A.Asha1* and G.S. Prabha Littis Malar2
and G.S. Prabha Littis Malar2
Department of Chemistry and Research Centre, Scott Christian College (Autonomous), Nagercoil. Affiliated to Manonmaniam Sundaranar University, Abhishekapatti, Tiruneveli, Tamil Nadu, India.
Corresponding Author E-mail: ashaanbaianmsc@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2503
Abstract
Cytotoxicity measurement is needed for all drug-loaded nanoparticles. Because, if the nanoparticles have toxicity means, the drug-loaded polymeric nanoparticles cannot be used for the drug delivery. Generally cell viability is measured in the cytotoxicity measurement. In this work, the nanoparticle have synthesized from the natural polymeric material. These nanoparticles have been prepared using a nano-precipitation technique. Drugs, Insulin and Curcumin are added to these synthesized nanoparticles. This drug was coated on the surface of the nanoparticles to enhance the biocompatibility. These drug-loaded polymeric nanoparticles are used for the drug delivery. L929 cells have been to prove the cytotoxicity of these drug loaded polymeric nanoparticles by Neutral red assay method. From the cytotoxicity assay TPIG, TPCG and CCIG, CCCG nanoparticles are not cytotoxic. Insulin-loaded Tapioca/pectin and a Casein/chitosan nanoparticle were used to study the anti- diabetic assay. Curcumin-loaded Tapioca/pectin and Casein/Chitosan nanoparticle were used for Anti-cancer studies, by making use of Human Osteosarcoma cells (HOS). From these studies, the Insulin and Curcumin-loaded Tapioca/pectin and Casein/chitosan nanoparticles are not cytotoxic, and they can be used for drug delivery.
Keywords
Casein; Drug; L929 cells; Nanoparticles; Pectin; Tapioca
Download this article as:| Copy the following to cite this article: Asha A, Malar G. S. P. L. Cytotoxicity, Antidiabetic and Anticancer Studies of Insulin and Curcumin-Loaded Polymeric Nanoparticles. Biomed Pharmacol J 2022;15(3). |
| Copy the following to cite this URL: Asha A, Malar G. S. P. L. Cytotoxicity, Antidiabetic and Anticancer Studies of Insulin and Curcumin-Loaded Polymeric Nanoparticles. Biomed Pharmacol J 2022;15(3). Available from: https://bit.ly/3v8JK54 |
Introduction
Polymers are used within the pharmaceutical industry to enhance and manage the delivery of medicinal drugs; they are also used as shielding agents, coating materials, binders and drug carrier etc 1. Natural polymers come under the class of polymeric materials, and are based on their source. Natural polymers have few crucial characters like biodegradability, safety, loss of toxicity, availability and low cost. Proteins, polysaccharides, and polynucleotides are included in the biopolymers 2. Proteins like wheat gluten, collagen, silk, and gelatin also have a crucial role within the pharmaceutical industry. Polysaccharides have some impivotal role in the pharmaceutical industry like coating agents, tablet formulation, emulsifying and gelling agents etc. 3.
The dimensions of nanoparticle (NP) formations for drug delivery should be within 1–100 nm, and then only are the nanoparticles active ingredients for drug delivery and also pharmaceutical applications 4. Insulin therapy is a crucial key within the control of diabetes, and diabetic individuals taking daily insulin injections 5. The importance of insulin delivery includes the real-time release of insulin in an appropriate dose in responsible manner over a prolonged period of time 6.
Curcumin is a bright yellow-orange diferuloylmethane, molecular weight of 368.39 g / mol, melting point 183 ° C and the chemical formula of curcumin is C21H20O6. Chemically, it exhibits keto-Enol tautomerism and it has a prominent keto form in neutral and acidic solutions, while the dominant form in solid form and alkali solution is its stable Enol form. This natural polyphenol is known worldwide as the “amazing drug of life” 7. Curcumin has been usually used as a food-coloring agent as well as in pharmaceutical research due to its anti-inflammatory, anti-oxidative, anti-carcinogenic and anti-microbial effects 8. Curcumin has antioxidant properties and it has free radical scavenging via the phenolic and methoxy groups on benzene rings and β-diketone. This antioxidant property of Curcumin has many pharmacological activities 9. The fundamental drawback of Curcumin is bad aqueous solubility 10.
Materials and Methods
Materials
Glacial acetic acid (molecular weight 60.052 g/Mol), Pectin (molecular weight 194.14 g/Mol), Casein , Low molecular weight Chitosan, Glutaraldehyde (Molar mass: 100.11 g/Mol), 10 % Fetal bovine serum, L-glutamine, Calcium chloride, Ethanol, Sodium bicarbonate were purchased from Merck, Germany. L929 cells were purchased from National Centre for Cell Sciences (NCCS), Pune, India.
Preparation of Tapioca starch powder
Tapioca roots were gathered from Kanyakumari District. After cleaning them properly they are dispersed of the sand present in the other facets of the tapioca roots. The tapioca roots were peeled nicely. Then the tapioca flesh was sliced into equal sizes and dried under the shadow for a week. The tapioca slices were dried properly; after they were grind into a fine powder in a wet grinder. The powder was then sieved for the same particle size. This powder is stored in desiccators for further studies.
Preparation of Tapioca/ Pectin nanoparticles
Four grams of pectin were dissolved in 100 ml glacial acetic acid. Five grams of Tapioca powder were dissolved in 100 ml aqueous. The dissolved pectin was drop-wise, added to the dissolved tapioca solution with stirring. After 30 mints 0.1 ml glutaraldehyde was added as a linking agent. This mixture was stirred for 3 hours till the mixture became homogeneous. Now the tapioca pectin nanoparticles were formed.
Preparation of Insulin loaded nanoparticles (TPIG)
100 ml of tapioca pectin nanoparticles mixture were separated and add 0.1 ml of insulin drop wise to the combination with nonstop stirring. The mixture was stirred constantly for 10 hrs; finally the Insulin-loaded nanoparticles were formed. This combination became centrifuged at 6000 rpm for 45 mints. Washed with water and the polymeric nanoparticles were stored as powder form for further research.
Preparation of Curcumin loaded nanoparticles (TPCG)
0.5mg of Curcumin was dissolved in 50 ml ethanol as a clear solution. This curcumin solution was drop-wise, added to 100 ml of tapioca pectin nanoparticles. This combination was stirred constantly for 10 hours; finally, the Curcumin-loaded nanoparticles were formed. This mixture was centrifuged at 6000 rpm for 45 mints. Washed with water and the polymeric nanoparticles were stored as powder form for further studies.
Preparation of Casein/ Chitosan nanoparticles
Four grams of casein was dissolved in 100 ml of Calcium Chloride and heated to boil for 20 mints. Five grams of Chitosan were dissolved in 100 ml of 1% acetic acid. The dissolved casein was drop-wise, added to the dissolved Chitosan solution with stirring. After 30 mints 0.1 ml Glutraraldehyde was added as a linking agent. This mixture was stirred for 3 hours till the mixture became homogeneous. Now the Casein Chitosan nanoparticles were formed.
Preparation of Insulin loaded nanoparticles (CCIG)
100 ml of Casein Chitosan nanoparticle mixture was separated and adds 0.1 ml of Insulin was added drop-wise to the combination with continuous stirring. The mixture was stirred continuously for 10 hrs; finally the Insulin-loaded nanoparticles were formed. This mixture was centrifuged at 6000 rpm for 45 mints. Washed with water the polymeric nanoparticles were stored as powder form for further studies.
Preparation of Curcumin loaded nanoparticles (CCCG)
0.5mg of Curcumin was dissolved in 50 ml ethanol as a clear solution. This Curcumin solution was drop-wise added to 100 ml of Casein Chitosan nanoparticles. This combination was stirred continuously for 10 hours; finally the Curcumin-loaded nanoparticles were formed. This mixture was centrifuged at 6000 rpm for 45 mints. Washed with water and the polymeric nanoparticles were stored as powder form for further studies.
Cytotocixity Assay
L292 cell lines were trypsinized and suspended in growth medium at 5 × 104 cells/ml density were seeded in 96-well plates (100 µl/ well). The stock solutions of Insulin-loaded and Curcumin loaded polymeric nanoparticles were prepared in dimethyl sulfoxide (DMSO) at a concentration of 1.0 µg/µL. Five different concentrations of giving drug loaded polymeric nanoparticles were prepared and added to the respective wells and incubated with atmosphere of 5% CO2 in the air at 37 °C. After 24 or 48 hours the cell viability was evaluated using Neutral red staining assay. 150 µl of the neutral red staining was added to solution to each well and incubated for 2 hours and washed with a solution. 150 µl of solubilisation solution added and placed the plate on a shaker for 15 mints at room temperature. The absorbance was readied at 540 nm.
% of cell viability= x-y/x×100.
whereas,
x=control
y=test sample
The regression graph was obtained from different concentration was plotted against the % of cell viability.
Cell Viability Assay
A human Osteosarcoma cells were cultured with Eagles Minimum Essential Medium. HOS (GRL 1543) cell-line culture medium contained 10% fetal bovine serum. Different concentrations of nanoparticle samples (10, 20, 40, 60, 80, 100µl/ml) were added to these cells and incubated in a CO2 incubator at 37°C with 5% CO2. At 24 h, the medium was sucked out and 5 mg/ml MTT in PBS solutions were added and in incubating for 3 h. Then the medium was solubilised and the absorbance was measured at 570nm using ELISA reader Denver Jasco Model 7800 UV/VIS Spectrophotometer, Jasco, Tokyo, Japan.
Invitro Anti-Diabetic Assay
α – Amylase inhibition assay
A total of 500 µl of reaction mixture containing TPIG and CCIG (1mg/ml) and standard drug (100-1000µg/ml) were added to 500 µl of 0.20 mm phosphate buffer (pH 6.9) containing α-amylase solution and incubated at 25°C for 10 min. Then 500 µl of a starch solution in sodium phosphate buffer was added in the tube. The reaction mixture was again incubated at 25°C for 10 min. The reaction was terminated with 1.0 ml of 3, 5 dinitrosalicylic acid color reagent. The test tube was again incubated in a boiling water bath for 5 min, and cooled to room temperature. 10 ml of distilled water was added to the reaction mixture and absorbance was measured at 540 nm against positive control (Acarbose) 11.
α – Glucosidase inhibition assay
The inhibition of 𝛼 -glucosidase activity of TPIG and CCIG was determined using the modified method. 1 mg of 𝛼 -glucosidase was dissolved in 100 ml of phosphate buffer containing 200 mg of bovine serum albumin. The reaction mixture of 10 μL of TPIG and CCIG were remixed with 490 μL phosphate buffer pH 6.8 and 250 μL of 5 mm 𝑝 -nitrophenyl 𝛼 -D-glucopyranoside was added to the mixture. Followed by preincubating at 37°C for 5 min, 250 μL 𝛼 -glucosidase (0.15 unit/mL) was added and incubated at 37°C for 15 min. The reaction was terminated by adding 2 mL 200 mM Na2CO3. 𝛼 -glucosidase activity was determined spectrophotometrically at 400 nm by measuring the quantity of 𝑝 -nitrophenol released from p-NPG. For 𝛼 -glucosidase inhibitor, Acarbose was used as a positive control 12.
The inhibitory percentage is calculated by the following equation:
whereas,
Ac control = Absorbance of control reaction,
Ap of sample = Absorbance in the presence of the samples of extracts.
Invitro Anti-Cancer Assay
The Human Osteosarcoma cells cultured in Eagle’s Minimum Essential Medium with 10% fetal bovine serum were sub cultured in 96 well flat bottom plates. Various concentrations of TPCG and CCCG samples were delivered to the cells and incubated for overnight at 37°C within the CO2 incubator. After incubation, 50 μl of MTT solution was added and again incubated for 3 hours. Then, 150 μl of the solubilization solution was introduced into each well, wrapped with aluminum foil and incubated for 15 mins in shaker. The absorbance of the resultant formation product was measured at 570 nm in the spectrophotometer 13.
Results and Discussion
Cytotoxicity assay for TPIG and TPCG nanoparticles
The cytotoxicity of insulin and Curcumin-loaded Tapioca/Pectin nanoparticles were investigated using L292 cells with the aid of the neutral red staining assay. Five different concentrations were brought to the cell-line plate. The percentage of cell viability of Insulin- loaded nanoparticles was 99.85±0.42 and Curcumin-loaded nanoparticles was 100.22±0.25 at the 10 µl concentrations.
Table 1: % of cell viability of TPIG nanoparticles.
| Sample Concentration
(µg/ml) |
Average OD | % of cell Viability |
| 10µl | 1.918 | 99.85±0.42 |
| 20µl | 1.910 | 99.56±0.33 |
| 40µl | 1.890 | 98.5±0.60 |
| 60µl | 1.898 | 98.93±0.3 |
| 80µl | 1.893 | 98.67±0.21 |
| 100µl | 1.882 | 97.7±0.6 |
| Positive Control | 0.097 | 5.10±0.37 |
| Negative Control | 1.920 | 99.99±0.30 |
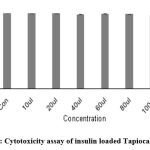 |
Figure 1: Cytotoxicity assay of insulin loaded Tapioca/Pectin NPs. |
Table 2: % of cell viability of TPCG nanoparticles.
| Sample Concentration
(µg/ml) |
Average OD | % of cell Viability |
| 10µl | 1.922 | 100.22±0.25 |
| 20µl | 1.896 | 98.86±0.58 |
| 40µl | 1.893 | 98.74±0.49 |
| 60µl | 1.872 | 97.66±0.53 |
| 80µl | 1.855 | 96.74±0.35 |
| 100µl | 1.844 | 96.12±0.21 |
| Positive Control | 0.98 | 5.2±0.40 |
| Negative Control | 1.920 | 99.99±0.31 |
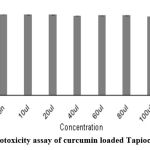 |
Figure 2: Cytotoxicity assay of curcumin loaded Tapioca/Pectin NPs. |
Cytotoxicity assay for CCIG and CCCG nanoparticles
The cytotoxicity of insulin and Curcumin-loaded Casein/Chitosan nanoparticles were investigated using L292 cells by neutral red staining assay. Five different concentrations were brought to the cell-line plate. The percentage of cell viability of Insulin-loaded nanoparticles was 99.85±0.3 and that of Curcumin-loaded nanoparticles was 99.59±0.38 at the 10 µl concentration.
Table 3: % of cell viability of CCIG nanoparticles.
| Sample Concentration
(µg/ml) |
Average OD | % of cell Viability |
| 10µl | 1.916 | 99.85±0.3 |
| 20µl | 1.914 | 99.75±0.65 |
| 40µl | 1.902 | 99.09±0.5 |
| 60µl | 1.885 | 98.26±0.28 |
| 80µl | 1.872 | 97.54±0.60 |
| 100µl | 1.847 | 96.29±1.14 |
| Positive Control | 0.097 | 5.10±0.37 |
| Negative Control | 1.920 | 99.99±0.31 |
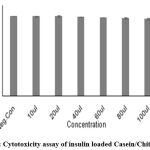 |
Figure 3: Cytotoxicity assay of insulin loaded Casein/Chitosan NPs. |
Table 4: % of cell viability of CCCG nanoparticles.
| Sample Concentration
(µg/ml) |
Average OD | % of cell Viability |
| 10µl | 1.911 | 99.59±0.38 |
| 20µl | 1.890 | 98.48±0.68 |
| 40µl | 1.888 | 98.26±0.31 |
| 60µl | 1.859 | 96.87±0.44 |
| 80µl | 1.868 | 97.35±0.20 |
| 100µl | 1.738 | 90.56±0.1 |
| Positive Control | 0.98 | 5.2±0.40 |
| Negative Control | 1.920 | 99.99±0.31 |
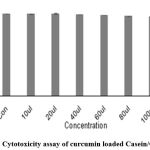 |
Figure 4: Cytotoxicity assay of curcumin loaded Casein/Chitosan NPs. |
Invitro Anti-Diabetic Assay
In vitro anti-diabetic activity was carried out in Insulin-loaded TPIG and CCIG polymeric nanoparticles using α- amylase and α- glucosidase enzymes. Acarbose was used as a positive control. Insulin-loaded TPIG and CCIG polymeric nanoparticles had much effect on α- amylase enzyme. Various concentrations of drug-loaded TPIG and CCIG nanoparticles were brought to α- amylase inhibitory assay. In α – amylase inhibitory assay, the maximum inhibition value of TPIG was 63.79% and CCIG was 51.72% obtained in 100µl/ml. The IC50 value of α- amylase inhibition assay of TPIG was 242 µl/ml and CCIG was 209.32 µl/ml. For α – Glucosidase assay, the maximum inhibition value of TPIG was 73.95% and CCIG was 80.43 %. The IC50 value of α- glucosidase inhibition assay of TPIG was 249.5 µl/ml and CCIG was 239 µl/ml. The polymeric nanoparticles TPIG and CCIG had better inhibition property against the metabolic enzymes, which were responsible for type II diabetic. Insulin-loaded polymeric nanoparticles exhibited better inhibition at lower concentration than Standard (Acarbose) positive control.
Table 5: α – amylase and α – glucosidase inhibition of TPIG and CCIG NPs.
| Sample
& Concentrations |
% of inhibition of
α – amylase (1mg/ml) |
IC 50 Value
(µg/ml) |
% of inhibition of α – glucosidase (1mg/ml) | IC 50 Value
(µg/ml) |
| TPIG
|
242.2 |
|
249.45 | |
| 200 | 12.75 | 14.79 | ||
| 400 | 25.51 | 29.58 | ||
| 600 | 38.27 | 44.37 | ||
| 800 | 51.03 | 59.16 | ||
| 1000 | 63.79 | 73.95 | ||
| CCIG
|
|
209.3 |
|
239 |
| 200 | 10.34 | 16.08 | ||
| 400 | 20.68 | 32.17 | ||
| 600 | 31.03 | 48.25 | ||
| 800 | 41.37 | 64.34 | ||
| 1000 | 51.72 | 80.43 | ||
| Acarbose (Positive Control) | 53.44 | 220.93 | 53.38 | 0.93 |
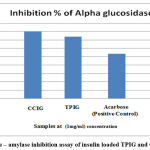 |
Figure 5: α – amylase inhibition assay of insulin loaded TPIG and CCIG NPs. |
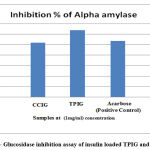 |
Figure 6: α- Glucosidase inhibition assay of insulin loaded TPIG and CCIG NPs. |
In Vitro Anti-Cancer Assay
The anti-proliferative effect of Curcumin-loaded TPCG and CCCG polymeric nanoparticles against HOS was inspected using MTT assay (Tab-6,7 and Fig -7,8). These Curcumin-loaded polymeric nanoparticles had cytotoxicity against Human Osteosarcoma cells based on the concentration. If the concentration of drug-loaded polymeric nanoparticles increased the % of cell viability also increased. The IC 50 value of TPCG was 62.88 µl/ml and CCCG was 66.039 µl/ml. The polymeric nanoparticles TPCG and CCCG were cytotoxic at the concentration of 60µl/ml. If the concentration of drug-loaded polymeric nanoparticles increased, the cytotoxicity effect also increased.
Table 6: Anti cancer activity of TPCG nanoparticles.
| Concentration of Sample TPCG (µg/ml) | Average value | % of cell Viability |
| 10µl | 2.073 | 92.27±3.35 |
| 20µl | 1.902 | 84.72±0.2 |
| 40µl | 1.902 | 84.54±0.18 |
| 60µl | 1.065 | 47.41±2.63 |
| 80µl | 0.608 | 27.07±0.28 |
| 100µl | 0.198 | 8.83±0.51 |
| Negative Control | 2.248 | 100.01±0.84 |
| Positive Control | 0.104 | 4.6±0.35 |
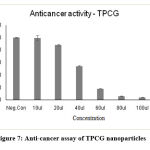 |
Figure 7: Anti-cancer assay of TPCG nanoparticles. |
Table 7: Anti cancer activity of CCCG NPs.
| Concentration of Sample CCCG (µg/ml) | Average value | % of cell Viability |
| 10µl | 2.135 | 95.03±2.40 |
| 20µl | 1.772 | 78.76±0.61 |
| 40µl | 0.626 | 27.87±0.31 |
| 60µl | 0.424 | 18.91±0.32 |
| 80µl | 0.260 | 11.60±0.28 |
| 100µl | 0.108 | 4.86±0.15 |
| Negative Control | 2.248 | 100.01±0.84 |
| Positive Control | 0.104 | 4.6±0.35 |
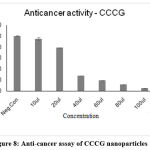 |
Figure 8: Anti-cancer assay of CCCG nanoparticles. |
Conclusion
Insulin and curcumin-loaded Tapioca/pectin and Casein/Chitosan polymeric nanoparticles were prepared by the nano-precipitation method. Those Insulin and Curcumin- loaded polymeric nanoparticles were evaluated by cytotoxicity assay. For the cytotoxicity assay L929 cells were used by the neutral red assay technique. From the report of the cytotoxicity assay it was proved that the drug Insulin and Curcumin-loaded Tapioca/Pectin and Casein/Chitosan polymeric nanoparticles were not cytotoxic up to the concentration of 100µl/ml. From the anti diabetic assay, it was proved that Insulin-loaded nanoparticles had better inhibition against metabolic enzymes and from the anticancer activity assay, Curcumin loaded nanoparticles acted as better cytotoxic against Human Osteosarcoma cells. From this cytotoxicity assay drug-loaded Tapioca/ Pectin and Casein/ Chitosan nanoparticles can be used for drug delivery.
Acknowledgement
The author thanks to Department of Chemistry and Research Centre, Scott Christian College (Autonomous), Nagercoil, Tamilnadu, IndiaThe author thanks to Department of Chemistry and Research Centre, Scott Christian College (Autonomous), Nagercoil, Tamilnadu, India.
Conflict of Interest
The authors have no conflict of interests regarding the publication of this article.
Funding Sources
There is no funding Source.
References
- Pawar Dipali Sanjay, Patrekar Prasad Vasantrao, Dhanawade Pooja Pandit, Dongare Sujata Dattaprasad and Mali Savita Shivaji. Polymers Used in Pharmaceuticals: A Brief Review. J .Phar. Chem. Rese., 2016; 2: 233-238.
- Kharkwal, H, Malhotra, B, and Janaswamy, S. Cellulose-based polymeric systems in drug delivery. Poly. Dru. Deli., 2017; Chap 2: 10.
CrossRef - J Elizabeth Bealer, J, Onissema-Karim, S, Ashley Rivera-Galletti, Maura Francis, Jason Wilkowski, David Salas-de la Cruz, and Xiao Hu. J. Protein–Polysaccharide Composite Materials: Fabrication and Applications. MDPI. Poly., 2020; 12: 1-28.
CrossRef - Wim H De Jong, and Paul JA Borm.. Drug delivery and nanoparticles: Applications and hazards. Inte.r J. Nano., 2016; 3: 133-149.
CrossRef - American Diabetes Association. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes. Diabetic Care., 2018; 41. S74: 573- 585.
- Katsarou, A, Gudbjörnsdottir, S, Rawshani, A, D. Dabelea, D, Bonifacio , E, Anderson, B.J, Jacobsen, L.M, Schatz, D.A, and Lernmark Å. Type 1 diabetes mellitus. Nat. revi. dis. pri., 2017; 3: 1-17.
CrossRef - Javad Sharifi-Rad, Yousef El Rayess. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Pharmacol., 2020; 11: 1-23.
CrossRef - Kamran Mansouri, Shna Rasoupoor. Clinical effects of curcumin in enhancing cancer therapy: A systematic review. BCM. Cancer., 2020; 20: 1-11.
CrossRef - Augustine Amalraj, Anitha Pius, Sreerag Gopi. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives – A review. J. Trad. Compl. Medicine., 2017;17: 205-233.
CrossRef - Sandhuli S. Hettiarachchi, Shashiprabha P. Dunuweera, Asiri N. Dunuweera, R. M. Gamini Rajapakse. Synthesis of Curcumin Nanoparticles from Raw Turmeric Rhizome. pub., 2012; 6: 8246- 8252.
CrossRef - Karan Khadayat , Bishnu P. Marasini , Hira Gautam , Sajani Ghaju and Niranjan Parajuli. Evaluation of the alpha-amylase inhibitory activity of Nepalese medicinal plants used in the treatment of diabetes mellitus. Phytosci., 2020; 6: 3-8.
CrossRef - Ali S. Alqahtani, Syed Hidayathulla , Md Tabish Rehman , Ali A. ElGamal. Alpha-Amylase and Alpha-Glucosidase Enzyme Inhibition and Antioxidant Potential of 3-Oxolupenal and Katononic Acid Isolated from Nuxia oppositifolia. Biomolecules., 2020; 10: 2-19.
CrossRef - Jinsha Koroth, Snehal Nirgude, Shweta Tiwari, Vidya Gopalakrishnan. Investigation of anti-cancer and migrastatic properties of novel curcumin derivatives on breast and ovarian cancer cell lines. BCM. Comp. Med. Ther., 2019; 19: 2-16.
CrossRef








