Sara A.Aldossary1* and Abdulla Al-Taher2
and Abdulla Al-Taher2
1Department of pharmaceutical sciences, college of clinical pharmacy, king Faisal university, alahsa, Saudi Arabia.
2Department of biomedical sciences, college of vetenery medicine, king Faisal university, alahsa, Saudi Arabia.
Corresponding Author E-mail: saldossary@kfu.edu.sa
DOI : https://dx.doi.org/10.13005/bpj/2472
Abstract
Arsenic is considered to be toxic when it is in an inorganic state and basic sources are contamination of water, smoking tobacco and irrigation of food crops. Sesamol is liposoluble lignans extraction that is used in rats to reduce skin papillomas. The main aim of the study is to study the effect of toxic arsenic on rats, usage of sesamol in treating hepato and nephrotoxicity in rats and analyze kidney tissue and liver tissues of the rats. The study primarily focuses on the effect of injecting arsenic and sesamol to the group of animals or injecting both and analyzing them to understand hepatotoxicity and nephrotoxicity. Sesamol along with arsenic powder have been used and the rats were kept in standard condition. The laboratory experiment has been carried out where both single and double oral treatments were provided. Animals were grouped into four groups where every 4 groups have eight rats each. SPPS software has been used to analyze the data collected. It has been shown treated rats with sesamol, counter act the toxicity effect of arsenic upon liver and kidney tissues. It has been found that sesamol has a protective effect upon arsenic induced liver and nephrotoxicity in rats.
Keywords
Arsenic; Hepato; Immunohistochem Kidney; Sesamol; Toxicity
Download this article as:| Copy the following to cite this article: Aldossary S. A, Al-Taher A. Effect of Sesamol on Arsenic Induced Hepato and Nephrotoxicity in Rats. Biomed Pharmacol J 2022;15(3). |
| Copy the following to cite this URL: Aldossary S. A, Al-Taher A. Effect of Sesamol on Arsenic Induced Hepato and Nephrotoxicity in Rats. Biomed Pharmacol J 2022;15(3). Available from: https://bit.ly/3Dd5vWu |
Introduction
Arsenic is considered to be a natural element in the crust of the earth and is distributed in an overall environment of air, water as well as land. It is toxic when it is inorganic and the most toxic inorganic forms include Arsenate and Arsenite that have various mechanisms depending on the state of valence. Though homicides of arsenic receive publicity from the media, the basic source of toxicity of arsenic to the public is through contamination of water and utilizing that water in preparation of food and irrigation of food crops, eating food that is contaminated and smoking tobacco. Exposure in a longer period to toxic arsenic through drinking water and food can lead to chronic arsenic poisoning 1-3. Characteristic effects include skin lesions and cancer. Arsenic release into the environment propagates via weathering and the process of mining along with other phenomena like volcanic activity. Compounds of inorganic arsenic are toxic at a high level whereas compounds of organic arsenic tend to be less harmful to human health. Due to the toxicity of arsenic, the symptoms of acute poisoning of arsenic are vomiting, abdominal pain along with diarrhea, muscle cramping and sometimes death in extreme cases 3-4. In case of long term exposure, higher degrees of arsenic in the inorganic form is detected in skin and have changes in pigmentation, skin lesion and patches on palm and foot sole. Along with skin cancer, it also leads to lungs cancer and bladder cancer. Other adverse effects of toxic arsenic include developmental impact, diabetes, pulmonary disease and disease of cardiovascular5. It also leads to adverse pregnancy and infant mortality affecting child health and in early childhood, it is linked with enhancement in mortality in young people because of various cancer, lung cancer, failure in kidney and heart attacks 5.
Sesamol is a liposoluble lignans extraction along with a prominent fragrance component in sesame oil that is used to demonstrate activities of potential anticancer. The water-soluble lignin sesamol which is a natural compound, is derived from Sesamum Indicum seed oil and is considered as a potent antioxidant along with a significant potent anticancer [6]. This sesamol protects against atherosclerosis, hypertension and ageing along with healing of the wound, antioxidant, anti-inflammatory and free radical activity of scavenging 5. In the case of rats, it was evident that sesamol provides a 50% reduction in skin papillomas within 20 weeks after providing 12-O-tetradecanoylphorbol 13-acetate 6. After treatment of sesamol, plasma cholesterol along with the level of triacylglycerol were reduced and thus it was possibly inhibited HMG CoA reductase activity or activity of lipoprotein lipase (Sesamol has significantly reduced tumor burden along with lipid peroxidation and raised the level of antioxidants. This leads to inhibition of the development of a tumor on the skin along with promotion. Apoptosis in cells of the tumor was evident to cause by downregulation of Bcl-2 and stimulation of Bcl-2 that is aligned with an expression of X protein and is administered with free sesamol and encapsulated sesamol 7.
The main objectives of the study are:
The effect of toxicity of arsenic on Sprague Dawley rats.
The usage of sesamol in treating hepatotoxicity and nephrotoxicity induced by arsenic in rats.
To examine Histopathology in the liver and kidney of rats.
Material and Method
Drugs
The powders along with Sesamol and arsenic were collected from Sigma Chemical Co., USA. In 1% of an aqueous solution of Tween 80, Sesamol was made whereas arsenic was made in normal saline which was stabilized through gum of 0.2%.
Animals and treatments
Male rat Sprague Dawley having weight approx. 200 g were collected from Animal House, College of Medicine, King Faisal University. These rats were preserved in the standard condition of approx. 24℃ temperature, approx. 45% humidity and 12hr light or dark cycle. They were then supplied along with standard laboratory chow as well as water ad libitum. The rats were kept being acclimatized for a week before the conduction of experiments.
Ten hours before the experiment, animals were grouped in random process in four groups with every group having eight rats. A single dose of oral treatment of normal saline is stabilized through 0.2% gum that was provided to the first group and is served as control. In the group of the second, hepato and nephrotoxicity was induced by injected arsenic in rats. 3rd group was provided with arsenic with twice the dose of oral which is about 10mg/kg. Moreover both the 2nd and 3rd group of rats recieved two injections of either sesamol of dose of 50mg/kg or vehicle of sesamol which is of 1% aqueous solution of Tween 80 after administration of arsenic. The 4th rats’ group were provided with sesamol in absence of arsenic hepato and nephrotoxicity induction.
The protocol of the experiment was approved through the Local Animal Care committee along with procedures conducted aligning with international guidelines based on care and utilization of laboratory species.
Sample preparation and biochemical studies
The animals were killed a day after the administration of arsenic. Gathering blood samples was conducted and they were left for 1 hour to clot. After that, blood was centrifuged for 10 minutes at 5000 rpm to get a pure serum that was then stored at 20-degree temperature. To evaluate serum aspartate aminotransferase as well as alanine aminotransferase degree based on manufacturer named Randox Laboratories Ltd. UK, recommending, colourimetric kits there were utilized.
Firstly the removal of the liver along with kidney tissue has been done, they were washed with ice-cold saline and were stored at 80-degree temperature. Utilizing cold potassium phosphate buffer, the liver and kidney were homogenized. Homogenates were separated at 5000 rpm for 10 minutes at 4℃ and the supernatant was utilized to determine the malondialdehyde and to minimize the glutathione degree and to view the activity of catalase utilizing colourimetric kits of the assay as framed by manufacturer’s instruction of bio-diagnostic 8. Depending on the manufacturer, Cayman Chemical Co, USA, instruction the degree of Nitric Oxide (NO) was assayed through utilizing a kit of the colourimetric assay.
Exam of Histopathological of liver tissue and Kidney tissue
The samples of tissues of liver and kidney from every rat were fixed in 10% formalin that were subjected to dehydration in grades of ascending of alcohol and after that, they were embedded in paraffin. Sections were cut into 4m, which were then marked with hematoxylin along with eosin and investigated through utilizing a light microscope via a pathologist not aware regarding treatment protocol 9.
Statistical analysis
To determine the data gathered, every value is expressed in mean ±S.E.M. one-way variance analysis was conducted followed by a Tukey test for multiple comparing. SPSS version 21 have been utilized and the difference is analyzed at the significance degree p<0.5.
Results
Impact of sesamol on the measured biochemical parameters.
Table 1: Effect of sesamol on level of antioxidants in Arsenic that is induced through hepatotoxicity in animals.
| GSH (mg/g tissue) | NO (nmol/100mg tissue) | MDA | CATALASE(u/g tissue) | Dose (mg/kg) | Treatment |
| 117.6±1.6 | 90.4±0.4 | 24.20±0.66 | 5.7±0.37 | Control | |
| 108±5.3 a | 105.3±1.15 a | 53.11±1.13 a | 3.2±0.52 a | 10mg/kg | Arsenic |
| 137.4±5.9 |
142.7±1.8 |
34.8±0.87 | 4.8±0.6 | 50mg/kg | Sesamol |
| 115.6±1.9 b | 119.7±1.04 b | 45.26±0.92 b | 5.5±0.34 b | Arsenic+sesamol |
*MDA:malondialdehyde , NO: nitric oxide, GSH: glutathione.
All the values are expressed as mean ±S.E.M., n = 8in each group
a P < 0.05vs.controlgroup.
b P < 0.05vs.arsenic group
The table above illustrates the effect of sesamol on antioxidants degree in arsenic which induced hepatotoxicity in animals. Glutathione in the case of treating with arsenic is found to be 108±5.3a and sesamol is about 137.4± 5.9. While in the case of treating both arsenic and sesamol, the Glutathione level is 115.6± 1.9b. This reveals that the level of effect of sesamol treatment is higher on an antioxidant degree in arsenic that leads to hepatotoxicity among animals.
Table 2: impact of sesamol on renal malondialdehyde (MDA), reduce glutathione (GSH) and NO level and catalase in rats which exposed to arsenic nephrotoxicity.
| Arsenic+sesamol | sesamol | arsenic | control | |
| 25.19±0.37 b | 30.78±0.24 | 37.6±0.33 a | 27.21±0.39 | Mda(nmol/g tissue) |
| 3.74±0.06 b | 4.01±0.15 | 3.2±0.12 a | 4.7±0.08 | GSH(mmol/g tissue) |
| 0.114±0.016 b | 0.118±0.013 | 0.129±0.02 a | 0.102±0.011 | No(nmol/g tissue) |
| 0.77±0.05 b | 1.28±0.05 | 0.54±0.07 a | 1.33±0.05 | Catalase(U/g tissue) |
All the values are expressed as mean ±S.E.M., n = 8ineachgroup.
a P < 0.05vs.controlgroup.
b P < 0.05vs.arsenic group
In table 2 it has been evident that while injecting arsenic, the level of MDA is 37.6± 0.33, in the case of sesamol it is 30.78±0.24 and while injecting both Arsenic and sesamol it is 25.19± 0.37b. The level of GSH while injecting arsenic is 3.2±0.12a, in the case of sesamol it is 4.01±0.15 and injecting both arsenic and sesamol is 3.74±0.06b.
Table 3: impact of sesamol treatment on blood urea nitrogen and level of serum creatinine in rats exposed to arsenic nephrotoxicity.
| Arsenic+sesamol | sesamol | arsenic | control | |
| 28.94±0.53 b | 16.14±0.57 | 37.87±0.72 a | 12.71±0.21 | BUN (mg/dl) |
| 1.85± 0.02 b | 1.49± 0.02 | 2.9± 0.04 a | 1.5± 0.02 | Serum creatinine (mg/dl) |
All the values are expressed as mean ±S.E.M., n = 8ineachgroup.
a P < 0.05vs.controlgroup.
b P < 0.05vs.arsenic group
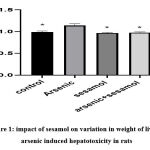 |
Figure 1: impact of sesamol on variation in weight of liver of arsenic induced hepatotoxicity in rats. |
From the above graph, it has been found through ‘t’ test that while injecting arsenic to rats exposed to hepatotoxicity, an inclination in weight has been observed in the liver than that of injecting sesamol and both arsenic and sesamol altogether.
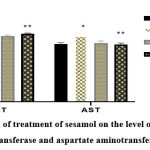 |
Figure 2: Effect of treatment of sesamol on the level of serum of alanine aminotransferase and aspartate aminotransferase in rats. |
The above graph depicts that in the case of injecting arsenic along with sesamol, the serum enzyme level of alanine aminotransferase increased and in the case of aspartate aminotransferase while injecting arsenic, the serum enzyme level increased.
Effects of sesamol on liver and kidney histopathology
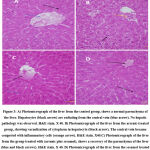 |
Figure 3: A) Photomicrograph of the liver from the control group, shows a normal parenchyma of the liver. Hepatocytes (black arrow) are radiating from the central vein (blue arrow). |
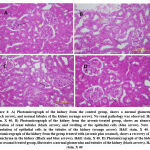 |
Figure 4: A) Photomicrograph of the kidney from the control group, shows a normal glomerulus(black arrow), and normal tubules of the kidney (orange arrow). No renal pathology was observed. |
In figure 4 the animals falling under the control group (figure 4,A)shows normal glomerulus and normal renal tubules of the kidney. There exists no dilation in renal tubules and no such cytoplasmic vacuolization of renal tubules was observed (fig4,A) 12.The animals falling under the group of arsenic-treated show abnormal dilation of renal tubules and there is a swelling of epithelial cells [13]. Cytoplasmic vacuolization of epithelial cells was observed (fig4, B). The animals falling under the group treated with arsenic along with sesamol show the recovery of kidney parenchyma (fig4, C). The animals falling under the sesamol treated group exhibit normal glomerulus along with tubules of the kidney (fig4, D).
Discussion
The results shows that while treating the rats with sesamol on renal malondialdehyde. There was a reduction in the level of glutathione as well as nitric oxide and catalase in rats that were exposed to arsenic nephrotoxicity 10. Table 3 shows the effect of sesamol on BUN and serum creatinine levels in rats when they were exposed to arsenic nephrotoxicity. It has been seen that when injecting arsenic, Blood urea nitrogen (BUN) increases and in the case of serum creatinine level it also increased. When injected with Sesamol both BUN and Serum Creatinine are higher among rat when exposed to arsenic nephrotoxicity. When both Arsenic and sesamol are injected to rats, both the level of BUN and Serum are increased than the standard range. Treatment of acute arsenic has led to marked damage of the liver like ballooning degeneration, centrilobular necrosis along with cytoplasmic vacuolation of hepatocytes with sinusoidal congestion (fig-3B). Markedly the treatment of sesamol attenuated the injury of tissue when induced with arsenic and restored the same kind of histopathological figure which was seen in the control group (fig-3C) 11. In the control group (fig3A) animals shows normal liver parenchyma. It was observed that there was radiation of hepatocytes from a central vein and no such hepatic pathology is being observed. Among the animals belonging to the group treated by arsenic, it was observed cytoplasmic vacuolization in hepatocytes. Moreover, it has been observed that the central vein has become congested with that of inflammatory cells. In the group, the animals are treated with both arsenic along with sesamol that indicates a recovery of liver parenchyma. Furthermore, the rats under the group of sesamol treated, exhibit normal liver parenchyma. The animals of hepatocyte were generally compared with the animals from the group of control. From the figure, it has been depicted that while treating acute arsenic there is marked damage of the liver such as ballooning degeneration, centrilobular necrosis and cytoplasmic vacuolation of hepatocytes as well as sinusoidal congestion. While treating with sesamol, the injury of tissue when injected with arsenic restores a similar kind of histopathological figure in the control group. Normal liver parenchyma has been seen along with the radiation of hepatocytes from a central vein and no hepatic pathology is observed. The group of animals who are treated with arsenic suffered from cytoplasmic vacuolization in hepatocytes 20. In this case, the central vein is congested along with inflammatory cells. Animals, when treated with both arsenic and sesamol, recover from liver parenchyma but when treated with only sesamol it leads to normal liver parenchyma. The animals of the control group having normal glomerulus and renal tubules of the kidney do not face dilation in renal tubules and also cytoplasmic vacuolization of renal tubules. The animals who are being treated with arsenic face abnormal dilation of renal tubules and thus leading to the swelling of epithelial cells. These animals suffer from Cytoplasmic vacuolization of epithelial cells. Those animals who are being treated with both arsenic and sesamol are found to be recovered from kidney parenchyma whereas when treated with sesamol normal glomerulus were found along with kidney tubules.
Conclusion
From the findings, it has been evident that there is a higher effect of sesamol treatment on the level of antioxidants in arsenic which leads to hepatotoxicity among the animals. This leads the liver to develop inflammation as the animal is exposed to toxic substances like arsenic. In such cases, the liver normally removes and breaks down the drugs along with chemicals from the bloodstream 14-16. The level of MDA after injecting arsenic tends to be higher leading to the decrease in the level of GSH and is lower in the case of injecting sesamol leading to the increase in GSH level 14. It has also been evident that at the time of treating with sesamol on renal malondialdehyde leads to the reduction of the degree of glutathione along with nitric oxide as well as catalase in rats which is exposed to arsenic nephrotoxicity17. This leads to a lower concentration of Glutathione in plasma from the vein of hepatic that was not higher than that of concentration in plasma from the portal vein and from the aorta that indicates depleted liver was not releasing glutathione into plasma 18-19. It was also evident that when the rat is exposed to nephrotoxicity while injecting arsenic BUN and serum creatinine is increased as well as injecting arsenic and sesamol both lead to an increase in the level of BUN and serum Creatinine. Through ‘t’ test it has been evident that while injecting arsenic to a rat that is exposed to hepatotoxicity they gained in weight of liver than that of injecting either sesamol or both arsenic and sesamol. There is also a rise in serum enzyme level of alanine aminotransferase when injecting arsenic and an increase in the level of serum enzyme of aspartate aminotransferase is seen in the case of injecting arsenic.
Conflict of Interest
There is no conflict of interest.
Funding Sources
There is no funding source
References
- Azab, A.E., Albasha, M.O. and Elsayed, A.S.I., 2017. Prevention of nephropathy by some natural sources of antioxidants. Yangtze Medicine, 1(04), p.235.
CrossRef - Hasanein, P. and Emamjomeh, A., 2019. Beneficial effects of natural compounds on heavy metal-induced hepatotoxicity. In Dietary Interventions in Liver Disease(pp. 345-355). Academic Press.
CrossRef - Kondamudi, P.K., Kovelamudi, H., Mathew, G., Nayak, P.G., Rao, M.C. and Shenoy, R.R., 2014. Investigation of sesamol on myeloperoxidase and colon morphology in acetic acid-induced inflammatory bowel disorder in albino rats. The Scientific World Journal, 2014.
CrossRef - Chi, L., Xue, J., Tu, P., Lai, Y., Ru, H. and Lu, K., 2019. Gut microbiome disruption altered the biotransformation and liver toxicity of arsenic in mice. Archives of toxicology, 93(1), pp.25-35.
CrossRef - Kumar, N., Mudgal, J., Parihar, V.K., Nayak, P.G., Kutty, N.G. and Rao, C.M., 2013. Sesamol treatment reduces plasma cholesterol and triacylglycerol levels in mouse models of acute and chronic hyperlipidemia. Lipids, 48(6), pp.633-638.
CrossRef - Liu, Z., Ren, B., Wang, Y., Zou, C., Qiao, Q., Diao, Z., Mi, Y., Zhu, D. and Liu, X., 2017. Sesamol induces human hepatocellular carcinoma cells apoptosis by impairing mitochondrial function and suppressing autophagy. Scientific reports, 7(1), pp.1-12.
CrossRef - Mahmoud, N., 2014. PROTECTIVE EFFECT OF ALLOPURINOL ON PARACETAMOL-INDUCED LIVER INJURY IN RATS. Al-Azhar Journal of Pharmaceutical Sciences, 50(2), pp.117-136.
CrossRef - com. 2021. Sesamol. [online] Available at: <https://www.mdedge.com/dermatology/article/163528/aesthetic-dermatology/sesamol> [Accessed 22 October 2021].
- Pavle Randjelovic, I., 2021. Gentamicin nephrotoxicity in animals: Current knowledge and future perspectives. [online] PubMed Central (PMC). Available at: <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5427480/> [Accessed 22 October 2021].
- Ren, B., Yuan, T., Zhang, X., Wang, L., Pan, J., Liu, Y., Zhao, B., Zhao, W., Liu, Z. and Liu, X., 2020. Protective effects of sesamol on systemic inflammation and cognitive impairment in aging mice. Journal of agricultural and food chemistry, 68(10), pp.3099-3111.
CrossRef - Liu, W., Wang, B., Zhao, Y., Wu, Z., Dong, A., Chen, H., Lin, L., Lu, J. and Hai, X., 2021. Pharmacokinetic characteristics, tissue bioaccumulation and toxicity profiles of oral arsenic trioxide in rats: implications for the treatment and risk assessment of acute promyelocytic leukemia. Frontiers in Pharmacology, 12.
CrossRef - Bodaghi-Namileh, V., Sepand, M.R., Omidi, A., Aghsami, M., Seyednejad, S.A., Kasirzadeh, S. and Sabzevari, O., 2018. Acetyl-l-carnitine attenuates arsenic-induced liver injury by abrogation of mitochondrial dysfunction, inflammation, and apoptosis in rats. Environmental toxicology and pharmacology, 58, pp.11-20.
CrossRef - Al Aboud, D., Baty, R.S., Alsharif, K.F., Hassan, K.E., Zhery, A.S., Habotta, O.A., Elmahallawy, E.K., Amin, H.K., Moneim, A.E.A. and Kassab, R.B., 2021. Protective efficacy of thymoquinone or ebselen separately against arsenic-induced hepatotoxicity in rat. Environmental Science and Pollution Research, 28(5), pp.6195-6206.
CrossRef - Ahmed, K.A., Korany, R.M.S., El Halawany, H.A. and Ahmed, K.S., 2019. Spirulina platensis alleviates arsenic-induced toxicity in male rats: biochemical, histopathological and immunohistochemical studies. Adv Anim Vet Sci, 7(8), pp.701-710.
CrossRef - Ramadan, S.S., Almeer, R., Albasher, G. and Abdel Moneim, A.E., 2021. Lycopene mitigates arsenic-induced nephrotoxicity with activation of the Nrf2 pathway in mice. Toxin reviews, pp.1-11.
CrossRef - WS, W.M.S., Norlelawati, A.T., Aung, S. and Zunariah, B., 2021. Histopathological Changes in Chronic Low Dose Organic Arsenic Exposure in Rats Kidney. IIUM Medical Journal Malaysia, 20(1).
CrossRef - Rahaman, M.S., Rahman, M.M., Mise, N., Sikder, T., Ichihara, G., Uddin, M.K., Kurasaki, M. and Ichihara, S., 2021. Environmental arsenic exposure and its contribution to human diseases, toxicity mechanism and management. Environmental Pollution, p.117940.
CrossRef - Tchounwou, P.B., Yedjou, C.G., Udensi, U.K., Pacurari, M., Stevens, J.J., Patlolla, A.K., Noubissi, F. and Kumar, S., 2019. State of the science review of the health effects of inorganic arsenic: perspectives for future research. Environmental toxicology, 34(2), pp.188-202.
CrossRef - Ezhilarasan, D., Ali, D. and Varghese, R., 2021. Sesamol induces cytotoxicity via mitochondrial apoptosis in SCC-25 cells. Human & experimental toxicology, p.09603271211047926.
CrossRef - Jeong, Y.H., Jeong, G.H., Jeong, Y.H. and Kim, T.H., 2020. Identification of sesamol byproducts produced by plasma treatment with inhibition of advanced glycation endproducts formation and ONOO− scavenging activities. Food chemistry, 314, p.126196.
CrossRef








