible memory and cognitive damage. These neuropathological features represent classical hallmarks of AD and are shared by T2DM 57. Mitochondrial dysfunction also contributes to insulin resistance and is shared by both diseases 58. Mitochondrial dysfunction induces energy deficiency59 and interferes with metabolic processes in the body60.
Studies have reported that GLP-1 has direct central actions on the brain, and possesses neurotrophic effects 54,61–65. DPP-4 inhibition improves glucose metabolism66 through the upregulation of insulin secretion66 and the suppression of glucagon release 66. Vildagliptin enhances glucose-dependent insulin secretion67 and lowers blood glucose67 in T2DM through GLP-167. Vildagliptin has prevented high-fat diet induced brain and hippocampal dysfunction in insulin-resistant rats61. Our research findings and available evidence points toward a multimodal approach of vildagliptin in attenuating neurodegenerative changes seen in Alzheimer’s disease. Treating AD with an antidiabetic drug may decrease the economic burden, and slow disease progression. It may improve the quality of life of patients as well as caregivers. However, studies are required to deduce the exact role vildagliptin plays in Alzheimer’s disease.
Conclusion
This study demonstrated that vildagliptin: i) improved spatial learning and memory retention ii) increased availability of acetylcholine and iii) increased pyramidal cell count in CA3 regions of the hippocampus, iv) attenuated neurodegeneration induced by aluminium chloride in Wistar rats. Based on our findings we conclude that vildagliptin may serve as a potential drug for attenuating neuropathological changes in AD.
Acknowledgment
The authors would like to acknowledge Manipal Academy of Higher education for the support.
Conflict of Interests
The authors declare that they have no conflict of interests.
Funding Sources
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
References
- Perl, D. P. Neuropathology of Alzheimer’s disease. Mt. Sinai J. Med. 77, 32–42 (2010).
- Carlesimo, G. A. & Oscar-Berman, M. Memory Deficits in Alzheimer’s Patients: A Comprehensive Review. Neuropsychology Review vol. 3 (1992).
- 2021 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 17, 327–406 (2021).
- Thal, D. R., Walter, J., Saido, T. C. & Fändrich, M. Neuropathology and biochemistry of Aβ and its aggregates in Alzheimer’s disease. Acta Neuropathol. 129, 167–182 (2015).
- Association, A. 2021 Alzheimer’s disease facts and figures: Race , Ethnicity and Alzheimer’s in America. Race, Ethn. Alzheimer’s Am. 13, 1–104 (2021).
- Lin, L. Commonality between Diabetes and Alzheimer’s Disease and a New Strategy for the Therapy. Clin. Med. Pathol. 1, CPath.S667 (2008).
- Peila, R., Rodriguez, B. L. & Launer, L. J. Type 2 Diabetes, APOE Gene, and the Risk for Dementia and Related Pathologies The Honolulu-Asia Aging Study. Diabetes vol. 51 http://diabetesjournals.org/diabetes/article-pdf/51/4/1256/372544/db0402001256.pdf (2002).
- Verdile, G., Fuller, S. J. & Martins, R. N. The role of type 2 diabetes in neurodegeneration. Neurobiol. Dis. 84, 22–38 (2015).
- Chornenkyy, Y., Wang, W. X., Wei, A. & Nelson, P. T. Alzheimer’s disease and type 2 diabetes mellitus are distinct diseases with potential overlapping metabolic dysfunction upstream of observed cognitive decline. Brain Pathology vol. 29 (2019).
- Sakr, H. F. Effect of sitagliptin on the working memory and reference memory in type 2 diabetic sprague-dawley rats: Possible role of adiponectin receptors 1. J. Physiol. Pharmacol. 64, 613–623 (2013).
- Kosaraju, J. et al. Vildagliptin: An anti-diabetes agent ameliorates cognitive deficits and pathology observed in streptozotocin-induced Alzheimer’s disease. J. Pharm. Pharmacol. 65, 1773–1784 (2013).
- Athauda, D. & Foltynie, T. The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson’s disease: Mechanisms of action. Drug Discov. Today 21, 802–818 (2016).
- Abdelsalam, R. M. & Safar, M. M. Neuroprotective effects of vildagliptin in rat rotenone Parkinson’s disease model: Role of RAGE-NFκB and Nrf2-antioxidant signaling pathways. J. Neurochem. 133, 700–707 (2015).
- Bae, C. S. & Song, J. The role of glucagon-like peptide 1 (GLP1) in type 3 diabetes: GLP-1 controls insulin resistance, neuroinflammation and neurogenesis in the brain. Int. J. Mol. Sci. 18, 16–18 (2017).
- Chakrabarty Mrinmoy, Bhat Priyanka, Kumari Sweta, D’souza Avin, Bairy K. L., Chaturvedi Abhishek. Natarajan Archana, Rao K.G. Mohandas, K. S. Cortico-hippocampal salvage in chronic aluminium induced neurodegeneration by mrinmoy.pdf. 161–171 (2012).
- Thippeswamy, A. H. et al. Evaluation of Bacopa monniera for its Synergistic Activity with Rivastigmine in Reversing Aluminum- Induced Memory Loss and Learning Deficit in Rats. 6, 208–213 (2013).
- Igwenagu, E., Igbokwe, I. O. & Egbe-Nwiyi, T. N. Fasting hyperglycaemia, glucose intolerance and pancreatic islet necrosis in albino rats associated with subchronic oral aluminium chloride exposure. Comp. Clin. Path. 29, 75–81 (2020).
- Buraimoh, A. A., Ojo, S. A., Hambolu, J. O. & Adebisi, S. S. Effects of Oral Administration of Aluminium Chloride on the Histology of the Hippocampus of Wistar Rats. Curr. Res. J. Biol. Sci. 3, 509–515 (2011).
- Kawahara, M. & Kato-Negishi, M. Link between aluminum and the pathogenesis of Alzheimer’s disease: The integration of the aluminum and amyloid cascade hypotheses. Int. J. Alzheimers. Dis. 2011, (2011).
- Vojdani, A. toxics Elevated IgG Antibody to Aluminum Bound to Human Serum Albumin in Patients with Crohn’s, Celiac and Alzheimer’s Disease. (2021) doi:10.3390/toxics9090212.
- Ayush Kumar, Sanchari Basu Mallik, Samita Rijal, Nilanjan Changdar, Jayesh Mudgal, R. R. S. Dietary Oils Ameliorate Aluminum Chloride-Induced Memory Deficit in Wistar Rats. Pharmacogn. Mag. 13 (Suppl, 179–188 (2017).
- Praveenkumar, S. E. et al. Amelioration of aluminium chloride (AlCl3) induced neurotoxicity by combination of rivastigmine and memantine with artesunate in Albino Wistar rats. Biomed. Pharmacol. J. 12, 703–711 (2019).
- Ahmed, H. H., Salem, A. M., Sabry, G. M., Husein, A. A. & Kotob, S. E. New insights in the horizon for the treatment of Alzheimer’s Disease: A proposal based on experimental study. Der Pharm. Lett. 7, 165–182 (2015).
- Abdel-Wahab, W. M. AlCl3-Induced Toxicity and Oxidative Stress in Liver of Male Rats: Protection by Melatoni. Life Sci. J. 9, (2012).
- Kalaiselvi, A., Reddy, G. A. & Ramalingam, V. Ameliorating Effect of Ginger Extract ( Z ingiber officinale Roscoe ) on Liver Marker Enzymes , Lipid Profile in Aluminium chloride Induced Male Rats. Int. J. Pharm. Sci. Drug Res. 7, 52–58 (2015).
- N.R. Naidu, S. Bhat, D. U. Effect of Long Term Administration of Aluminium Chloride on. Int. J. Pharm. Biol. Sci. 3, 616–622 (2013).
- Kumar, A., Prakash, A. & Dogra, S. Neuroprotective effect of carvedilol against aluminium induced toxicity: Possible behavioral and biochemical alterations in rats. Pharmacol. Reports 63, 915–923 (2011).
- Konda, V. et al. Effect of aluminum chloride on blood glucose level and lipid profile in normal, diabetic and treated diabetic rats. Indian J. Pharmacol. 49, 357–365 (2017).
- Nivsarkar, M. et al. Reduction in Aluminum Induced Oxidative Stress by Meloxicam in Rat Brain. 10, 151–155 (2006).
- Yassin, N. A. Z. et al. Effect of Boswellia serrata on Alzheimer ’ s disease induced in rats. 8, 1–11 (2013).
- Chakrabarty, M. et al. Cortico hippocampal salvage in chronic aluminium induced neurodegeneration by Celastrus paniculatus seed oil : Neurobehavioural , biochemical , histological study. 3, 161–171 (2012).
- Fernandes, J. et al. N-acetyl-L-tryptophan, a substance-P receptor antagonist attenuates aluminum-induced spatial memory deficit in rats. Toxicol. Mech. Methods 28, 328–334 (2018).
- Ellman L. George, Courtney, K. D. & Francisco, S. A new and rapid colorimetric of acetylcholinesterase determination. Biochem. J. 7, 88–95 (1961).
- Hossain, M., Chetana, M. & Uma Devi, P. Late effect of prenatal irradiation on the hippocampal histology and brain weight in adult mice. Int. J. Dev. Neurosci. 23, 307–313 (2005).
- Madhyastha, S., Somayaji, S. N., Rao, M. S., Nalini, K. & Bairy, K. L. Hippocampal brain amines in methotrexate-induced learning and memory deficit. Can. J. Physiol. Pharmacol. 80, 1076–1084 (2002).
- Suzanne M. de la Monte. Insulin resistance and Alzheimer’s disease. BMB Rep. 42, 475–481 (2009).
- Nguyen, T. T. et al. Type 3 Diabetes and Its Role Implications in Alzheimer’s Disease. Int. J. Mol. Sci. 21, (2020).
- Whitehouse, P. J. et al. Alzheimer’s disease and senile dementia: Loss of neurons in the basal forebrain. Science (80-. ). 215, 1237–1239 (1982).
- Whitehouse, P. J., Price, D. L., Clark, A. W., Coyle, J. T. & DeLong, M. R. Alzheimer disease: Evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann. Neurol. 10, 122–126 (1981).
- Dash Devasrita , Chogtu Bharti, K. B. L. Metformin Attenuates Cognitive Deficits in Experimentally Induced Alzheimer’s Disease. J. Glob. Pharma Technol. 1, 8–12 (2017).
- Tayebati, S. K., Di Tullio, M. A. & Amenta, F. Effect of treatment with the cholinesterase inhibitor rivastigmine on vesicular acetylcholine transporter and choline acetyltransferase in rat brain. Clin. Exp. Hypertens. 26, 363–373 (2004).
- Sharma, K. Cholinesterase inhibitors as Alzheimer’s therapeutics (Review). Mol. Med. Rep. 20, 1479–1487 (2019).
- Bailey, J. A., Ray, B., Greig, N. H. & Lahiri, D. K. Rivastigmine lowers Aβ and increases sAPPα levels, which parallel elevated synaptic markers and metabolic activity in degenerating primary rat neurons. PLoS One 6, (2011).
- Ali, M. A., El-Abhar, H. S., Kamel, M. A. & Attia, A. S. Antidiabetic Effect of Galantamine: Novel Effect for a Known Centrally Acting Drug. (2015) doi:10.1371/journal.pone.0134648.
- Ali, M. A., El-Abhar, H. S., Kamel, M. A. & Attia, A. S. Antidiabetic effect of galantamine: Novel effect for a known centrally acting drug. PLoS ONE vol. 10 (2015).
- Xiao, L. et al. Neurotrophic factor-α1, a novel tropin is critical for the prevention of stress-induced hippocampal CA3 cell death and cognitive dysfunction in mice: comparison to BDNF. Transl. Psychiatry 11, (2021).
- Salim, S. Minireviews Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. J Pharmacol Exp Ther 360, 201–205 (2017).
- Cherian SB,, Bairy KL, R. M. Effect of chronic prenatal restraint stress on hippocampal neuronal cell density in male and female wistar rats at weaning. Int. J. Recent Sci. Res. 8, 17153–17158 (2017).
- Adelodun, S. T. et al. Aluminium chloride-induced hippocampal damage: CA3 hippocampal subfield involvement and the neuroprotective role of Buchholzia coriacea ethanolic seed extract. Phytomedicine Plus vol. 1 100104 (2021).
- Barkur, R. R. & Bairy, L. K. Evaluation of passive avoidance learning and spatial memory in rats exposed to low levels of lead during specific periods of early brain development. Int. J. Occup. Med. Environ. Health 28, 533–544 (2015).
- Chalichem, N. S. S., Gonugunta, C., Krishnamurthy, P. T. & Duraiswamy, B. DPP4 Inhibitors Can Be a Drug of Choice for Type 3 Diabetes: A Mini Review. American Journal of Alzheimer’s Disease and other Dementias vol. 32 (2017).
- Rizzo, M. R. et al. Dipeptidyl peptidase-4 inhibitors have protective effect on cognitive impairment in aged diabetic patients with mild cognitive impairment. Journals Gerontol. – Ser. A Biol. Sci. Med. Sci. 69, 1122–1131 (2014).
- Perry, T. & Greig, N. Enhancing Central Nervous System Endogenous GLP-1 Receptor Pathways for Intervention in Alzheimers Disease. Curr. Alzheimer Res. 2, 377–385 (2005).
- During, M. J. et al. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat. Med. 9, 1173–1179 (2003).
- Khalaf, S. S., Hafez, M. M., Mehanna, E. T., Mesbah, N. M. & Abo-Elmatty, D. M. Combined vildagliptin and memantine treatment downregulates expression of amyloid precursor protein, and total and phosphorylated tau in a rat model of combined Alzheimer’s disease and type 2 diabetes. Naunyn. Schmiedebergs. Arch. Pharmacol. 392, 685–695 (2019).
- Jamshidnejad-Tosaramandani, T., Kashanian, S., Babaei, M., Al-Sabri, M. H. & Schiöth, H. B. The potential effect of insulin on ache and its interactions with rivastigmine in vitro. Pharmaceuticals 14, 1–11 (2021).
- Zina Kroner, D. The Relationship between Alzheimer’s Disease and Diabetes: Type 3 Diabetes? Altern. Med. Rev. 14, 373 (2009).
- Montgomery, M. K. & Turner, N. Mitochondrial dysfunction and insulin resistance : an update. Endocr. Connect. 4, R1–R15 (2015).
- Xu, F. et al. Aluminum chloride caused liver dysfunction and mitochondrial energy metabolism disorder in rat. J. Inorg. Biochem. 174, 55–62 (2017).
- Moreira, P. I., Carvalho, C., Zhu, X., Smith, M. A. & Perry, G. Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochim. Biophys. Acta – Mol. Basis Dis. 1802, 2–10 (2010).
- Pintana, H., Apaijai, N., Chattipakorn, N. & Chattipakorn, S. C. DPP-4 inhibitors improve cognition and brain mitochondrial function of insulin-resistant rats. J. Endocrinol. 218, 1–11 (2013).
- Yuriko et al. The dipeptidyl peptidase-4 inhibitor linagliptin ameliorates high-fat induced cognitive decline in tauopathy model mice. Int. J. Mol. Sci. 20, (2019).
- Perry, T. & Greig, N. A New Alzheimers Disease Interventive Strategy: GLP-1. Curr. Drug Targets 5, 565–571 (2004).
- Zhang, D. D. et al. Vildagliptin, a DPP4 inhibitor, alleviates diabetes-associated cognitive deficits by decreasing the levels of apoptosis-related proteins in the rat hippocampus. Exp. Ther. Med. 15, 5100–5106 (2018).
- Li, Y., Zheng, M., Sah, S. K., Mishra, A. & Singh, Y. Neuroprotective influence of sitagliptin against cisplatin-induced neurotoxicity, biochemical and behavioral alterations in Wistar rats. Mol. Cell. Biochem. 455, (2019).
- Pujadas, G. et al. The dipeptidyl peptidase-4 (DPP-4) inhibitor teneligliptin functions as antioxidant on human endothelial cells exposed to chronic hyperglycemia and metabolic high-glucose memory. Endocrine 56, (2017).
- Abbas, T., Faivre, E. & Hölscher, C. Impairment of synaptic plasticity and memory formation in GLP-1 receptor KO mice: Interaction between type 2 diabetes and Alzheimer’s disease. Behav. Brain Res. 205, 265–271 (2009).