Manuscript accepted on :10-06-2022
Published online on: 25-08-2022
Plagiarism Check: Yes
Reviewed by: Dr. Ocholi Edogbanya
Second Review by: Dr. Salman Ahmed
Final Approval by: Dr. H Fai Poon
Thabiso Katlego Teffo1* , Shalini Dukhan1
, Shalini Dukhan1 , Phillemon Ramalepe2
, Phillemon Ramalepe2 and Ida Risenga1
and Ida Risenga1
1School of Animal, Plant and Environmental Sciences, University of the Witwatersrand, Johannesburg, South Africa.
2Wits School of Education, University of the Witwatersrand, Johannesburg, South Africa
Corresponding Author E-mail: 1081325@students.wits.ac.za
DOI : https://dx.doi.org/10.13005/bpj/2469
Abstract
Bulbine abyssinica is a succulent medicinal plant indigenous to South Africa. The species has been commonly used traditionally by indigenous people for the treatment of various skin related ailments as well as the management of diabetes mellitus. To date, limited studies have been conducted on the underground stems and roots, as well as comparative analyses across the different plant parts of the species. Methanolic extracts of B. abyssinica leaves, underground stems and roots were used to perform phytochemical screening, quantitative phytochemical analyses, antioxidant and antibacterial assays. The leaves contained most of the phytochemical groups tested, as well as higher total phenolic (1841.7 ± 4.8 mg/100g GAE), total flavonoid (809.2 ± 75.6 mg/100g QE), total tannin (2850 ± 70.01 mg/100g GAE) and total proanthocyanidin (636.67 ± 1.67 mg/100g CE) contents compared to the underground stems and roots. The antioxidant activity results showed that the roots exhibited the strongest scavenging power against 2, 2 diphenylpicryhydrazyl (DPPH) (0.105 ± 0.01 mg/ml), whereas the leaves showed a higher antioxidant power against hydrogen peroxide (0.66 ± 0.07 mg/ml) and metal chelating radicals (2.68 ± 0.16 mg/ml). All three plant parts showed intermediate zones of inhibition (10 - 19 mm) against Staphylococcus aureus and Escherichia coli. The current study validates the use of different plant parts of B. abyssinica in the traditional medicine context, and suggests the plant’s potential application in the pharmaceutical and cosmetic industries.
Keywords
Antibacterial; Antioxidant; Bulbine abyssinica; Phytochemical; Roots, Underground Stems
Download this article as:| Copy the following to cite this article: Teffo T. K, Dukhan S, Ramalepe P, Risenga I. Comparative Phytochemical Analysis, Antioxidant and Antibacterial Activities in the Leaves, Underground Stems and Roots of Bulbine abyssinica. Biomed Pharmacol J 2022;15(3). |
| Copy the following to cite this URL: Teffo T. K, Dukhan S, Ramalepe P, Risenga I. Comparative Phytochemical Analysis, Antioxidant and Antibacterial Activities in the Leaves, Underground Stems and Roots of Bulbine abyssinica. Biomed Pharmacol J 2022;15(3). Available from: https://bit.ly/3TjvEbx |
Introduction
Traditional medicine is known to be the oldest form of healthcare in the world used for the prevention of physical and mental illnesses 1. Medicinal plants are utilised for the management and treatment of numerous ailments as a result of the natural bioactive compounds such as phenolics, flavonoids, saponins, alkaloids and terpenoids 2, 3 and the high nutrient content required for drug development 4. According to the World Health Organization (WHO), about 80% of the global population, of which majority of these are Third World countries, rely on traditional healers and their medicines, rather than western pharmaceutical products due to their affordability and immediate availability 5, 6. One of the commonly used medicinal plant species in South Africa is Bulbine abyssinica.
This succulent medicinal plant species belongs to the Asphodelaceae family, similar to the genus Aloe. B. abyssinica is commonly distributed across the Eastern Cape and Kwa Zulu-Natal provinces, and can also be found in other African countries such as ESwatini, Lesotho, Angola and Ethiopia 3. The whole parts of B. abyssinica (leaves, stems, roots and flowers) have medicinal uses in the traditional sector that range from the treatment of skin-related conditions such as wounds, scars and burns, to vaginal and bladder infections, dysentery, rheumatism, bilharzia, infertility as well as the management of diabetes mellitus related illnesses3. In addition to the traditional uses of B. abyssinica, the species is also used in ethnoveterinary medicine for the treatment of gastrointestinal parasites, blood cleansing and internal sores in animals, specifically cattle 7-9. Several reports have been made on the phytochemical and biological activities of B. abyssinica leaves 10- 14. However, till date, limited studies have been conducted on the phytochemical and medicinal potential of the underground stems and roots, as well as comparative analyses across the different plant parts of B. abyssinica. Thus, the aim of this study was to determine the phytochemical profile, total phenolic, total flavonoid, total tannin and total proanthocyanidin content, antioxidant and antibacterial activities of the leaves, underground stems and roots of B. abyssinica. The purpose of the study is to contribute new knowledge and provide understanding on how each plant part varies across their respective phytochemical, antioxidant and antibacterial potential. All parts of the species are used in both traditional and ethnoveterinary medical practices. Therefore, findings from this study may also contribute knowledge to the pharmaceutical industry with the aim to bioprospect and use this plant for the manufacturing of medicinal and cosmetic products.
Materials and Methods
Plant material collection and preparation
Potted B. abyssinica plants were collected from the Wild Flower Nursery in the North West Province, South Africa, and then maintained in the rooftop greenhouse where the plants were watered (200 ml per potted plant) every second day, at the University of the Witwatersrand, Johannesburg, South Africa until they fully matured. Once the plants had fully acclimatised, the leaves, underground stems and roots were harvested and rinsed thoroughly using distilled water prior to laboratory analysis. The harvested material was then dried in a hot air-drier (Binder, for three days at 40˚C and then crushed into fine powder with an electric blender.
Sample extraction
The extraction of phytochemical compounds was adopted from the method described by Siddhuraju et al. (2003) 15 and Pakade et al. (2013) 16. To produce crude extracts, three grams of the powdered leaf, underground stem and root samples were mixed with 25 ml of 80% methanol, respectively. The extracts were then sonicated at 50 ˚C in a water bath for a period of 25 minutes. A volume of 20 ml of 80% acetone was added in the water bath. The extracts were then filtered using Whatman® number 1 qualitative filter paper, and the supernatant of all the extracts was collected. This method was repeated for re-extraction and fusion of the supernatants. Upon the completion of the extraction process, the crude extracts were stored in the refrigerator at 16 ˚C prior to phytochemical, antioxidant and antibacterial analyses.
Qualitative phytochemical screening
The phytochemical screening of the methanolic leaf, underground stem and root extracts of B. abyssinica were used to test for the presence of ten compound groups, as detailed below, using methods from various authors.
Test for Tannins (FeCl3 test)
To test for the presence of tannins, 2 ml of the plant extract was mixed with 2 ml of distilled water and another 2 ml of 5% ferric chloride. A green-blue colour indicated the presence of tannins 17.
FeCl3 Test for Phenolics
One millilitre of crude plant extract was placed into a test tube and mixed with a few drops of 10% ferric chloride. The appearance of a green blue or violet colour was an indication of phenolic compounds 18.
Test for Flavonoids (HCl test)
A volume of 0.5 ml of crude plant extract was mixed with five drops of concentrated hydrochloric acid (HCl). The development of a red colour indicated the presence of flavonoids 19.
Test for Steroids
Two millilitres of the crude plant extract was placed in a test tube and mixed with 2 ml of chloroform. A further 2 ml of concentrated H2SO4 was added to the test tube. A reddish-brown ring at the junction indicated the presence of steroids 20.
Test for Terpenoids
One millilitre of the crude plant extract was mixed with 0.5 ml of chloroform. Then, a few drops of concentrated H2SO4 acid was added to the mixture. The formation of a reddish-brown precipitate indicated presence of terpenoids 18.
Salkowski’s Test for Glycosides
For this method, 0.5 ml of crude plant extract was placed in a test tube and mixed with 2 ml of H2SO4. The presence of a reddish-brown colour forming indicated the presence of the steroidal aglycone part of the glycoside 21.
Froth test for Saponins
A volume of 0.5 ml of crude plant extract is placed in a test tube with 5 ml of distilled water. The test tube is shaken vigorously. Three drops of olive oil were placed inside the test tube and shaken vigorously. The presence of a stable foam was an indication of the presence of saponins 21.
Test for Coumarins
One millilitre of crude plant extract was placed in a test tube and mixed with 1 ml of 10% sodium hydroxide. The formation of a yellow colour indicated the presence of coumarins 17.
Test for Phlobatannins
One millilitre of crude plant extract was mixed with a few drops of 2% HCl. The appearance of red precipitate indicated the presence of phlobatannins 17.
Test for Volatile Oils
One millimetre of crude plant extract was placed in a test tube and mixed with 0.2 ml of 1% w/v sodium hydroxide. The presence of a precipitate indicated presence of volatile oils 22.
Quantitative phytochemical analysis
Total phenolic content (TPC) analysis
The Folin-Ciocalteu reagent assay was used for the approximation of TPC using the methanol extracts of B. abyssinica leaves, underground stems and roots. A solution of 750 μl1:10 Folin-Ciocalteu reagent and 2.5 mL of 7.5% aqueous sodium carbonate was added to methanol extracts of B. abyssinica (300 μL) which reacted in an Erlenmeyer flask closed at the opening with a rubber stopper [16]. The mixture was diluted with 7 ml of distilled water, followed by placing the mixture in a dark compartment for two hours at ambient temperature. A spectrophotometer (Thermo-Fisher Scientific Genesys (GEN10S UV-Vis)) was used to determine the absorbance of the phenolic content in the extracts at a wavelength of 765 nm 23. The following linear equation inherited from the calibration curve formulated by using gallic acid working stock solution was used to calculate the TPC from the methanol extracts:
y=0.0495x – 0.0259, r2= 0.9994
Total Flavonoid Content (TFC) analysis
The existing methodology developed by Siddhuraju et al. (2003) 15 that was slightly modified by Pakade et al. (2013) 16 was adopted for the approximation of TFC. A measurement of 0.3 ml of crude extracts was diluted with 4 ml of deionised water using a 10 ml volumetric flask. A volume of 0.3 ml of sodium nitrate (NaNO3) was added and thoroughly mixed, followed by 3 ml of aluminium chloride (AlCl3) solution after 5 minutes. After six minutes, 2 ml of 1 M sodium hydroxide solution was placed inside the volumetric flask. Following the addition of the chemicals in sequential order, the reaction was made up to 10 ml, with deionised water, and stirred for 15 minutes. A UV-visible spectrophotometer was used to measure the TFC of the extract mixture at a wavelength of 510 nm 23. A linear equation inherited from the calibration curve formulated using quercetin standards was used to calculate the TFC from different methanolic extractions:
y= 0.2388x – 0.0019, r2 = 0.9997
Total Tannin Content (TTC) analysis
The method modified by Lahare et al. (2021) 24 was used to determine the concentration of total tannins in the methanolic extracts of B. abyssinica. Aliquots of 0.1 ml of the extracts were dissolved in 7.5 ml of distilled water, followed by the addition of 0.5 ml of Folin-Ciocalteu reagent. A volume of 1 ml of 35% sodium carbonate was added into the mixtures, and then diluted to 10 ml using distilled water. The mixtures were shaken thoroughly, and incubated at room temperature for 30 minutes. The absorbance of the mixtures and gallic acid (standard) were recorded at a wavelength of 725 nm using a spectrophotometer. A linear equation obtained from the gallic acid curve was used to determine the concentration of total tannins:
y= 0.046x– 0.0264, r2 = 0.9833.
Total proanthocyanidin content (TPAC)
The total proanthocyanidin content was determined using the method by Oyedemi et al. (2010) 25. A volume of 0.5 ml of the methanolic extracts of B. abyssinica were mixed with 3 ml of 4% vanillin-methanol solution, followed by 1.5 ml of HCl. The mixtures were vortexed and incubated for 15 minutes at room temperature prior to measuring the absorbance at a wavelength of 500 nm. The linear equation from the catechin calibration curve was used to determine the concentration of total proanthocyanidins:
y= 0.9554x + 0.0003, r2 = 0.9927.
In vitro antioxidant activity analysis
diphenylpicryhydrazyl (DPPH) scavenging activity assay
A DPPH free radical assay developed by Brand-Williams et al. (1995) 26 was followed with some modifications. In this method, the methanolic extracts of B. abyssinica were used to determine the scavenging potential of DPPH. Fifty milligrams (mg) of DPPH was added to 100 ml of 80% methanol to prepare the stock solution. The stock solution was then diluted with 80% methanol where the dilution mixture had a ratio of 1:5 (stock solution: 80% methanol). A reaction mixture was made containing 10, 20, 30, 40 and 50 μL of the extracts and 700 μL of the work solution which was filled with 80% methanol to make up a volume of 1 ml. The mixture was then stored in the dark for 45 minutes, followed by the measurement of the absorbance at a wavelength of 517 nm using a spectrophotometer. A blank solution was formulated using 80% methanol and the control solution was prepared using the work solution and 80% methanol. The inhibition (%) was determined using the following equation:
Scavenging (%) = [(Ab sample – Ab blank)/Ab control] ✕ 100.
Ab represents the absorbance values for the sample, blank and control solutions.
Hydrogen (H2O2) peroxide assay
The potential of B. abyssinica to scavenge hydrogen peroxide was determined using the method by Ruch et al. (1989) 27. The 40mM solution of hydrogen peroxide was prepared by adding phosphate buffer (pH 7.4) and 30% aqueous hydrogen peroxide solution. The methanolic leaf, and root extracts of B. abyssinica (10, 20, 30, 40 and 50 μL) were added to the hydrogen peroxide solution (600 μL, 40mM) and then the absorbance of each solution was recorded following a 10 minute incubation at a wavelength of 230 nm using a spectrophotometer. The blank solution used contained the phosphate buffer without hydrogen peroxide and the positive control contained the 40mM hydrogen peroxide solution. The scavenging activity of the extracts were calculated using the following equation:
% scavenged H2O2 = [(Ac – As)/Ac] ✕ 100,
Ac is the absorbance of the control and As is the absorbance of the extract mixed with 40mM hydrogen peroxide.
Metal chelating assay
The method by Dinis et al. (1994) 28 was modified and used to assess the chelate scavenging power of the methanolic leaf, underground stem and roots of B. abyssinica. Varying concentrations (10, 20, 30, 40 and 50 μL) of the extracts were added to a solution of 2 mM ferric (II) chloride (50 μL). A reaction was initiated by the addition of 200 μL of ferrozine ((3-(2-pyridyl)-5, 6-dipheny-1, 2, 4-triazine-p, p’-disulfonic acid monosodium salt hydrate)) (5 mM). The mixture was vigorously shaken and then left to stand at room temperature for 10 minutes. The absorbance of the solutions was measured spectrophotometrically at 562 nm, with the blank solution being 80% methanol and the positive control being the mixed ferric chloride and ferrozine solutions without the extracts. The percentage chelating effect of the extracts were calculated using the following equation:
% chelating effect = [(A0 – A1)/A0] ✕ 100
A0 is the absorbance of the control and A1 is the absorbance in the presence of the sample of the extracts.
All antioxidant assay results were presented in IC50 values, which indicates the amount of an extract that is required to inhibit 50% of a particular biological process [29].
Antibacterial assay
Test organisms
Gram-positive Staphylococcus aureus (ATCC 25923) and Gram-negative Escherichia coli (ATCC 25922), as well as the Muller-Hinton and Baird-Parker agar were obtained from Thermo-Fisher Laboratory specialties (Pty) Ltd, Johannesburg, South Africa. Gram-positive S. aureus and Gram-negative E. coli were sub-cultured in two media, namely Muller-Hinton (E. coli) and Baird Parker (S. aureus) agar respectively.
Qualitative screening of antibacterial activity
The agar well diffusion method formulated by Okeke et al. (2001) 30 and adapted by Jain et al. (2015) 31 with slight modifications was used in this study. Bacterial plates containing S. aureus and E. coli were incubated at 37 ˚C for 24 hours. Following the growth of the bacteria on the agar plates, six millimetre diameter wells were bored on the plates using a sterile borer. A 100 μL volume of the respective hot air-dried extracts were impregnated into the wells. In order to achieve efficient diffusion of the extracts, the plates were allowed to stand for one hour in the fridge before transferring them into the incubator for another 24 hours at 37 ˚C. A zone of inhibition, which indicates the potency of the extract against the bacterial strains, was measured in millimetres (mm) using a ruler. Dimethyl sulfoxide (DMSO) was used as a negative control.
Statistical analysis
The collected data from this experiment was analysed by one-way analysis of variance (ANOVA) using R Studio. Values for total phenolic content, total flavonoid content, antioxidant and antibacterial activities were expressed in mean ± SE. A Tukey HSD post-hoc test was performed to determine where the significant difference lies following the one-way ANOVA.
Results
Phytochemical screening
The results from the phytochemical screening provided an overview of the presence of selected secondary metabolites in the methanolic extracts of B. abyssinica as summarised in Table 1. The results from the extracts of B. abyssinica showed the presence of seven phytochemical groups, namely tannins, phenolics, flavonoids, steroids, terpenoids, glycosides and saponins. The leaf extract presented a moderate presence of the aforementioned phytochemicals, except for glycosides and saponins, which only displayed a low presence. The underground stems and roots only had a low presence of the listed phytochemicals, with the stems having absence of phenolics and flavonoids, and tannins in addition for the roots. Interestingly, all three plant parts did not show any presence of coumarins, phlobatannins, and volatile oils.
Table 1: Phytochemical screening of the methanolic extracts of B. abyssinica.
| Phytochemical group | Leaves | Stems | Roots |
| Tannins | ++ | + | – |
| Phenolics | ++ | – | – |
| Flavonoids | ++ | – | – |
| Steroids | ++ | + | + |
| Terpenoids | ++ | + | + |
| Glycosides | + | + | + |
| Saponins | + | + | + |
| Coumarins | – | – | – |
| Phlobatannins | – | – | – |
| Volatile oils | – | – | – |
(+): low presence; (++): moderate presence; (+++): high presence; (-): absence
Quantitative phytochemical analysis
The total phenolic content of the methanolic extracts of the leaves, underground stems and roots of B. abyssinica is shown in Figure 1. The highest TPC was obtained from the leaves of B. abyssinica (1841.7 ± 4.8 mg/100g GAE), followed by the roots (1652.8 ± 19.2 mg/100g GAE) and the underground stems (1328.7 ± 7.2 mg/100g GAE). All three plant parts showed significant differences (p<0.05). The total flavonoid results showed a similar pattern as the TPC (Figure 2). The leaf extracts of B. abyssinica had a higher TFC (809.2 ± 75.6 mg/100g QE), when compared to the roots (231.7 ± 33.2 mg/100 QE) and the underground stems (226.7 ± 0.5mg/100g QE) (Fig. 2). Significant differences were only observed between the leaves and stems as well as the leaves and roots (p<0.05) respectively.
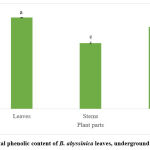 |
Figure 1: Total phenolic content of B. abyssinica leaves, underground stems and roots. |
Values are represented in mean ± SE. Means of different letters (a-c) show a significant difference (Tukey’s test; p<0.05).
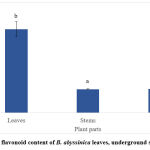 |
Figure 2: Total flavonoid content of B. abyssinica leaves, underground stems and roots. |
Values are represented in mean ± SE. Means of different letters (a-b) show a significant difference (Tukey’s test; p<0.05).
The results from the total tannin content of B. abyssinica revealed the leaves to contain the highest concentration of total tannins (2850 ± 70.01 mg/100g GAE), then the underground stems (2678.3 ± 55.01 mg/100g GAE) followed by the roots (2393 ± 55.97 mg/100g GAE) (Figure 3). However, the leaves and stems showed no significant difference (p>0.05). The total proanthocyanidin content of B. abyssinica showed a similar trend with that of total tannins (Figure 4). The leaves had the highest TPAC at 636.7 ± 1.67 mg/100g CE. This was followed by the underground stems (429.5 ± 0.76 mg/100g CE) and then the roots (403.8 ± 2.03 mg/100g CE). Significant differences were observed across all plant parts (p<0.05).
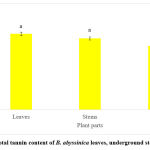 |
Figure 3: Total tannin content of B. abyssinica leaves, underground stems and roots. |
Values are represented in mean ± SE. Means of different letters show a significant difference (Tukey’s test; p<0.05).
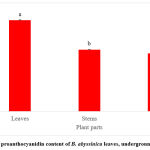 |
Figure 4: Total proanthocyanidin content of B. abyssinica leaves, underground stems and roots. |
Values are represented in mean ± SE. Means of different letters show a significant difference (Tukey’s test; p<0.05).
In-vitro antioxidant activity analysis
The DPPH, hydrogen peroxide and metal chelating scavenging activities of the methanolic leaf, underground and root extracts of B. abyssinica were assessed and the results were presented in IC50 values Figures 5-7. The lower the IC50, the greater the scavenging power of the extracts. The DPPH antioxidant activity results of B. abyssinica are presented below (Figure 5). B. abyssinica roots displayed the lowest IC50 value of 0.11 ± 0.01 mg/ml, followed by the underground stems (0.46 ± 0.04 mg/ml) and the leaves (4.79 ± 0.35 mg/ml). Only the leaves against the stems and roots respectively, showed significant differences (p<0.05).
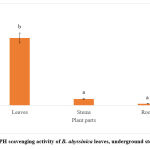 |
Figure 5: DPPH scavenging activity of B. abyssinica leaves, underground stems and roots. |
Values are represented in mean ± SE. Means of different letters (a-b) show a significant difference (Tukey’s test; p<0.05).
The H2O2 scavenging assay was used to evaluate the potential of B. abyssinica extracts against the radical (Figure 6). The IC50 values for both species were all under 1 mg/ml, which indicates an excellent scavenging power against hydrogen peroxide. The leaves of B. abyssinica showed the lowest scavenging activity at 0.66 ± 0.07 mg/ml when compared to the roots (0.73 ± 0.07 mg/ml) and the underground stems (0.82 ± 0.05 mg/ml). No significant differences were observed for the hydrogen peroxide assay (p>0.05).
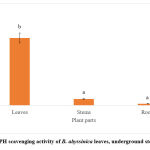 |
Figure 6: Hydrogen peroxide scavenging activity of B. abyssinica leaves, underground stems and roots. |
Values are represented in mean ± SE. Means of the same letter (a) show no significant difference (Tukey’s test; p>0.05).
The extracts of B. abyssinica were assessed for their potential to chelate iron radicals (Figure 7). All plant parts of B. abyssinica had IC50 values greater than 2 mg/ml, with the leaves having the lowest value of the three parts (2.68 ± 0.16 mg/ml) followed by the underground stems (2.69 ± 0.13 mg/ml) and then the roots (3.02 ± 0.13 mg/ml). There was no significant difference reported for this assay (p>0.05). The results show that B. abyssinica has an overall good antioxidant activity.
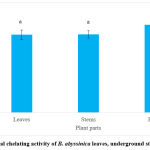 |
Figure 7: Metal chelating activity of B. abyssinica leaves, underground stems and roots. |
Values are represented in mean ± SE. Means of the same letter (a) show no significant difference (Tukey’s test; p>0.05).
Antibacterial activity
The leaf, underground stem and root extracts of B. abyssinica showed intermediate zones of inhibition against both bacterial strains. The leaves had a higher inhibition against S. aureus, with a diameter of 19.33 ± 0.88 mm, while the underground stems presented the highest inhibition against E. coli at 15.00± 0.58 mm. Significant difference was only reported against S. aureus, between the leaves and roots (p<0.05). No significant difference was observed against E. coli across all plant parts (p>0.05).
Table 2: Antibacterial activity of the methanolic extracts of B. abyssinica.
| Plant part | S. aureus | E. coli |
| Leaves | 19.33a± 0.88 | 13.67a± 0.88 |
| Stems | 15.00b± 1.73 | 14.67a± 0.88 |
| Roots | 12.67b± 0.88 | 13.67a± 0.33 |
Values are represented in mean ± SE (mm). Means of different letters within a column (a-b) show a significant difference (Tukey’s test; p<0.05).
Discussion
The presence of the tested phytochemical groups in this study provides some evidence that supports the traditional uses of B. abyssinica 32. The leaves of this species presented majority of the phytochemicals as compared to the underground plant parts. Phenolic compounds are known to be the largest and most abundant group of phytochemicals, making up 45% 33. Phenolic compounds are comprised of one or more hydroxyl groups with an aromatic ring 32, 34. They play various roles in plants, such as in the production of colour, odour and the protection of plants against both environmental stress and pathogens32. This is probably the reason why phenolic compounds are also studied for their pharmacological properties 32, 35, 36.
In the present study, the total phenolic content was determined in the methanolic leaf, underground stems and roots B. abyssinica. The results showed that the plant parts did show a rich quantity of phenolics, with the leaves presenting the highest TPC compared to its other plant parts. This indicates that the leaves could be more beneficial in terms of its medicinal properties in comparison to the underground stems and roots. In addition, B. abyssinica leaves may be extremely useful to industry as the results from this current study show higher concentrations of phenolics. While previous studies by Idamokoro and Afolayan (2020)14 showed yields of TPC from water (16 mg/g) and ethanol (29.38 mg/g) leaf extracts, the present study showed higher TPC yields in the methanolic extracts of leaves, underground stems and roots. The extraction technique, as well as the use of different solvents such as methanol, ethanol and water, play a role in the extraction yield of phytochemical compounds. Phytochemicals have different solubility properties in different solvents, and this is also dependent on the plant material used and the preparation method followed prior to extraction37. The current TPC results for all parts of B. abyssinica yielded higher results compared to the aqueous (16 mg/g), ethanol (29.38 mg/g) and methanol (16.31 mg/ml) extracts of B. abyssinica leaves from a study by Idamokoro and Afolayan (2020)14.
Flavonoids are the largest group of phenolic compounds best known for their antioxidant properties associated with illnesses such as Alzheimer’s disease, atherosclerosis and cancer 33, 38. Flavonoids consist of over 4000 compounds 32, 39 and are greatly used in pharmaceutical, medical and cosmetic industries as a result of their anti-inflammatory and anti-carcinogenic properties 38. It has also been discovered that flavonoids possess enzyme inhibition, antimicrobial, estrogenic, anti-allergic and cytotoxic anti-tumour properties 33.
Tannins have multiple medicinal applications, ranging from cancer therapy, to antimicrobial, antioxidant, wound-repair, diabetes treatment and antidiarhoic properties 40. Subbotina et al. (2003)41 and Russo et al. (2018)42 both reported that tannins are potent antidiarrhoics, with results showing that the compounds significantly reduced the duration of acute diarrhoea in children, compared to standard rehydration treatments. The uses of tannins are also explored in structural bone repair by the application in spray-dried powder (PTSDs) with the aim of replacing bone in vivo 40, 43. The high concentration of tannins in the leaves, underground stems and roots of B. abyssinica indicate that the species is a rich source of these beneficial compounds, and their application in various industries would be a great contribution to improving the overall well-being of humans.
Proanthocyanidins have been reported to having a wide range of medicinal properties, from protection against UV radiation from the sun, the improvement of vision to the strengthening of blood vessels 44, 45. Proanthocyanidins have also been reported to possess antioxidant, antibacterial, antiviral, anti-carcinogenic, anti-allergic and blood clotting potential 45, 46. The presence of proanthocyanidins in B. abyssinica infers that the species does have promising benefits in improving human health, and their isolation from this species could be useful in the production of medicines using naturally sourced compounds.
Plants have a variety of antioxidants that have the potential to inhibit the oxidant process under the influence of either atmospheric or reactive oxygen species 47. Phytochemicals have been shown to exhibit antioxidant activities due to their scavenging properties 14, 48. The current study revealed that the underground stems and roots have the strongest antioxidant activity against DPPH compared to the leaves. The hydrogen peroxide assay results have shown excellent scavenging activity as IC50 values were all below 1 mg/ml. B. abyssinica seems to have a slightly weaker activity against iron radicals in the metal chelating assay, with all values being above 2 mg/ml, however, the results indicate a good scavenging power. Overall, the results show that B. abyssinica possesses a good antioxidant activity, however, the underground stems and roots had better scavenging powers with IC50 values lower than 1 mg/ml in both DPPH and hydrogen peroxide assays. This indicates that the underground plant parts are better radical scavengers than the leaves, indicating a greater antioxidant source. The application of the underground stems and roots in the pharmaceutical industry could provide improved management and treatment options for the above mentioned illnesses associated with oxidative stress.
The antibacterial activity of the methanolic extracts of B. abyssinica showed intermediate zones of inhibition against Gram-positive S. aureus and Gram-negative E. coli. All plant parts exhibited growth inhibition against S. aureus and E. coli. Ahmed et al. (2020) 32 stated that the difference in the structure of the cell wall between Gram-positive and Gram-negative bacteria contributes to the bacteria’s susceptibility to the extracts. Gram-negative bacteria consist of a liposaccharide outer membrane, increasing the impermeability of substances, in this case, phytochemicals, whereas Gram-positive bacteria have a peptidoglycan outer membrane, making the bacteria highly permeable 32. As impermeable as E. coli is, because of its bacteriology, all the extracts of B. abyssinica were able to inhibit its growth. This could potentially present an opportunity for B. abyssinica leaves, underground stems and roots to be used in the manufacturing of antibacterial products such as antibiotic medication, as well as cosmetic products such as antibacterial creams, soaps and hand sanitizers.
The results from this study contributes information on the phytochemical analysis, antioxidant and antibacterial activity of the leaves, underground stems and roots of B. abyssinica, as there are currently limited studies on the comparative analyses of the different plant parts. This information could be extremely valuable to traditional communities and farmers who rely on natural remedies as they can be made aware of the benefits that each plant part offers in comparison to using the plant as a whole for its use in traditional and ethnoveterinary medicines. Pharmaceutical and cosmetic industries could also make use of this knowledge to make use of plant parts which could be beneficial to promote human health, depending on the products being manufactured.
Conclusion
The leaf extracts of B. abyssinica had a greater presence of the tested phytochemical groups, compared to their respective underground organs. The total phenolic, total flavonoid, total tannin and total proanthocyanidin contents of B. abyssinica showed a similar pattern in terms of the presence of various phytochemical groups, which were higher in the leaves than the other plant parts. The antioxidant activity assays showed a good activity against DPPH, hydrogen peroxide and metal chelating radicals, with the plant parts varying in scavenging potential. The antibacterial results showed an overall intermediate inhibition against S. aureus and E. coli, therefore the results validate the use of B. abyssinica in traditional and ethnoveterinary medicine, and further provides evidence that these plant species can be used in pharmaceutical and cosmetic industries.
Acknowledgement
Authors would like to extend thanks to the National Research Foundation (NRF) for their financial support, and the University of the Witwatersrand, Johannesburg school of Animal, Plant and Environmental Sciences Medicinal Plants laboratory for providing the facilities to conduct this research.
Conflict of Interest
There is no conflict of interest.
Funding Sources
There is no funding source.
References
- Yuan H, Ma Q, Ye L and Piao G. The traditional medicine and modern medicine from natural products. Molecules. 2016; 21: 559.
CrossRef - Bodede O and Prinsloo G. Ethnobotany, phytochemistry and pharmacological significance of the genus Bulbine (Asphodelaceae). Journal of Ethnopharmacol. 2020; 260: 112986.
CrossRef - Teffo T. K, Dukhan S, Ramalepe P and Risenga I. Possible implications of climate change on the medicinal properties of Bulbine species with a particular focus on Bulbine abyssinica, Bulbine frutescens and Bulbine natalensis in South Africa. J Pharmacogn Phytochem. 2021; 10: 49-56.
CrossRef - Gangwar K. K, Deepali G. R and Gangwar R. S. Ethnomedicinal plant diversity in Kumaun himalaya of Uttarakhand, India. Nat Sci. 2010; 8: 66-78.
- Srivastava J, Lambert J and Vietmeyer N. Medicinal plants: An expanding role in development. WB; 1996; 1-18.
CrossRef - Gurib-Fakim A. Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol Asp Med. 2006; 27:1-93.
CrossRef - Maphosa V and Masika P. J. Ethnoveterinary uses of medicinal plants: A survey of plants used in the ethnoveterinary control of gastro-intestinal parasites of goats in the Eastern Cape Province, South Africa. Pharm Biol. 2010; 48: 697-702.
CrossRef - McGaw LJ, Famuyide IM, Khunoana ET, Aremu AO. Ethnoveterinary botanical medicine in South Africa: A review of research from the last decade (2009 to 2019). J Ethnopharmacol. 2020; 257: 112864.
CrossRef - Rizwan H, Sajid M, Shamim A, Abbas H, Qudoos A and Maqbool M, et al. Sheep parasitism and its control by medicinal plants: A review. PUJ. 2021; 14: 112-21.
CrossRef - Mocktar C. Antimicrobial and chemical analyses of selected Bulbine species(Doctoral dissertation). University of Kwa Zulu-Natal. 2000; 1-78.
- Kibiti C. M and Afolayan A. J. Preliminary phytochemical screening and biological activities of Bulbine abyssinica used in the folk medicine in the Eastern Cape Province, South Africa. Evid Based Complement Alternat Med. 2015; 1-12.
CrossRef - Kibiti C. M and Afolayan A. J. Antifungal activity and brine shrimp toxicity assessment of Bulbine abyssinica used in the folk medicine in the Eastern Cape Province, South Africa. Bangladesh J Pharmacol. 2016; 11: 469-77.
CrossRef - Odeyemi SW, Afolayan AJ. Identification of antidiabetic compounds from polyphenolic-rich fractions of Bulbine abyssinica A. rich leaves. Pharmacogn Res. 2018; 10: 72.
- Idamokoro E. M and Afolayan A. J. In vitro Evaluation of the Phytochemical and Antioxidant Properties of Bulbine abyssinica Extracts. Int J Agric Biol. 2020; 24: 1781-7.
- Siddhuraju P, Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J Agric Food Chem. 2003; 51: 2144-55.
CrossRef - Pakade V, Cukrowska E and Chimuka L. Comparison of antioxidant activity of Moringa oleifera and selected vegetables in South Africa. S Afr J Sci. 2013; 109: 1-5.
CrossRef - Roghini R and Vijayalakshmi K. Phytochemical screening, quantitative analysis of flavonoids and minerals in ethanolic extract of Citrus paradisi. Int J Pharm Sci & Res. 2018; 9: 4859-64.
- Prabhavathi R. M, Prasad M. P and Jayaramu M. Studies on qualitative and quantitative phytochemical analysis of Cissus quadrangularis. Adv Appl Sci Res. 2016; 7: 11-7.
- Tepal P. Phytochemical screening, total flavonoid and phenolic content assays of various solvent extracts of tepal of Musa paradisiaca. Malaysian J Anal Sci. 2016; 20: 1181-90.
CrossRef - Yadav R. N and Agarwala M. Phytochemical analysis of some medicinal plants. J Phytol. 2011. 3: 10-14.
- Gul R, Jan S. U, Faridullah S, Sherani S and Jahan N. Preliminary phytochemical screening, quantitative analysis of alkaloids, and antioxidant activity of crude plant extracts from Ephedra intermedia indigenous to Balochistan. Sci World J. 2017; 1-7.
CrossRef - Ayoade E. T, Akinyemi A. O and Oyelere F. S. Phytochemical profile of different morphological organs of Moringa oleifera plant. J phytopharm. 2019; 8: 295-8.
CrossRef - Iqbal S and Bhanger M. I. Effect of season and production location on antioxidant activity of Moringa oleifera leaves grown in Pakistan. J Food Compost Anal. 2006; 19: 544-51.
CrossRef - Lahare R. P, Yadav H. S, Bisen Y. K and Dashahre A. K. Estimation of Total Phenol, Flavonoid, Tannin and Alkaloid Content in Different Extracts of Catharanthus roseus from Durg District, Chhattisgarh, India. 2021; 7: 1-6.
CrossRef - Oyedemi S. O, Bradley G and Afolayan A. J. In-vitro and-vivo antioxidant activities of aqueous extract of Strychnos henningsii Gilg. Afr J Pharm Pharmacol. 2010; 4: 070-8.
- Brand-Williams W, Cuvelier M. E and Berset C. L. Use of a free radical method to evaluate antioxidant activity. LWT. 1995; 28: 25-30.
CrossRef - Ruch R. J, Cheng S. J and Klaunig J. E. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. J Carcinog. 1989; 10: 1003-8.
CrossRef - Dinis T. C, Madeira V. M and Almeida L. M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994; 315: 161-9.
CrossRef - Martinez-Morales F, Alonso-Castro A. J, Zapata-Morales J. R, Carranza-Álvarez C and Aragon-Martinez O. H. Use of standardized units for a correct interpretation of IC50 values obtained from the inhibition of the DPPH radical by natural antioxidants. Chem Pap. 2020; 74: 3325-34.
CrossRef - Okeke M. I, Iroegbu C. U, Eze E. N and Okoli A. S, Esimone CO. Evaluation of extracts of the root of Landolphia owerrience for antibacterial activity. J Ethnopharmacol. 2001; 78: 119-27.
CrossRef - Jain I, Jain P, Bisht D, Sharma A, Srivastava B and Gupta N. Comparative evaluation of antibacterial efficacy of six Indian plant extracts against Streptococcus mutans. J Clin Diagn Res: . 2015; 9:ZC50.
CrossRef - Ahmed S, Moni B. M, Ahmed S, Gomes D. J and Shohael A. M. Comparative phytochemical, antioxidant, and antibacterial study of different parts of Doigota plants (Bixa orellana L.). Bull Natl Res Cent. 2020; 44: 1-0.
CrossRef - Saxena M, Saxena J, Nema R, Singh D and Gupta A. Phytochemistry of medicinal plants. J Pharmacogn Phytochem. 2013; 1, 168-182.
- Sulaiman CT, Balachandran I. Total phenolics and total flavonoids in selected Indian medicinal plants. Indian J Pharm Sci. 2012; 74: 258.
CrossRef - Huang W. Y, Cai Y. Z and Zhang Y. Natural phenolic compounds from medicinal herbs and dietary plants: potential use for cancer prevention. Nutr Cancer. 2009; 62: 1-20.
CrossRef - Zarza-García A. L, Sauri-Duch E, Raddatz-Mota D, Cuevas-Glory L. F, Pinzón-López L. L, Rivera-Cabrera F and Mendoza-Espinoza J. Pharmacological, phytochemical and morphological study of three Mayan accessions of Bixa orellana L. leaves. Emir J Food Agric. 2017; 163-9.
CrossRef - Truong, D.H., Nguyen, D.H., Ta, N.T.A., Bui, A.V., Do, T.H. and Nguyen, H.C., 2019. Evaluation of the use of different solvents for phytochemical constituents, antioxidants, and in vitro anti-inflammatory activities of Severinia buxifolia. J. Food Qual.2019; 1-9.
CrossRef - Panche A. N, Diwan A. D and Chandra S. R. Flavonoids: an overview. J Nutr Sci. 2016; 5, 1-15.
CrossRef - Ren W, Qiao Z, Wang H, Zhu L and Zhang L. Flavonoids: promising anticancer agents. Med Res Rev. 2003; 23: 519-34.
CrossRef - Pizzi A. Tannins medical/pharmacological and related applications: A critical review. Sustain Chem Pharm. 2021; 22: 100481.
CrossRef - Subbotina M. D, Timchenko V. N, Vorobyov M. M, Konunova Y. S, Aleksandrovih Y. S and Shushunov S. Effect of oral administration of tormentil root extract (Potentilla tormentilla) on rotavirus diarrhea in children: a randomized, double blind, controlled trial. Pediatr Infect Dis J. 2003; 22:706-11.
CrossRef - Russo M, Coppola V, Giannetti E, Buonavolontà R, Piscitelli A and Staiano A. Oral administration of tannins and flavonoids in children with acute diarrhea: a pilot, randomized, control-case study. Ital J Pediatr. 2018; 44: 1-6.
CrossRef - Abdalla S, Pizzi A and Bahabri F. S. Macro porous tannin spray-dried powder scaffolds with stem cells for bone engineering. Mater Chem Phys. 2020; 239: 121980.
CrossRef - Shi J, Yu J, Pohorly J. E and Kakuda Y. Polyphenolics in grape seeds-biochemistry and functionality. J Med Food. 2003; 6: 291-9.
CrossRef - Rauf A, Imran M, Abu-Izneid T, Patel S, Pan X, Naz S, Silva A. S, Saeed F and Suleria H. A. Proanthocyanidins: A comprehensive review. Biomed Pharmacother. 2019, 116: 108999.
CrossRef - Fine A. M. Oligomeric proanthocyanidin complexes: history, structure, and phytopharmaceutical applications. Alt Med Rev: a journal of clinical therapeutic. 2000. 5: 144-51.
- Pisoschi A. M and Negulescu G. P. Methods for total antioxidant activity determination: a review. Biochem Anal Biochem. 2011; 1:106.
CrossRef - Zheng W and Wang S. Y. Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem. 2001; 49:5165-70.
CrossRef







