Himangshu Mahato1* , Vaswati Das2
, Vaswati Das2 and Supreeti Biswas3
and Supreeti Biswas3
1pharmacology, the West Bengal University of Health Sciences, Siliguri, India.
2pathology, the West Bengal University of Health Sciences, Siliguri, India.
3pharmacology, the West Bengal University of Health Sciences, Kolkata, India.
Corresponding Author E-mail: drhimangshu27@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2499
Abstract
Background: Reduction of health cost burden with existing low-cost drug and thereby improving patient compliance is utmost necessary. Keeping in mind the above, we started with low cost, broad spectrum, WHO enlisted essential drug amikacin. We tried to revaluate it with another two low-cost drugs, L-carnitine, and Cholecalciferol. Objectives: Measurement of amikacin induced nephrotoxicity by means of abnormal renal biochemical parameters on albino rats and comparison of improvement after administration of L-carnitine & Cholecalciferol along with renal histopathology examination (HPE) of amikacin treated rats and causality assessment of amikacin induced adverse drug reactions (ADR) in hospitalized patient. Materials and Methods: Healthy albino male rats (N=40) were taken from Institutional animal house of Burdwan medical College and Hospital (BMCH) and were randomly divided into 4 groups. CPCSEA acclimatization guideline followed. IEAC and CREC clearances taken. Renal biochemical parameters from blood samples were analysed. Sterile water for injection was given to all group. Group I is control (only vehicle), Amikacin added to Group II, III and IV. L carnitine & Cholecalciferol was added to Group III & Group IV respectively. Post test measurement of renal biochemical parameters and HPE were done. Clinical observation of amikacin treated hospitalised patients and collection of their ADR in BMCH were done to find out correlations with animal experiment. Results: Statistical analyses were done using Graph Pad Prism version.4 software. Minimisation of amikacin induced nephropathy were seen, more in Group IV than Group III. HPE found the same conclusion. WHO UMC causality assessment revealed, 94.35% ADR were “probable/likely” whereas 5.65% were “possible”. The Naranjo’s adverse reaction probability scale revealed almost the same. Conclusion: Interventional animal experiment, biochemical parameters, histopathology along with open label, non-interventional, prospective observational study clearly indicates cholecalciferol is significantly better than L carnitine to minimise the effects of amikacin induced nephropathy.
Keywords
Amikacin; Amikacin induced nephropathy; Cholecalciferol; L-Carnitine
Download this article as:| Copy the following to cite this article: Mahato H, Das V, Biswas S. Comparative Experimental Evaluation of L-carnitine and Cholecalciferol on Amikacin Induced Nephrotoxicity and Clinical Evaluation of Amikacin Induced Adverse Drug Reactions. Biomed Pharmacol J 2022;15(3). |
| Copy the following to cite this URL: Mahato H, Das V, Biswas S. Comparative Experimental Evaluation of L-carnitine and Cholecalciferol on Amikacin Induced Nephrotoxicity and Clinical Evaluation of Amikacin Induced Adverse Drug Reactions. Biomed Pharmacol J 2022;15(3). Available from: https://bit.ly/3oheRYj |
Introduction
The kidney functions as the major excretory system, which makes it particularly vulnerable to nephrotoxic injury 1. Nephrotoxicity is a deleterious effect on the kidney due to some toxic chemicals, medication, or both and therefore some medications with predominantly renal excretion need their dose to be adjusted during compromised renal function.
There are so many useful drugs which have nephrotoxic potential like aminoglycosides, cisplatin, non-steroidal anti-inflammatory drugs, lithium, rifampicin etc. and they exert their toxic effects by altered intra glomerular hemodynamic, tubular cell toxicity, oxidative injury, inflammation, crystal nephropathy and thrombotic microangiopathy. 2, 3 Renal proximal tubule epithelial cells (RPTECs) comprise the bulk of the renal parenchyma and are the primary targets of a large variety of ischemic and toxic insults.4
Drug-induced toxicity has accounted for 20% of all primary aetiologies in renal injury. Pharmacological agent induced nephrotoxicity is further shown to be one of the chief aetiologies in intrinsic renal failure aside from the second most common cause, infection.2 In the era of modern medicine, patients are exposed to an expanding variety of drugs for diagnostic and therapeutic purposes. Unfortunately, some of these agents cause adverse drug effects linked with systemic toxicity, including impairment of renal function.5 Reduction of drug induced chronic kidney disease (CKD) requires attention to the unique pharmacologic considerations along with continuous assessment of the costs and benefits of any therapeutic intervention or diagnostic test. Maintaining patient safety within reasonable cost is likely to be as important as any currently available therapy for the disease, with just as great as benefit.6
Aminoglycoside antibiotics (AGs) are a critical component of the current antibacterial arsenal and widely used in meningitis, cystic fibrosis, multi drug resistant tuberculosis and bacterial sepsis in infants etc. 7 AGs still constitute the most effective therapeutic alternative against bacteria insensitive to other antibiotics because of their broad spectrum activity, fast bactericidal effect, favourable chemical and pharmacokinetic properties, synergy with beta lactam antibiotics, little resistance, and low cost. 8 Thus, despite undesirable nephrotoxic and ototoxic adverse effects, AGs like amikacin continues being frequently used as first as well as second choice drug in a vast variety of clinical situations.
Among different antioxidant supplements, L carnitine a quaternary ammonium compound, associated with various biological processes, including (1) improved insulin sensitivity (2) the acetylation of histones and (3) anti-inflammatory and (4) antioxidant processes and widely known for its involvement in the transport of long chain fatty acids into the mitochondria for energy production in peripheral tissues. 9,10 It is taken into cell by organic cation transporters. In humans and rats, these organic cation transporters are localized in the brain, heart, intestine, kidney, liver, lung, pancreas, placenta, thyroid, and trachea. 11 It has been suggested that L carnitine prevents impairment of fatty acid beta oxidation in mitochondria and plays a part in removing potentially toxic metabolites associated with the β-oxidation of fatty and this mechanism is essential in sequestering and subsequent removal of abnormal organic acids in several organic academia and to protect tissues from damage. 12 Inflammation and reactive oxygen substances play an important role in acute kidney injury pathophysiology.
Vitamin D is a pleiotropic hormone with several endocrine, paracrine, and autocrine effects on multiple tissues and organs, beyond skeletal homeostasis maintenance 13. Several studies have shown positive therapeutic efficacy of vitamin D and their analogues to reduce inflammation and proteinuria 14,15. A large, randomized placebo controlled clinical trial (the VITAL Study, 𝑛 = 281) confirmed that vitamin D analogue was able to reduce albuminuria and blood pressure in patients with diabetic nephropathy 16. Together, these clinical data provide a strong case to argue in favour of vitamin D analogues as a complementary therapy for treatment of proteinuria in drug induced nephropathy. Vitamin D and VDR (vitamin D receptor activation) preserves mitochondrial morphology and integrity in the renal tissue, and they play an essential role in podocyte preservation,17 although tubular effect of vitamin D is still debateable.
There are so many studies which showed L carnitine has nephroprotective role in aminoglycoside induced nephrotoxicity. Recently Vitamin D analogues are emerging as a nephroprotective pleotropic hormone, though there is paucity in data regarding their role in drug induced nephropathy.
In 2015, with the latest estimates, 176 million Indians were living in extreme poverty. 18 Therefore, it automatically follows the several public health-related challenges like tuberculosis, malnutrition, various chronic diseases etc. 19,20 Majority of the Indian population can’t afford growing health expenditure, so review or analysis of previously used low-cost medicines as well as new medicines both are necessary to reduce health burden.
With this backdrop a study was planned to evaluate nephroprotective role of cost-effective antioxidant L carnitine and a pleotropic hormone Cholecalciferol on amikacin treated experimental rat model through evaluation of some renal biochemical parameters and antioxidative biomarkers. Renal histopathology was also done to make the conclusion more specific. Our aim was to find the better alternative in between L carnitine and Cholecalciferol to minimise the nephrotoxic effects of amikacin. In our present study one of our objectives was to observe and assess adverse drug reaction (ADR) of amikacin through collection of biochemical renal parameters from amikacin treated hospitalised patients and collection of the same from amikacin treated experimental rat model.
We preferentially chosen amikacin in our study as it is on the World Health Organization’s List of Essential Medicines, a list of the most important medication needed in a basic health system.21
Materials and Methods
Paper materials
Informed Consent Form (ICF) in vernacular languages were used along with predesigned and pretested Case Record Form (CRF) including adverse drug reactions (ADR) check list.
Drugs and chemicals
Amikacin, L Carnitine, Cholecalciferol, and Pentothal for induction of anaesthesia were used in animal experiment. Amikacin [TABLE1] vials were gathered from hospital supplied samples. L carnitine [TABLE 1] & Cholecalciferol [TABLE 1] were taken from nearby medicine shop after proper checking of batch no and expiry date etc. All chemicals and reagents used for biochemical blood parameter analysis were available in the Department of Biochemistry. Pentothal was collected from the departmental laboratory and was used for rat scarification.
Equipment
Rat cage as per the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). 22 Disposable 2cc, 5cc, Tuberculin 1ml and 0.005ml glass syringes for intraperitoneal injection and for blood collection. Blood collecting container for urea and creatinine and labeller. Centrifuge machine (Electra-BL, India) to separate serum from the collected blood. Randox Autoanalyzer (rx Daytona+, Ireland) to measure urea and creatinine value. Dissecting tray with proper sterile animal dissecting instruments. Formalin containing airtight container to dispatch animal viscera for histopathology examination. Spectronic 21 Spectrophotometer (Milton Roy Company, United States) for Malondialdehyde (MDA) analysis and Microscope (Olympus CX21i, Japan)).
Table 1: Test Drugs Details.
| Brand Name | Volume | Presentation | Manufactured by | Batch No. | Expiry Date |
| AMIKACIN SULPHATE* | |||||
| Endocin-500 | 2ml | Endocin-500 INJ. | Adley Formulations
(Hospital supply) |
AVS-3082 | 12/2015 |
| L-CARNITINE | |||||
| Carnisure-1g | 5ml | Carnisure-1gm/5ml INJ | Elder Pharmaceuticals | B69773 | 01/2016 |
| CHOLECALCIFEROL | |||||
| Vitanova D3 3L | 1ml | Vitanova D3 3L
Injection IP |
Zuventus Healthcare Ltd. | DLA15001 | 03/2016 |
* All amikacin sulphate injection (10 vial) were of same batch number and expiry date.
Methodology
The Study was carried out in the Department of Pharmacology in collaboration with The Department of Biochemistry, Pathology, and various clinical departments of BMCH. Approval from The Institutional Animal Ethics Committee (IAEC) and Clinical Research Ethics Committee (CREC) were obtained prior to initiation of study.
Animal study
Adult healthy male albino rats (N=40) with weight of 100 – 150 grams and those previously not investigated for the same purpose were taken from the institutional animal house, BMCH and kept in The Department of Pharmacology laboratory for acclimatization for a week under environmentally controlled conditions with free access to food and water (ad libitum).They were kept in standard metallic clean rat cages after maintaining the temperature within a range of (25-28 0C) and in 12 hour light /12 hour dark cycle. Experiments were carried out as per CPCSEA guidelines.
Animals were randomly divided into four groups of 10 rats each as follows.
Group-I (n1=10) [Control]: Sterile water for injection used as a vehicle and was given in same volume to each rat through intra peritoneal route (i.p). Amikacin was given for the same duration of 5 days. Sterile water was used from the same sealed pack of amikacin injection with same batch number.
Group-II (n2=10) [Amikacin + Vehicle]: Amikacin was given intra peritoneally (i.p) at a single dose of 1.2gm / kg body weight (b.w)/day for 5 days. [23] Volume of the injection was determined as per the body weight of the individual rat.
Group-III (n3=10) [Amikacin + L carnitine + Vehicle]: L Carnitine was given intra peritoneally along with amikacin and vehicle at a single dose of 200mg/kg b.w/day for 5 days. [24] Volume of the injection was determined as per the body weight of the individual rat.
Group-IV (n4=10) [Amikacin + Cholecalciferol + Vehicle]: Cholecalciferol was given intra-peritoneally along with Amikacin and vehicle at a single dose of 250 IU/100gm b.w/day for 5 days. [25] Volume of the injection was determined as per the body weight of the individual rat.
Collection of blood
Blood was collected twice: one at baseline (before giving injection) from retro orbital plexus to measure biochemical profile, and second after completion of injection (sixth day after starting therapy) as per the group requirements. This time blood was collected from the heart of the animal, after sacrificing them as per the study need. The blood samples were collected into non heparinized micro well vials and were allowed to coagulate at room temperature. Thereafter, centrifuged at 3000 rpm for 20 minutes to extract the sera and stored at -20°C. Serum urea and creatinine were measured in autoanalyzer. MDA were analysed from the above clotted blood using spectrophotometer (spectronic-21).
Renal tissues were taken out after scarification and formalin preserved. All the tissues were sent to the Department of Pathology for histopathology examination (HPE) after proper labelling.
Serum urea
Serum urea was measured by using enzymatic assay (Berthelot method). 26 Urease catalyses the conversion of urea into ammonia and CO2. Released ammonia reacts with salicylate in presence of nitroprusside and hypochlorite, giving a blue green coloured complex.
Absorbance of the coloured solution is directly proportional to the urea concentration, when measured at 578 nm. 27
Serum creatinine
Serum creatinine was measured by modified Jaffe’s method. Most routine serum creatinine assays in current use have evolved from the reaction first described by Jaffe in 1886. 28 Creatinine reacts with picric acid at alkaline pH to form a yellow orange complex. 29
Creatinine + Alkaline Picrate →→→→ Yellow Orange Complex
Kinetic Jaffe’s method involves mixing serum with alkaline picrate and reading the rate of change in absorption spectrophotometer at 520 nm. This not only standardized the procedure, but also removed the need for sample deproteinization. 30
Serum Malondialdehyde (MDA)
Thiobarbituric acid test (TBA) 31 was done. Polyunsaturated fatty acids react with molecular oxygen to undergo free radical mediated auto oxidation. Numerous non-volatile peroxides and aldehyde compounds are formed during this process and decomposed to form MDA and remaining other substances react with TBA to form a pink colour compound. Then the product is readily extractable into butanol and read absorbance at 532mm.
Estimation of MDA in samples was done as follows:
The tube containing 0.5 ml of freshly prepared serum and 2.5ml of TCA was allowed to stand for 10minutes at room temperature. The tube was stirred thoroughly after admixing 2.5ml of sulphuric acid, thereafter 3.5 ml of TBA reagent was added to this. The coupling of lipid peroxide with TBA was carried out by heating in boiling water bath for 30 minutes and then cooled in water. N butanol amounting 4.0ml was added and chromogen was extracted to organic phase by vigorous shaking.
Separation of the organic phase by centrifugation at 3000rpm for 10minutes was done and the supernatant organic phase was pipetted to a clean test tube. Its absorbance was determined at 532nm wavelength by spectrophotometer (spectronic 21). N butanol was used as blank to assure zero reading. The optical density was noted, and level of MDA was calculated from standard curve. MDA values were expressed in nmole/ml in case of serum.
Preparation of histopathological slide
After completion of the treatment period according to experimental design, the animals were anesthetized using Pentothal sodium and sacrificed for tissue collection. 32 Kidneys were dissected out and collected carefully. Organs were fixed in 10% neutral buffer formalin for 24 hour and grossing was done after careful removal of adherent fat tissues. 33 Followed by fixation, dehydration of the tissues was conducted by immersing the tissue in a series of gradually increasing concentrations of alcohol (50%, 70%, 80%, 95% and absolute alcohol) and embedded into paraffin wax for making blocks. Sectioning of the tissue (thickness around 5 microns) was performed by using a microtome (Leica, Germany). 34 Small ribbon of different tissue sections were placed on microscopic slide with help of warm distilled water containing few drops of Mayer’s albumin and deparaffinised with xylene solution. Staining had been done by Harris haematoxylin & eosin yellow solution using standard procedure for preparing permanent slide. 35 Histopathological changes were observed and interpreted under 20x of a light microscope and significant sections were photographed. Special stain like PAS stain for histopathology was not done due to non availability of reagent during the study period.
Statistical analysis
Microsoft Excel 2010 and Graph Pad Prism version.4 were used for statistical analysis. One way analysis of variance (ANOVA) followed by paired-t test was carried out to compare continuous parametric values within groups.
Clinical observation
It was designed as an open label, non-interventional, prospective, observational study. Patients admitted in various in-patient department of a tertiary care teaching hospital (BMCH), were recruited as per inclusion and exclusion criteria and after collection of written informed consent. Illiterate patients gave their fingerprint (left thumb impression) in presence of appropriate witness. All indoor patient of both gender and of all ages and otherwise healthy, who would receive amikacin chemotherapy, were under inclusion criteria. No changes were made in the treatment decision, schedule, or duration during the study period.
Patient who was unable or not willing to give consent. Patients who were receiving chemotherapy regimens other than amikacin. Patients who were pregnant or were willing to go for pregnancy. Patients who were with renal impairment and with very poor general condition. Patients who were with any other major systemic ailment according to physician’s discretion. Those who were participated in any other clinical trial within past 3 months. Those who were receiving medication which might interact with the study medication. All of these were under the exclusion criteria.
Amikacin injection doses used in hospital were 500mg twice daily I.V with adequate hydration. All hospitalized patients of our study received full course of 5 days amikacin chemotherapy as per the directions given in bed head ticket (BHT) by the treating physician.
Baseline laboratory values such as serum urea and creatinine were collected from the BHT, before and after the completion of amikacin therapy. Increased serum urea, increased serum creatinine, oliguria and swelling of face were taken as indicator of nephropathy whereas fullness in ear, tinnitus and loss of hearing were taken as indicator of ototoxicity. Collection of the above data was done in specified case report format (CRF), as a part of ADR information associated with our study.
Table 2: Assessment parameters for clinical observation.
| Parameters | Laboratory parameters | Base line visit | After completion of therapy |
| Medical history | Urea | √ | √ |
| Clinical examination | Creatinine | √ | √ |
| Adverse effects | Concomitant medications | √ | √ |
Concomitant medication
Comorbid patients with other medications were allowed to be take their medication, provide that they did not have any significant interaction with the study drugs.
Causality assessment
Causality assessment of ADR were done through WHO UMC causality assessment scale and Naranjo’s algorithm.
Results and Discussion
Animal experiment
A total 40 adult healthy male albino rats with weight of 100 grams to 150 grams and those previously not investigated for the same purpose were randomly divided into 4 groups, 10 rats each. Blood samples were collected group wise from each rat before initiation of the treatment and taken as baseline values (B). Blood samples were again collected after completion of the treatment and recorded as Post-test value (E). Urea, Creatinine (Cr) and Malondialdehyde (MDA) were measured using specific standard procedure mentioned before and analysed [Table 3] thereafter.
Table: 3: Mean Baseline Score.
| Parameters | GrI (n1=10) | GrII (n2=10) | GrIII (n3=10) | GrIV (n4=10) | P value |
| UREA | |||||
| Range | 21-33 | 23-33 | 21-33 | 23-33 | |
| Median | 27 | 26.50 | 26.50 | 29.00 | |
| Mean±SEM | 27.3±1.274 | 27±1.116 | 26.3±1.212 | 28.6±1.097 | 0.5766 (NS) |
| CREATININE | |||||
| Range | 0.4-0.6 | 0.4-0.6 | 0.4-0.6 | 0.4-0.6 | |
| Median | 0.5 | 0.5 | 0.5 | 0.5 | |
| Mean±SEM | 0.5±0.02582 | 0.48±0.02494 | 0.48±0.02494 | 0.49±0.02769 | 0.9373 (NS) |
| MDA | |||||
| Range | 1.760-2.470 | 1.430-2.320 | 1.250-2.110 | 1.230-2.310 | |
| Median | 1.945 | 1.905 | 1.660 | 1.840 | |
| Mean±SEM | 2.037±0.08146 | 1.928±0.09344 | 1.686±0.07671 | 1.809±0.1276 | 0.0800 (NS) |
n=Sample size, SEM=Standard error of mean, NS= Not significant.
P values calculated from ANOVA test. Post test P values were not calculated because the P value was greater than 0.05. ANOVA assumes that the data are sampled from populations with identical SDs. This assumption is tested using Bartlett method.
Bartlett statistic (corrected) = 2.871. The P value is 0.4120. Bartlett’s test suggests that the difference among the SDs is not significant.
ANOVA assumes that the data are sampled from populations that follow Gaussian distributions. This assumption is tested using Kolmogorov and Smirnov (KS) method.
Table 4 Summary of Data.
| Group | Sample size | Mean | SD | SEM | Median |
| GrI (MDA)B | 10 | 2.037 | 0.2576 | 0.08146 | 1.945 |
| GrII (MDA)B | 10 | 1.928 | 0.2955 | 0.09344 | 1.905 |
| GrIII (MDA)B | 10 | 1.686 | 0.2426 | 0.07671 | 1.660 |
| GrIV (MDA)B | 10 | 1.809 | 0.4034 | 0.1276 | 1.840 |
| Group | Minimum | Maximum | 95% Confidence Interval | ||
| From | To | ||||
| GrI (MDA)B | 1.760 | 2.470 | 1.853 | 2.221 | |
| GrII (MDA)B | 1.430 | 2.320 | 1.717 | 2.139 | |
| GrIII (MDA)B | 1.250 | 2.110 | 1.512 | 1.860 | |
| GrIV (MDA)B | 1.230 | 2.310 | 1.520 | 2.098 | |
MDA=Malondialdehyde, SD=Standard deviation, SEM=Standard error of mean.
Table 5: GROUP I (Control) [SUMMARY] [Student paired t test].
| PARAMETERS | Gr I-(B) | Gr I-(E) | P value |
| UREA | |||
| Range | 21-33 | 23-32 | |
| Median | 27 | 26.50 | |
| Mean±SEM | 27.3±1.274 | 27.1±1.016 | 0.6618 (NS) |
| CREATININE | |||
| Range | 0.4-0.6 | 0.4-0.7 | |
| Median | 0.5 | 0.5 | |
| Mean±SEM | 0.5±0.02582 | 0.53±0.03000 | 0.1934 (NS) |
| MDA | |||
| Range | 1.760-2.470 | 1.750-2.480 | |
| Median | 1.945 | 1.955 | |
| Mean±SEM | 2.037±0.08146 | 2.046±0.08358 | 0.1467 (NS) |
Table 6: GROUP II (Amikacin+ Vehicle) [SUMMARY] [Student paired t test]
| PARAMETER | Gr II-(B) | Gr II-(E) | P value |
| UREA | |||
| Range | 23-33 | 53-65 | |
| Median | 26.50 | 59 | |
| Mean±SEM | 27±1.116 | 59.4±1.194 | *** P ˂ 0.0001 |
| CREATININE | |||
| Range | 0.4-0.6 | 1.020-1.100 | |
| Median | 0.5 | 1.085 | |
| Mean±SEM | 0.48±0.02494 | 1.074±0.009214 | *** P ˂ 0.0001 |
| MDA | |||
| Range | 1.430-2.320 | 6.970-8.630 | |
| Median | 1.905 | 7.775 | |
| Mean±SEM | 1.928±0.09344 | 7.741±0.1914 | *** P ˂ 0.0001 |
Table 7: GROUP III (Amikacin + L Carnitine + Vehicle) [summary] [Student paired t test]
| PARAMETER | GrIII-(B) | GrIII-(E) | P value |
| UREA | |||
| Range | 21-33 | 41-51 | |
| Median | 26.50 | 47.50 | |
| Mean±SEM | 26.3±1.212 | 47.4±1.013 | *** P ˂ 0.0001 |
| CREATININE | |||
| Range | 0.4-0.6 | 0.7-1.0 | |
| Median | 0.5 | 0.85 | |
| Mean±SEM | 0.48±0.02494 | 0.85±0.02687 | *** P ˂ 0.0001 |
| MDA | |||
| Range | 1.250-2.110 | 2.720-3.790 | |
| Median | 1.660 | 3.120 | |
| Mean±SEM | 1.686±0.07671 | 3.178±0.1176 | *** P ˂ 0.0001 |
Table 8: GROUP IV (Amikacin + Cholecalciferol + Vehicle)[summary][Student paired t test]
| PARAMETER | GrIV (B) | GrIV (E) | P value |
| UREA | |||
| Range | 23-33 | 32-39 | |
| Median | 29 | 36 | |
| Mean±SEM | 28.6±1.097 | 35.4±0.7024 | ***P=0.0002 |
| CREATININE | |||
| Range | 0.4-0.6 | 0.5-0.9 | |
| Median | 0.5 | 0.7 | |
| Mean±SEM | 0.49±0.02769 | 0.7±0.03651 | ***P ˂ 0.0001 |
| MDA | |||
| Range | 1.230-2.310 | 2.980-4.640 | |
| Median | 1.840 | 3.830 | |
| Mean±SEM | 1.809±0.1276 | 3.78±0.1800 | ***P ˂ 0.0001 |
Table 9: Comparison between Group I, Group II, Group III and Group IV [Post test values].
| Parameters | GrI (n1=10) | GrII (n2=10) | GrIII (n3=10) | GrIV(n4=10) | P value |
| UREA | |||||
| Range | 23-32 | 53-65 | 41-51 | 32-39 | |
| Median | 26.5 | 59 | 47.5 | 36 | |
| Mean±SEM | 27.1±1.016 | 59.4±1.194 | 47.4±1.013 | 35.4±0.7024 | *** P ˂0.0001 |
| CREATININE | |||||
| Range | 0.4-0.7 | 1.02-1.10 | 0.7-1.0 | 0.5-0.9 | |
| Median | 0.5 | 1.085 | 0.85 | 0.7 | |
| Mean±SEM | 0.53±0.030 | 1.074±0.0092 | 0.85±0.027 | 0.7±0.036 | *** P ˂0.0001 |
| MDA | |||||
| Range | 1.75-2.48 | 6.97-8.63 | 2.72-3.79 | 2.98-4.64 | |
| Median | 1.95 | 7.77 | 3.12 | 3.83 | |
| Mean±SEM | 2.046±0.083 | 7.741±0.191 | 3.178±0.117 | 3.78±0.180 | *** P ˂0.0001 |
*** considered extremely significant.
UREA [Post test values]
Tukey Kramer Multiple Comparisons Test: If the value of q is greater than 3.813 then the P value is less than 0.05.
Table 10: Group I, Group II, Group III and Group IV [Urea Post test].
| Comparison | Mean Difference | q | P value |
| GrI (Urea)-E vs GrII (Urea)-E | -32.300 | 32.386 | *** P˂ 0.001 |
| GrI (Urea)-E vs GrIII (Urea)-E | -20.300 | 20.354 | *** P˂ 0.001 |
| GrI (Urea)-E vs Gr IV (Urea)-E | -8.300 | 8.322 | *** P˂ 0.001 |
| GrII (Urea)-E vs GrIII (Urea)-E | 12.000 | 12.032 | *** P˂ 0.001 |
| GrII (Urea)-E vs Gr IV (Urea)-E | 24.000 | 24.064 | *** P˂ 0.001 |
| GrIII (Urea)-E vs Gr IV (Urea)-E | 12.000 | 12.032 | *** P˂ 0.001 |
| Difference | Mean Difference | 95% confidence interval | |
| From | To | ||
| GrI (Urea)E – GrII (Urea)E | -32.300 | -36.103 | -28.497 |
| GrI (Urea)E– GrIII (Urea)E | -20.300 | -24.103 | -16.497 |
| GrI (Urea)E − GrIV (Urea)E | -8.300 | -12.103 | -4.497 |
| GrII (Urea)E − GrIII (Urea)E | 12.000 | 8.197 | 15.803 |
| GrII (Urea)E − GrIV (Urea)E | 24.000 | 20.197 | 27.803 |
| GrIII (Urea)E − GrIV (Urea)E | 12.000 | 8.197 | 15.803 |
Assumption test: Kolmogorov and Smirnov (KS) method have been used.
| Group | KS | P value | Passed normality test? |
| GrI (Urea)E | 0.1433 | ˃ 0.10 | YES |
| GrII (Urea)E | 0.1422 | ˃ 0.10 | YES |
| GrIII (Urea)E | 0.1694 | ˃ 0.10 | YES |
| GrIV (Urea)E | 0.2065 | ˃ 0.10 | YES |
(Urea) E=Urea post test values.
Creatinine [Post Test Values]
Tukey Kramer Multiple Comparisons Test
Table 11: Group I, Group II, Group III and Group IV [Creatinine Post test].
| Comparison | Mean Difference | q | P value |
| GrI (Cr)E vs GrII (Cr)E | -0.5440 | 19.732 | ***P˂ 0.001 |
| GrI (Cr)E vs GrIII (Cr)E | -0.3200 | 11.607 | ***P˂ 0.001 |
| GrI (Cr)E vs Gr IV (Cr)E | -0.1700 | 6.166 | ***P˂ 0.001 |
| GrII (Cr)E vs GrIII (Cr)E | 0.2240 | 8.125 | ***P˂ 0.001 |
| GrII (Cr)E vs Gr IV (Cr)E | 0.3740 | 13.565 | ***P˂ 0.001 |
| GrIII (Cr)E vs Gr IV (Cr)E | 0.1500 | 5.441 | **P˂ 0.01 |
| Difference | Mean Difference | 95% confidence interval | |
| From | To | ||
| GrI (Cr)E – GrII (Cr)E | -0.5440 | -0.6491 | -0.4389 |
| GrI (Cr)E– GrIII (Cr)E | -0.3200 | -0.4251 | -0.2149 |
| GrI (Cr)E− GrIV (Cr)E | -0.1700 | -0.2751 | -0.06489 |
| GrII (Cr)E − GrIII (Cr)E | 0.2240 | 0.1189 | 0.3291 |
| GrII (Cr)E − GrIV (Cr)E | 0.3740 | 0.2689 | 0.4791 |
| GrIII (Cr)E − GrIV (Cr)E | 0.1500 | -0.04489 | 0.2551 |
Assumption test: Kolmogorov and Smirnov (KS) method have been used.
| Group | KS | P value | Passed normality test? |
| GrI (Cr)E | 0.2441 | ˃0.10 | YES |
| GrII (Cr)E | 0.2085 | ˃0.10 | YES |
| GrIII (Cr)E | 0.2218 | ˃0.10 | YES |
| GrIV (Cr)E | 0.2000 | ˃0.10 | YES |
(Cr) E=Creatinine post test values
MDA [Post test values]
Tukey Kramer Multiple Comparisons Test
Table 12: Group I, Group II, Group III and Group IV [MDA Post test]
| Comparison | Mean Difference | q | P value |
| GrI (MDA)E vs GrII (MDA)E | -5.695 | 37.995 | ***P˂ 0.001 |
| GrI (MDA)E vs GrIII (MDA)E | -1.132 | 7.552 | ***P˂ 0.001 |
| GrI (MDA)E vs Gr IV (MDA)E | -1.734 | 11.569 | ***P˂ 0.001 |
| GrII (MDA)E vs GrIII (MDA)E | 4.563 | 30.442 | ***P˂ 0.001 |
| GrII (MDA)E vs Gr IV (MDA)E | 3.961 | 26.426 | ***P˂ 0.001 |
| GrIII (MDA)E vs Gr IV (MDA)E | -0.6020 | 4.016 | **P˂ 0.05 |
| Difference | Mean Difference | 95% confidence interval | |
| From | To | ||
| GrI (MDA)E – GrII (MDA)E | -5.695 | -6.266 | -5.124 |
| GrI (MDA)E– GrIII (MDA)E | -1.132 | -1.703 | -0.5605 |
| GrI (MDA)E− GrIV (MDA)E | -1.734 | -2.305 | -1.163 |
| GrII (MDA)E − GrIII (MDA)E | 4.563 | 3.992 | 5.134 |
| GrII (MDA)E − GrIV (MDA)E | 3.961 | 3.390 | 4.532 |
| GrIII (MDA)E − GrIV (MDA)E | -0.6020 | -1.173 | -0.03053 |
Assumption test: Kolmogorov and Smirnov (KS) method have been used.
| Group | KS | P value | Passed normality test? |
| GrI (MDA)E | 0.1839 | ˃0.10 | YES |
| GrII (MDA)E | 0.1617 | ˃0.10 | YES |
| GrIII (MDA)E | 0.1657 | ˃0.10 | YES |
| GrIV (MDA)E | 0.1695 | ˃0.10 | YES |
(MDA) E=MDA post test values.
Table 13: Differences of Mean of individual variables of individual group before test (B) and after test(E).
| Parameters | Group I | Group II | Group III | Group IV |
| UREA | ||||
| Mean (B) | 27.3 | 27 | 26.3 | 28.6 |
| Mean (E) | 27.1 | 59.4 | 47.4 | 35.4 |
| Difference (E-B) | -0.2 | 32.4 | 21.1 | 6.8 |
| CREATININE | ||||
| Mean (B) | 0.5 | 0.48 | 0.48 | 0.49 |
| Mean (E) | 0.53 | 1.074 | 0.85 | 0.70 |
| Difference (E-B) | 0.03 | 0.594 | 0.37 | 0.21 |
| MDA | ||||
| Mean (B) | 2.037 | 1.928 | 1.686 | 1.809 |
| Mean (E) | 2.046 | 7.741 | 3.178 | 3.78 |
| Difference (E-B) | 0.009 | 5.813 | 1.492 | 1.971 |
Histopathological Analysis
After scarification of each group of rat and preparation of renal HPE slides through different stages told above, following significant changes are observed. All samples were examined by the same pathologist who was unaware of which samples originated from which group. Tubular degeneration, tubular necrosis and tubular interstitial inflammation were evaluated semi-quantitatively and graded as mild, moderate, severe, and very severe based on histopathological features of representative field area. Tubular degeneration, tubular necrosis and tubular interstitial inflammation were defined as following.
Tubular degeneration (TD) was defined as vacuolization and increasing acidophilic cytoplasm of the proximal tubule epithelial cells. Tubular necrosis (TN) was defined as loss of nucleus in the epithelial cells, dark acidophilic cytoplasm, and loss of tubular epithelial cells into tubular lumen, maintaining the outline of the tubules. Infiltration of inflammatory cells in perivascular and interstitial areas were considered as Tubulointerstitial inflammation (TII) and it is also a predictor of progression to renal failure.36 Additionally, proteinaceous casts and medullary vascular congestion (MVC) were also included. 37 Presence of vascular congestion and erythrocytes were considered as medullary vascular congestion. 38 Histopathology slides were examined using Olympus CX 21i microscope and photographs were captured by Leica DFC 450 digital camera.
Group I (Control).
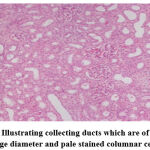 |
Figure 1: Illustrating collecting ducts which are of relatively large diameter and pale stained columnar cells. |
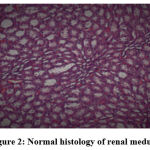 |
Figure 2: Normal histology of renal medulla. |
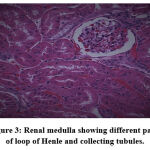 |
Figure 3: Renal medulla showing different parts of loop of Henle and collecting tubules. |
Group II (Amikacin + Vehicle).
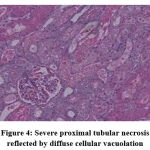 |
Figure 4: Severe proximal tubular necrosis reflected by diffuse cellular vacuolation. |
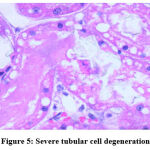 |
Figure 5: Severe tubular cell degeneration. |
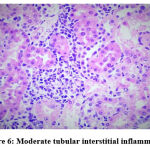 |
Figure 6: Moderate tubular interstitial inflammation. Click here to view figure |
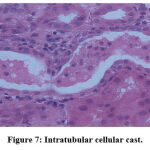 |
Figure 7: Intratubular cellular cast. |
Group III (Amikacin + Vehicle + L-Carnitine).
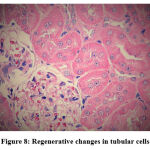 |
Figure 8: Regenerative changes in tubular cells. |
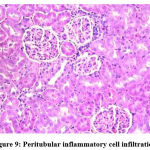 |
Figure 9: Peritubular inflammatory cell infiltration. |
Group IV (Amikacin + Vehicle + Cholecalciferol).
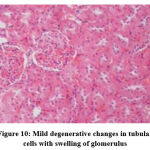 |
Figure 10: Mild degenerative changes in tubular cells with swelling of glomerulus. |
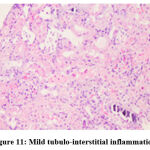 |
Figure 11: Mild tubulo-interstitial inflammation. |
Table 14: Histopathological analysis.
| HP Slides | GrI (n1=10) | GrII (n2=10) | GrIII (n3=10) | GrIV (n4=10) | |
| Parameters examined | Semi quantitative grading | ||||
| TD | Mild (0-10%) | 0 | 0 | 2 | 2 |
| Moderate (10-25%) | 0 | 1 | 1 | 0 | |
| Severe (25-50%) | 0 | 7 | 0 | 0 | |
| Very severe (˃ 50%) | 0 | 2 | 0 | 0 | |
| TN | Mild (0-10%) | 0 | 0 | 2 | 0 |
| Moderate (10-25%) | 0 | 4 | 0 | 0 | |
| Severe (25-50%) | 0 | 4 | 0 | 0 | |
| Very severe (˃ 50%) | 0 | 2 | 0 | 0 | |
| TII | Mild (0-10%) | 0 | 2 | 1 | 0 |
| Moderate (10-25%) | 0 | 7 | 0 | 0 | |
| Severe (25-50%) | 0 | 1 | 0 | 0 | |
| Very severe (˃ 50%) | 0 | 0 | 0 | 0 | |
| MVC | Mild (0-10%) | 0 | 2 | 2 | 0 |
| Moderate (10-25%) | 0 | 6 | 0 | 0 | |
| Severe (25-50%) | 0 | 2 | 0 | 0 | |
| Very severe (˃ 50%) | 0 | 0 | 0 | 0 | |
| CASTS | Diffuse | 0 | 4 | 0 | 0 |
| Focal | 0 | 6 | 0 | 0 | |
n= sample size
Clinical Observation
Causality Assessment of Amikacin induced ADRs
Most frequent ADRs were increased urea, increased creatinine, oliguria, swelling of face, tinnitus and feeling of fullness in ear. No patient reported hearing loss. Adverse reaction profiles of the patients are depicted in Fig [12]. Causality assessment showed all 71 subjects developed ADRs. Total ADRs were 230. Causality assessment revealed that 217 ADRs (94.35%) belonged to “probable/likely” category, whereas 13 (5.65%) were of “possible” type according to the WHO-UMC scale. Figure [13] gave a pictorial view of this observation. No case could be labelled “certain”, as rechallenge was not attempted by the attending physician. The Naranjo’s adverse reaction probability scale also revealed almost the similar finding i.e., 95.22% were in “probable” category and 4.78% were in “possible” category.
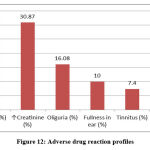 |
Figure 12: Adverse drug reaction profiles. |
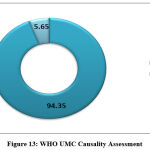 |
Figure 13: WHO UMC Causality Assessment. |
Analysis
Amikacin is a commonly used antibacterial drug that can cause significant nephrotoxic effects in both human and experimental animals. It has been reported that one mechanism of the toxic effects of aminoglycoside antibiotic are the result of oxidative reactions.39
Animal Experiment
In this study, we investigated whether the oxidative stress parameters of experimental groups were statistically significant or not among the tissues. MDA was measured from rat blood to see the protective role of L Carnitine & Cholecalciferol on amikacin induced nephropathy. In addition, serum creatinine and urea were determined as indicators of nephropathy. There are so many studies where antioxidants were used with aminoglycosides to see their nephroprotective potential, but in all such cases antioxidants were used few hour/days before giving amikacin [40,41,42] which may increase patient’s hospital stay and therefore hospital related expenses, keeping in mind the above we tried to use L Carnitine and Cholecalciferol along with the Amikacin for same duration.
We made an analysis with those baseline values (B) of individual variables in all 4 groups and found insignificant P values [Table 3] which were quite expected as chemotherapy was not started that time. We again collected group wise blood samples after completion of the chemotherapy schedule (E) for further analysis of the same variables (urea, creatinine, and MDA).Paired t test of group wise individual variables (B & E) was done using Graph Pad Prism version.4.
We know that p values < 0.05 is significant. We found, P values in group I were insignificant for all variable [Table: 5] as per expectations, as only sterile water for injection was given to them. In group II, group III and in group IV, all p values found were significant for each variable [Table: 6-8]. The paired t test clearly denotes that amikacin, amikacin and L Carnitine, amikacin and cholecalciferol all increase urea, creatinine, and MDA but our objective was to find a better combination which is least nephrotoxic. Therefore again, we started analysis in between all groups [Table 9] and taking all individual post-test values using ANOVA. Here we found extremely significant p value of ˂ 0.0001, in each group and in each variable [TABLE 10-12]. Variation among column means it is significantly greater than expected by chance, therefore comparison of post-test values of urea, creatinine, and MDA were done in between all 4 groups by means of Tukey Kramer Multiple Comparisons Test and found significant p values in every group. All baseline (B) and post-test values (E) were tested for normality through assumption test and passed. Mean of each variable in each group before and after the therapy was tabulated and difference between them was calculated [TABLE 13]. We found that, baseline mean of urea were almost equal for each group whereas mean of post test values were highest, second highest, third highest and least in group II, III, IV and in group I, respectively. Least post test mean value of urea was quite expected in group I, as no medication was used there. It denotes that, group IV (amikacin + vehicle + cholecalciferol) is less nephrotoxic than group III (amikacin + vehicle + L Carnitine). Indirectly it makes cholecalciferol a better drug to use with amikacin, than L Carnitine. In case of creatinine, baseline values were almost equal for each group whereas mean of post test values were highest, second highest, third highest and least in group II, III, IV and I respectively. Again, least post-test mean value of creatinine in group I, can easily be explained as no medication was used in this group. It denotes that, group IV is less nephrotoxic than group III. Indirectly it gives cholecalciferol again a higher position than L Carnitine in respect to nephroprotective potential. Like urea and creatinine, result of MDA was no exception. Though, post-test mean value of MDA in group III (3.178) and group IV (3.78) was close enough but statistically it was significant to reach to an opinion [TABLE 13] that Cholecalciferol is slightly better than L Carnitine here to combat oxidative stress.
Biochemical parameters are basically indirect evidence of nephropathy, so we planned for renal histopathology which is more specific and direct evidence of nephropathy. In control group (group I), all histopathology slides were normal [ Fig. 1-3] as expected because no medications were used there. Group II showed enormous changes like severe proximal tubular necrosis, severe tubular cell degeneration [Fig.5] reflected by diffuse cellular vacuolation [Fig.4], moderate tubular interstitial inflammation [Fig.6] and intratubular cellular cast [Fig.7] etc. which clearly indicates nephropathy. As amikacin were used in group II and vehicle had no effects on kidney. Group II histopathology and biochemical parameter analysis clearly indicates amikacin as the culprit. Amikacin administration led to granulovacuolar tubular degeneration in light microscopic examination whereas myeloid bodies, mitochondrial electron dense material deposition and mitochondrial swelling in electron microscopic evaluation.[39] That study also suggests that in elderly and in compromised renal condition, doses of amikacin should be lower than those administered to young adults. Nevertheless, administration interval may have to be prolonged to guarantee no toxic accumulation of amikacin. Regenerative changes in tubular cells [Fig.8] and restoration of normal microanatomy to some extent with Peritubular inflammatory cell infiltration [Fig.9] were found in group III histopathology where L Carnitine was used along with the amikacin, and it clearly showed beneficial effects of L Carnitine. Group IV histopathology report showed mild degenerative changes in tubular cells with swelling of glomerulus [Fig.10] and mild tubulointerstitial inflammation [Fig.11] where we used cholecalciferol in place of L Carnitine. The degenerative and inflammatory changes found here were milder enough than group II. It concluded that cholecalciferol is also beneficial for kidney to some extent if we use it with amikacin. In our study one of our primary objectives was to find the better alternative in between L Carnitine and Cholecalciferol, therefore we again tabulated the histopathology based on tubular degeneration, tubular necrosis, tubular interstitial inflammation, Medullary vascular congestion, and presence of diffuse or focal casts [Table 14]. Semi quantitative gradation was done based on percentage of area affected per representative field (Mild: 0-10%, Moderate: 10-20%, Severe: 25-50%, Very severe: >50%). Here we found that the severity of degeneration, inflammation and necrosis were much more in group II or amikacin group, whereas least in group I (control group) and group IV found much more beneficial than group III.
After the completion of animal experiment, we concluded that, cholecalciferol is better drug than l-carnitine. Biochemical as well as histopathological data significantly proved better nephroprotective potential of cholecalciferol in terms of controlling amikacin induced nephropathy.
Some rodent studies are reassuring, revealing no evidence of teratogenicity or IUGR despite the use of amikacin doses higher than those used clinically. [43,44] Those study also opine that aminoglycoside can damage the foetal kidney presumably because of delayed clearance, and irreversible failure has been reported after some aminoglycosides, but not amikacin.
Clinical Observation
In our secondary objective we tried to correlate our observational findings of amikacin induced ADR with the primary objective and to find out what type of ADR were happening mostly due to amikacin. Analysis was done through WHO UMC causality assessment scale [Fig.13]. Oliguria, swelling of face, increased urea, increased creatinine, fullness of ear and tinnitus were found & reported accordingly but there were no hearing loss and new ADR apart from nephropathy and ototoxicity. Blood samples of all the patient had been sent for urea and creatinine analysis before and after the amikacin therapy by the attending physician. We found 271 ADR from 71patient who were in amikacin therapy during our study period. Increased urea and creatinine were found in all 71 patient (30.87%), oliguria was found in 16.08%, fullness of ear was 10%, tinnitus found in 7.4% and swelling of face was in 4.78%. There were 9 patients with swelling of face, who denied IV fluid and were eating bottled water even though adequate hydration was advised in all BHT. Some reports were not taken into consideration as they willingly left the treatment in midway after signing the discharge on request form and few was absconded. WHO UMC causality assessment showed 94.35% were probable and the rest were possible. Naranjo’s probability scale showed almost the same result, here 95.22% was probable and possible was 4.78%.
In our present study, MDA was determined from animals only as there was short supply of reagent. All other biomarkers for oxidative stress (superoxide dismutase etc.) were not done, because it could not be possible to arrange huge amount of blood from a tiny animals like rat. PAS stain for histopathology was not done due to reagent scarcity. Tissue homogenizer was not present in our present set up; therefore, we could not arrange for homogenization of tissues which might be helpful to do another biomarker test. Both the biochemical and histopathology study showed cholecalciferol is better than L Carnitine to ameliorate amikacin induced nephropathy. Hence increased serum urea and creatinine were found in both clinical observation and animal experiment after amikacin therapy. Hence in this study clinical and experimental findings were at par with each other.
Conclusions
Interventional animal experiment, biochemical parameters, histopathology along with open label, non interventional, prospective observational study clearly indicates cholecalciferol is significantly better than l-carnitine to minimise the effects of amikacin induced nephropathy.
Acknowledgement
SWATI BHATTACHARYYA, Professor, Department of Pharmacology, R.G. Kar Medical College, Kolkata.
SABYASACHI MALLICK, Associate Professor, Department of Biochemistry, Burdwan Medical College, Burdwan.
All the faculty members and all other staffs of the Department of Pharmacology, Department of Biochemistry, Department of Medicine, Department of Surgery and Department of Gynaecology, Burdwan Medical College. DR. SWAPAN KUMAR DUTTA, DR. SUDIPTA SIL.
Conflict of Interest
All the authors here have no conflict of interest.
Funding Source
There is no funding Source.
References
- Raduly Zsolt, Price G Robert, Dockrell E C Mark, Csernoch Laszlo, Pocsi Istvan. Urinary Biomarkers of Mycotoxin Induced Nephrotoxicity-Current Status and Expected Future Trends. Toxins. 2021 Nov; 13:848.
CrossRef - Patel JB, Sapra A. Nephrotoxic Medications. [Updated 2021 Sep 18]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK553144.
- Ghane Shahrbaf F, Assadi F. Drug-induced renal disorders. J Renal Inj Prev. 2015;4(3):57-60.
- Seiji Kishi, Craig R. Brooks, Kensei Taguchi, Takaharu Ichimura, Yutaro Mori, et al. Proximal tubule ATR regulates DNA repair to prevent maladaptive renal injury responses. J Clin Invest. 2019;129(11):4797-4816.
CrossRef - Pazhayattil GS, Shirali AC. Drug-induced impairment of renal function. Int J Nephrol Renovasc Dis. 2014 Dec 12; 7:457-68.
CrossRef - Wagner LA, Tata AL, Fink JC. Patient safety issues in CKD: core curriculum 2015. Am J Kidney Dis. 2015 Jul;66(1):159-69.
CrossRef - Krause M Kevin, Serio W Alisa, Kane R Timothy. and Connolly E Lynn. Aminoglycosides: An Overview. Cold Spring Harb Perspect Med. 2016; 6: a027029.
CrossRef - Krause KM, Serio AW, Kane TR, Connolly LE. Aminoglycosides: An Overview. Cold Spring Harb Perspect Med. 2016 Jun 1;6(6): a027029.
CrossRef - Gnoni Antonio, Longo Serena, Gnoni V Gabriele. and Giudetti M Anna. Carninitine in Human Muscle Bioenergetics: Can Carnitine Supplementation Improve Physical Exercise ?. Molecules. 2020;25: 182.
CrossRef - Carillo MR, Bertapelle C, Scialò F, Siervo M, Spagnuolo G, et al. L-Carnitine in Drosophila: A Review. Antioxidants (Basel). 2020 Dec 21;9(12):1310.
CrossRef - Tamai I. Pharmacological and pathophysiological roles of carnitine/organic cation transporters (OCTNs: SLC22A4, SLC22A5 and Slc22a21). Biopharm Drug Dispos. 2013;34: 29–44.
CrossRef - Virmani MA, Cirulli M. The Role of l-Carnitine in Mitochondria, Prevention of Metabolic Inflexibility and Disease Initiation. Int J Mol Sci. 2022 Feb 28;23(5):2717.
CrossRef - Saponaro Federica, Saba Alessandro, Zucchi Riccardo. An Update on Vitamin D Metabolism. Int. J. Mol. Sci. 2020 Sep; 21:6573.
CrossRef - Delrue Charlotte, Speeckaert Reinhart, Delanghe R Joris, Speeckaert M Marijn. The Role of Vitamin D in Diabetic Nephropathy: A Translational Approach. Intl J Mol Sci. 2022 Jan; 23:807.
CrossRef - Fishbane S, Chittineni H, Packman M, Dutka P, Ali N, et al. Oral paricalcitol in the treatment of patients with CKD and proteinuria: a randomized trial. Am J Kidney Dis. 2009 Jul;54(4):647–652.
CrossRef - Zeeuw DD, Agarwal R, Amdahl M, Audhya P, Coyne D, et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. The Lancet. 2010 Nov 6; 376(9752): 1543–1551.
CrossRef - Gembillo Guido, Siligato Rossella, Amatruda Michela, Conti Giovanni, Santoro Domenico. Vitamin D and Glomerulonephritis. Medicina. 2021 Feb; 57:186.
CrossRef - worldbank.org/poverty[internet]. Poverty & Equity Brief, South Asia, India: 2020 April.
- India’s rank improves to 55th position on global hunger index. The Economic Times. 2014 Oct 13.
- India tops world hunger list with 194 million people. The Hindu. 2015 May 29.
- World Health Organization Model List of Essential Medicines, 21st List, 2019. Geneva: World Health Organization; 2019. Licence: CC BY-NC-SA 3.0 IGO. https://creativecommons.org/licenses/by-nc-sa/3.0/igo.
- nic.in/division/committee-purpose-control-and-supervision-experiments-animals-cpcsea [Internet]. New Delhi: Committee for the Purpose of Control and Supervision on Experiments on Animals, Ministry of Environment, Forests and Climate change, Government of India; [updated 2015 Sep 28; cited 2015 Sep 29]. Available from: http://cpcsea.nic.in
- Selim A, Khalaf M, Gad MA, Ei-Raouf AMO. Evaluation of the possible nephroprotective effects of vitamin E and rosuvastatin in amikacin-induced renal injury in rats. Journal of Biochemical and Molecular Toxicology. 2017 July; 31(11): e21957.
CrossRef - Mohamed M. Sayed-Ahmed, Meshan Lafi Aldelemy, Mohamed M. Hafez, et al. Inhibition of Gene Expression of Organic Cation/Carnitine Transporter and Antioxidant Enzymes and Cellular Longevity. Oxidative Medicine and Cellular Longevity, 2012 Mar; Article ID 452902:13 pages doi: 10.1155/2012/452902.
CrossRef - Nakano A, Abe H, Suzuki A. Effects of cholecalciferol and calcium on experimental hepatic osteodystrophy in rats. J Bone Miner Metab.1996 Sep; 14(3):158-166.
CrossRef - Allain CC, Poon LS, Chan CSG, Richmond W, Fu PC. Enzymatic determination of total serum Urea. Clin Chem. 1974 Apr; 20(4): 470–475.
CrossRef - Nicolaouand CK, Montagnon T. Molecules That Changed The World.Wiley-VCH. Applied Organometallic Chemistry. 2008 Apr 14; 22(5):286.
CrossRef - Jaffe M. Uber den niederschlag, welchen pikrinsaure in normalen hrn erzeugt und uber eine neue reaction des kreatinins. Z Physiol Chem. 1886; 10:391–400.
CrossRef - Bishop ML. Clinical Chemistry: Principles and Correlations. 2nd Philadelphia: J. B. Lippincott and Company, 1992. p. 441.
- Delanghe JR, Speeckaert MM. Creatinine determination according to Jaffe—what does it stand for?. NDT Plus. 2011 Jan 27; 4(2):83-86.
CrossRef - Moselhy HF, Reid RG, Yousef S, Boyle SP. A specific, accurate, and sensitive measure of total plasma malondialdehyde by HPLC. J Lipid Res. 2013 Mar; 54(3):852-858.
CrossRef - Ghosh MN. Fundamentals of experimental pharmacology: Anaesthetics used in laboratory animals. 5th Kolkata: Hilton & company; 2011.
- Bancroft JD, Gamble M. Theory and practice of histological techniques: Fixation of tissues. 6th ed. Philadelphia: Elsevier; 2008.
- Bancroft JD, Gamble M. Theory and practice of histological techniques: Microtomy: paraffin and frozen. 6th ed. Philadelphia: Elsevier; 2008.
- Bancroft JD, Gamble M. Theory and practice of histological techniques: The hematoxylins and eosin. 6th ed. Philadelphia: Elsevier; 2008.
- Clark MR, Trotter K, Chang A. The Pathogenesis and Therapeutic Implications of Tubulointerstitial Inflammation in Human Lupus Nephritis. Semin Nephrol. 2015 Sep;35(5):455-64.
CrossRef - Ari E, Kedrah AE, Alahdab Y, Bulut G, Eren Z, Baytekin O, et al. Antioxidant and renoprotective effects of paricalcitol on experimental contrast-induced nephrotoxicity model. The Brit J Radiol. 2011 Sep 7; 85:1038-1043.
CrossRef - Sarah R. McLarnon, Katie Wilson, Bansari Patel, Jingping Sun, Christina L. Sartain, et al. Lipopolysaccharide Pretreatment Prevents Medullary Vascular Congestion following Renal Ischemia by Limiting Early Reperfusion of the Medullary Circulation. 2022 April; 33(4):769-785.
CrossRef - Kubra Kaynar, Semih Gul, Safak Ersoz, Feyyaz Ozdemir, Hulya Ulusoy, et al. Amikacin-Induced Nephropathy: Is There Any Protective Way?. Renal Failure, 2007; 29(1), 23-27.
CrossRef - Ozer MK, Asci H, Oncu M, Yesilot S, Savran M, et al. Effects of pentoxifylline on amikacin-induced nephrotoxicity in rats. Ren Fail. 2009 Jul 7;31(2):134-139.
CrossRef - Kopple JD, Ding H, Letoha A, Ivanyi B, Qing DPY, Dux L, et al. L-carnitine ameliorates gentamicin-induced renal injury in rats. Nephrol Dial Transplant. 2002; 17(12):2122-2131.
CrossRef - Mishra P, Mandlik D, Arulmozhi S. et al. Nephroprotective role of diosgenin in gentamicin-induced renal toxicity: biochemical, antioxidant, immunological and histopathological approach. Futur J Pharm Sci. 2021; 7:
CrossRef
- Jagtap SV, Aher V, Gadhiya S, Jagtap SS. Gestational Trophoblastic Disease – Clinicopathological Study at Tertiary Care Hospital. J Clin Diagn Res. 2017;11(8):EC27-EC30.
CrossRef - Carl P. Weiner MD, MBA, FACOG, Catalin Buhimschi MD, in Drugs for Pregnant and Lactating Women (Second Edition), 2009.
CrossRef










