Manuscript accepted on :14-09-2022
Published online on: 21-09-2022
Plagiarism Check: Yes
Reviewed by: Dr. Pankaj Singh
Second Review by: Dr. Ammar Almulathanon
Final Approval by: Dr. Jihan Seid Hussein
Endang Nurcahyani1* , Novita Herliani2
, Novita Herliani2 and M Kanedi2
and M Kanedi2
1Applied Biology Study Program, Faculty of Mathematics and Natural Sciences, University of Lampung, Bandar Lampung 35145, Lampung, Indonesia
2Biology Study Program, Faculty of Mathematics and Natural Sciences, University of Lampung, Bandar Lampung 35145, Lampung, Indonesia.
Corresponding author E-mail: endang.nurcahyani@fmipa.unila.ac.id
DOI : https://dx.doi.org/10.13005/bpj/2496
Abstract
Hyperuricemia is a disease caused by an increase in uric acid levels in the blood over the normal level. Increased uric acid levels happen because the levels of purines in the body are quite high, so that the breakdown of purines into uric acid increases. This study used dried vanilla fruit extract using 60% ethanol solvent. The test animals used were 24 mice which were divided into 6 groups namely group Kn (standard feed), group K- (induced with suspension of chicken liver), group K + (induced by suspension of chicken liver and given allopurinol 10 mg / kgBB), group treatments P1, P2, and P3 were induced by suspension of chicken liver and vanilla fruit extract with doses of 50 mg / kgBB, 100 mg / kgBB, and 200 mg / kgBB respectively. Statistical data analysis using ANOVA (Analysis of Variance) through the SPSS 15.0 program with a level of α = 5% and continued with Duncan test at the level of α = 5%. The results of this study showed that vanilla fruit extract treatment P1, P2, and P3 had potential activity of antihyperuricemia, because it can reduce blood uric acid levels in mice induced by the chicken liver. The antihyperuricemia activity of vanilla fruit extract is comparable to the standard allopurinol chemical drug in reducing blood uric acid levels in mice statistically.
Keywords
Allopurinol; Antihyperuricemia; Uric Acid; Vanilla planifolia Andrews; Xanthine oxidase
Download this article as:| Copy the following to cite this article: Nurcahyani E, Herliani N, Kanedi M. Antihyperuricemia Activity of Vanilla (Vanilla planifolia Andrews) Fruits Ethanol Extract to Male Mice (Mus musculus L.). Biomed Pharmacol J 2022;15(3). |
| Copy the following to cite this URL: Nurcahyani E, Herliani N, Kanedi M. Antihyperuricemia Activity of Vanilla (Vanilla planifolia Andrews) Fruits Ethanol Extract to Male Mice (Mus musculus L.). Biomed Pharmacol J 2022;15(3). Available from: https://bit.ly/3LwXtK6 |
Introduction
Uric acid is the final production of the breakdown of purines originating from the body itself or foods containing purines 1. Hyperuricemia is a disease caused by an increase in uric acid levels above the normal level that occurs due to excessive uric acid production and reduced uric acid excretion or a combination of both. If hyperuricemia continues to occur it can cause arthritis gout 2. A bad lifestyle such as consuming foods that contain high purine substances can increase the risk of gout. Examples of foods with high levels of purine are vegetables (long beans and spinach), Gnetumgnemonseeds, meat, chicken innards (liver and intestines), seafood (shellfish and shrimp) and others 3. There are foods that are high in purines and some are low. Foods that contain high purine substances must be avoided, or not consumed excessively because it can increase hyperuricemia. Apart from food, the body itself produces of purines, which causes synthesis of substances such as CO2, glutamine, glycine, aspartic acid, and folic acid in the body 2.
The kidneys are associated with increased levels of uric acid in the blood, because uric acid in the blood is carried to the kidneys for excretion. One of the important causes of hyperuricemia is that kidney unable to excrete uric acid through urine. This can occur as a result of kidney problems such as the influence of drugs or the influence of some nutrients that can inhibit uric acid excretionurinary 1. Normal uric acid levels in men are 3.4-7.0 mg / dL, in women 2.4 – 5.7 mg / dL and children 2.8 – 4.0 mg / dL 4.
Vanilla fruit contains chemical compounds of flavonoids and alkaloids [5], besides that vanilla fruit also contains antioxidants 6 and anti-inflammatory 7. So far there has been no research on vanilla extract as an antihyperuricemic agent.This study aimed to determine the antihyperuricemia activity of vanilla (Vanilla planifolia Andrews) fruit extract on male mice and compared to standard allopurinol chemical drugs. This research is expected to be used as a scientific reference for further research so that it can be used by the public as a standard herbal medicine to reduce uric acid levels (antihyperuricemia).
Materials and Methods
This research is an experimental study using 24 mice (Mus musculus L.) test animals, aged 2-3 months with a weight of 30-40 grams. The tools used to measure uric acid levels was strips measuring uric acid levels and easy touch Glucose, Cholesterol, Uric Acid (GCU).
Extraction
Making vanilla extract uses a one-stage maceration method [8]. The plant materials used in this study were: 200 grams of dried vanilla fruit cut into small pieces and put into a glass beaker then added 2 liters of 60% ethanol solvent for 3 repetitions of maceration. Each maceration process is carried out 24 hours at 20 OC- 30 OC. The results of maceration was filtered and used using a rotatory evaporator at 600 OC, speed of 150 rpm for 14 hours.
Making Pathological Conditions for Hyperuricemia
Test animals were induced using the suspension of chicken liver to increase the uric acid level of mice blood to hyperuricemia. On day 0 the test animal was fasted for 12 hours, then measured the initial uric acid level, as the normal uric acid level of the test animal. The treatment groups II, III, IV, V, and VI were fed a standard feed of mice and chicken liver suspension at a dose of 25 ml/kg weight given once daily orally for 14 days to approach the condition of hyperuricemia.
Giving Treatment
The following are the details of each treatment group as follows.
Group I : Normal control (standard feed)
Group II : Negative control (induced by suspension of chicken liver)
Group III : Positive control (induced and given allopurinol suspension 10 mg/kg BB)
Group IV : Treatment 1 (induced and given vanilla fruit extract at a dose of 50 mg/kg BB)
Group V : Treatment 2 (induced and given vanilla fruit extract at a dose of 100 mg/kg BB)
Group VI : Treatment 3 (induced and given vanilla fruit extract at a dose of 200 mg/kg BB)
Measuring Uric Acid
Measurements of uric acid levels in mice were carried out on days 0, 15 and 30. Blood collection was carried out on the mice’s tail, by cutting the tip of the tail up to 0.1-0.2 cm using scissors that had been sterilized with alcohol, then blood is dripped on the uric acid strip which has been attached to the GCU easy touch tool and the tool will read the blood uric acid levels of mice in mg/dL. The data obtained were analyzed using ANOVA (Analysis of Variance) through the SPSS version 15.0 program at a significance level of 5% and continued with the Duncan test to see the differences between each treatment.
Results and Discussion
The antihyperuricemia activity of vanilla fruit extract on male mice in this study showed that on day 15 all treatment groups (K-, K +, P1, P2, P3) multiplied the increase in uric acid levels compared to the normal control group (Kn) not induced (Table 1). On the 30th day after treatment of vanilla P1 fruit extract treatment group at a dose of 50 mg/kg weight, P2 with a dose of 100 mg / kg weight, and P3 at a dose of 200 mg / kg weight and a positive control group (K +) decreased compared with the negative control group (K-). To clarify the average results of measurement of uric acid levels from each treatment group can be seen in Figure 1..
Table 1: Average of Total Uric Acid in Mice
| Treatment | Uric Acid Levels Mice ± SD (mg / dL) | ||
| Preliminary data
Day- 0 |
Improved data
15th day |
Decrease Data
30th day |
|
| Kn (standard feed without induction) | 1.40±0.00 | 1.40±0.00 a | 1.87±0.95a |
| K- (standard feed after induction) | 1.70±0.60 | 3.65±0.69 b | 4.77±1.89a |
| K+ (allopurinol 10 mg / kg weight) | 1.80±0.80 | 3.87±0.69 b | 2.02±1.25a |
| P1 (vanilla fruit extract 50 mg / kg weight) | 1.40±0.00 | 4.05±0.61 b | 2.45±1.23a |
| P2 (vanilla fruit extract 100 mg / kg weight) | 1.40±0.00 | 3.70±0.58b | 2.20±0.92a |
| P3 (vanilla fruit extract 200 mg / kg weight) | 1.40±0.00 | 3.67±0.69b | 2.02±0.77b |
Description: Numbers followed by different superscripts show significant differences based on Duncan’s test 5%
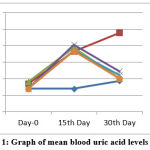 |
Figure 1. Graph of mean blood uric acid levels in mice |
The difference in decline from each group (Table 2) shows that in the treatment group P1, P2, P3 and K + experienced a higher decline compared to groups Kn and K-, but each treatment group P1, P2, P3 and K + has almost the same decline in value. The following table shows the average difference between all groups in Table 2.
Table 2: Mean Difference in Decreased Uric Acid Levels of Mice
| Treatment | Average Decrease Difference (mg / dL) |
| Kn (standard feed without induction) | -0.47a |
| K- (standard feed after induction) | -1.12a |
| K+ (allopurinol 10 mg / kg weight) | 1.85b |
| P1 (vanilla fruit extract 50 mg / kg weight) | 1.60b |
| P2 (vanilla fruit extract 100 mg / kg weight) | 1.50b |
| P3 (vanilla fruit extract 200 mg / kg weight) | 1.65b |
Description: Numbers followed by different superscripts show significant differences based on Duncan’s test 5%
In this study, the antihyperuricemia activity test carried out vanilla fruit extract (Vanilla planifolia Andrews) on male mice (Mus musculus L.) induced using chicken liver suspension. Chicken liver used for induction given once daily orally for 14 days has been shown to increase blood uric acid levels in mice. The results of the analysis show that the suspension of chicken liver can increase the total uric acid level of the treatment group K-, K +, P1, P2, and P3 compared to the normal control (Kn) which is only given standard feed (Table 1). But the five groups of induction treatments K- and K +, P1, P2, and P3 showed statistically large increases. On the 30th day the treatment groups P1, P2, and P3 and the positive control group (K +) decreased compared to the negative control group (K-), although the average decrease in uric acid levels did not reach the initial level of uric acid , but the result of the decrease is close to the initial uric acid level (Table 1). The results obtained in this study prove that vanilla fruit extract has antihyperuricemia activity because it can reduce blood uric acid levels in mice.
Normal uric acid levels of the test animals (Mus musculus L.) ranged from 0.5 to 1.4 mg/dL, and experienced hyperuricemia if uric acid levels in the blood reached 1.7-3.0 mg/dl [10]. The increase in uric acid levels of mice after induction varied, i.e. in the range of 2.3-7.5 mg/dl, this was due to the different conditions of individual mice. The induction of mice aims to condition mice to undergo hyperuricemia, which is done by administering High Purine Diet Foods (MDPT) in the form of suspension of chicken liver. According to Diantari and Candra [3], innards have high purine content which can increase uric acid levels in the blood of mice.
The results of the analysis (Table 2) prove that from each group P1, P2, P3 and positive control (K +) the mean decrease was almost as large, and showed that the vanilla fruit extract group P1, P2, and P3 had activity comparable to the drug chemistry of allopurinol (K +) in statistically reducing uric acid levels. The mean difference in decline for each group from high to low was as follows, in the positive control group (K +) the highest difference is 1.85 mg / dL, the vanilla P3 fruit extract group at a dose of 200 mg/kg has a difference of 1.65 mg/dL, group P1 with a dose of 50 mg/kgBB has a decrease in difference of 1.60 mg/dL, and the P2 group with a dose of 100 mg/kg weight has a mean difference in decrease of 1.60 mg/dL. Whereas in the normal control (Kn) it has a mean difference in decline of -1.125 mg/dL, and in the negative control group (K-) the mean difference in decline is -0.475 mg/dL, this occurs because in the negative control group (K-) and the normal control group (Kn) did not decrease uric acid levels, but there was an increase in the mean uric acid level.
Uric acid is the final production of the solution and disposal, which comes from purines produced by the body itself or from foods containing purines 1. Uric acid normally functions as an antioxidant in the human body 11. Uric acid levels in the blood can increase continuously beyond normal limits if the purine levels in the body increase and trigger the plasma in the blood to become very saturated, causing hyperuricemia due to excessive uric acid production and decreased uric acid excretion or a combination of both. Other diseases that can be caused are gout, a due to of monosodium urate (MSU) crystals in soft tissue, socket, and cartilage. Gout is an inflammation of the joints due to the accumulation of monosodium urate (MSU) crystals that form deposits continuously and cause acute inflammation such as acute or chronic joint inflammation called rheumatic gout or arthritis gout 2.
Purines are amines part of the proteins that make up the body of living things, even our own body’s metabolic system also produces purines. Purines aided by the xanthine oxidase enzyme will be catabolized to uric acid which is the final product of purine metabolism [2]. The xanthine oxidase enzyme is an enzyme that plays a role in the synthesis of uric acid, which catalyzes the oxidation of hypoxanthine to xanthine and subsequently catalyzes the oxidation of xanthine to uric acid 11. Based on the content of secondary metabolites, if the plants have antioxidant an activity, then they have the opportunity to have activityxanthine oxidase inhibitor 12.
Flavonoids in vanilla fruit extract 5 have a working mechanism that inhibits the action of the xanthine oxidase enzyme, which can reduce uric acid levels in the blood of mice. Vanilla fruit also acts as an anti-inflammatory 7 so it can help healing due to inflammation in the joints caused by the buildup of monosodium urate (MSU), due to high levels of uric acid in the blood. Flavonoids are useful as anti-inflammatory, protect cell structures, increase the effectiveness of vitamin C, prevent bone loss and as an antibiotic 13. According to Cos et al. 5, flavonoids can inhibit xanthine oxidase enzymes and have superoxide cleansers. This xanthine oxidase inhibition activity causes a decrease in blood uric acid levels.
In this study, the positive control used was the standard chemical drug allopurinol, which is one of the drugs that is often used by the public in treating gout. The working principle of allopurinol is to inhibit the activity of the xanthine oxidase enzyme to convert hypoxanthine to xanthine and then to uric acid 14. Allopurinol inhibits enzyme activity irreversibly by being a specific inhibitor and substrate for the xanthine oxidase enzyme. The function of this drug is as an analog substrate (purine) which will occupy the active site of the xanthine oxidase enzyme. In the liver xanthine oxidase will metabolize allopurinol and produce its active metabolite, oxypurinol (alloxantine) which also plays a role in inhibiting xanthine oxidase so that it can reduce blood uric acid levels maximally 15.
Conclusion
Based on the results of the study it can be concluded that:
Vanilla fruit extract (Vanillaplanifolia Andrews) has the potential as an antihyperuricemia drug for mice induced by a suspension of chicken liver.
Vanilla fruit extract (Vanillaplanifolia Andrews) has activity comparable to the chemical drug allopurinol (K +) in reducing blood uric acid levels in mice statistically.
Acknowledgement
Thanks to the technicians in the Botanical laboratory, especially the in vitro culture room, Department of Biology, Faculty of Mathematics and Natural Sciences, University of Lampung, Bandar Lampung, Indonesia, who have assisted in the analysis of this research.
Conflict of Interest
There is no conflict of interest.
References
- Krisnatuti D., Yenrina R., and Uripi V. Menu Planning for Patients with Gout Disorders. Penebar Swadaya. Jakarta, 2012; pp 11-15.
- Dalimartha S. Recipes of Medicinal Plants for Gout Revised Edition. Penebar Swadaya. Jakarta, 2008; pp 6-12.
- Diantari E., and Candra A.. The Effect Of Purine And Liquid Intake On Uric Acid Levels In Women Aged 50-60 Years In Gajah Mungkur District, Semarang. Journal of Nutrition College, 2013; 2 (1): 44-49.
CrossRef - Lingga L. Gout Disease Free Without Drugs. PT. Agromedia Pustaka. Jakarta. 2012; pp. 2.
- Shanmugavalli N., Umashankar V., and Raheem. Anitmicrobial activity of Vanilla planifolia. Indian Journal of Science and Technology. 2009; 2 (3): 37-40.
CrossRef - Shyamala B. N., Madhava N.M., Sulochanamma G., and Srinivas P. Studies on the Antioxidant Activities of Natural Vanilla Extract and Its Constituent Compounds through in Vitro Models. Journal of Agric. Food Chem. 2007; 55 (19): 7738–7743.
CrossRef - Niazi J., Kaur N., Sachdeva R. K.., Bansal Y., and Gupta V. Anti-inflammatory and Antinociceptive Activity of Vanillin. Drug Development and Therapeutics Journal. 2014; 5 (2):145-157.
CrossRef - Setyaningsih D., Rusli M. S., Melawati, and Mariska I. Optimization of Vanilla (Vanilla planifolia Andrews) Maceration of Modified Curing Process. Teknol. dan Industri Pangan. 2006; 17 (2):87-96.
- Fitrya and Muharni. Effects of hyperuricemia from the ethanolic extract of the roots of the tunjuk Langit plant (Helminthostachys zaylanica Linn Hook) on male swiss strain mice. Traditional Medicine Journal. 2014; 19 (1): 14-18.
- Itahana Y., Han R., Barbier S., Lei1 Z., Rozen S., and Itahana K.. The uric acid transporter SLC2A9 is a direct target gene of the tumor suppressor p53 contributing to antioxidant defense. Oncogene. 34: 1799-1810.
CrossRef - Bustanji Y., Hudaib M., Tawaha, Mohammad M. K., Almasri I., Hamed S., and Oran S. In Vitro Xanthine Oxidase Inhibition by Selected Jordanian Medicinal Plants. Jordan Journal of Pharmaceutical Sciences. 2011; 4 (1): 49-56.
- Song Y. S., Kim S. H., Sa J. H., Jin C., Lim C. J., and Park E. H. Anti-angiogenic, antioxidant and xanthine oxidase inhibition activities of the mushroom Phellinus linteus. Ethnopharmacol J. 88 (1): 113–116.
CrossRef - Lumbessy M. Total Flavonoid Test on Several Traditional Medicinal Plants in Waitina Village, East Mangoli District, Sula Archipelago Regency, North Maluku Province. Jurnal Mipa Unsrat Online. 2013; 2(1): 50-55.
CrossRef - Kristiani R. and Subarnas A. Antihyperuricemic Activity of Tangkur Fern Root (Polypodium feei) Ethanol Extract in Male Mice. Bionatural-Jurnal Ilmu-Ilmu Hayati dan Fisik. 2013; 15 (3): 174-177.
- Pacher P., Nivorozhkin A., and Szabo C. Therapeutic Effects of Xanthine Oxidase Inhibitors: Renaissance Half A Century After the Discovery of Allopurinol. Pharmacol. 2006; 58 (1): 87–114.
CrossRef







