Manuscript accepted on :27-07-2022
Published online on: 28-07-2022
Plagiarism Check: Yes
Reviewed by: Dr. Alamgir Ahmad Dar
Second Review by: Dr. Madiha Amjad
Final Approval by: Dr. Kishore Kumar Jella
Priya Durai Raj, Palagati Rohith Kumar Reddy, Palaniyandi Thiruvanavukkarasu, Sindhu Rajesh and Rajeswary Hari*
Dr. M.G.R. Educational and Research Institute University, Chennai-95. Tamil Nadu, India
Corresponding Author E-mail: rajihar@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2466
Abstract
The plant derived compounds possess several medicinal property including anticancer activities. In the present investigation molecular docking analysis was performed to identify a suitable antagonistic ligand from the phyto ligands of Carica pappya leaves which can inhibit the tumor progressive proteins PIK3CA, BCL 2. The molecular Docking analysis was performed using Autodock 4.2. The protein PIK3CA, BCL 2 structures were retrieved from PDB, and by GC-MS analysis the phyto molecules were identified. The ligand chemical structures were drawn using Chem sketch. The enzyme and ligand interaction were obtained as docking score using the Arguslabs server. Based on the docking score the best ligand was selected from the phyto constituents of Carica papaya ethanolic leaf extract and their inhibitory potential was analyzed in terms of their interactions with the amino acid residues present in the active site which were visualized and further confirmed by PYMOL. The standard drug Doxorubicin was also subjected to docking for comparison in the present study. Based on the docking score the phytochemicals namely Hexadecanoic acid, ethyl ester, Coumarine 3-(2,4-dinitrophenol), Androst-4-en-3-one,17-methoxy, 3-methoxime serves as the best antagonistic ligand in terms of their interaction with amino acids as well as inhibition of the particular tumour progressive proteins.
Keywords
Carica papaya; Doxorubicin; Ethanolic extract; Insilico; Ovarian cancer; Phyto ligands
Download this article as:| Copy the following to cite this article: Raj P. D, Reddy P. R. K, Thiruvanavukkarasu P, Rajesh S, Hari R. Anticancer Activity of Phyto Ligands from Carica papaya Leaves by Suppression of PI3CKA and BCL2 Proteins- An insilico Approach. Biomed Pharmacol J 2022;15(3). |
| Copy the following to cite this URL: Raj P. D, Reddy P. R. K, Thiruvanavukkarasu P, Rajesh S, Hari R. Anticancer Activity of Phyto Ligands from Carica papaya Leaves by Suppression of PI3CKA and BCL2 Proteins- An insilico Approach. Biomed Pharmacol J 2022;15(3). Available from: https://bit.ly/3oCLIHg |
Introduction
Ovarian cancer is the fifth leading cause of cancer-related deaths among women in the world. There has not been a major therapeutic advance in the treatment of ovarian cancer in over 30 years. One reason ovarian cancer remains such a difficult disease is that it is usually discovered at an advanced stage, when metastases have already occurred. Frequently, this metastatic spread occurs in the peritoneal cavity, where tumor cells dislodge from the primary tumor, find an accommodating microenvironment, and re-establish themselves. Though the first line of treatment for the ovarian cancer starts with platinum derived compounds, in several cases it becomes a challenge due to the chemo resistance produced by the cancer cells to the drug 1. Therefore researchers have forced to develop effective and novel therapeutic molecules to combat with the cancer cells.
The phosphatidylinositol 3-kinases (PI3Ks) belongs to the kinase family of enzymes which plays an prominent role in cell proliferation, transformation and cell survival via PI3K/AKT signaling pathway in the cells. Somatic mutations in the gene encoding this PI3K/AKT proteins as well as other components of this signaling system are found to be dysregulated in several cancers including ovarian cancer 2. Another protein the BcI-2, which is the direct gene product of proto-oncogene Bcl-2 is considered as cell death suppressor gene which inhibits the apoptosis 3. It is accepted by several researchers that in majority of tumors which are resistant to chemotherapy in the form of cytotoxic drugs or radiation showed high levels of this Bcl-2 protein expression.
There are several plant derived phyto compounds which has the potential to kill the cancer cells and exhibits anticancer property. But the exact mechanism of action is poorly understood. Drug discovery through insilico approaches have recently made the cancer drug discovery process more successful through the identification of novel specific inhibitors against these dysregulated proteins 4.
Carcinogenesis is a multi-step process characterized by progressive changes in the amounts or activity of proteins that regulate cellular proliferation, differentiation and survival 5. The cost of research and development in the pharmaceutical industry has been rising steeply and steadily to achieve any process in the last decade but the amount of time required in bringing new products to market take around ten to fifteen years. Insilico technique is an inexpensive technique that shortens the length of time spending in testing the efficacy of drug. In the present study we made an attempt to identify a suitable antagonistic ligand which can inhibit the tumor progressive proteins PIK3CA, BCL 2, from the phyto constituents of the ethanolic extract of Carica papaya leaves through molecular docking studies.
Materials and Methods
Collection of plant material
The leaves of Carica papaya collected from Chennai, Tamilnadu India. The plant leaf sample was dried in hot air oven at 40°C for 24 hours and ground into powder.
Sample preparation for GCMS analysis
About 5g of powdered material of plant was taken in a clean, flat-bottomed glass container and soaked in 30mL of 70% methanol. The container with its content was sealed and kept for a period of seven days accompanying occasional shaking and stirring. The whole mixture then underwent a coarse filtration by a piece of clean, white cotton material. Then, it was filtered through Whatmann filter paper. The filtrate (ethanolic extract) obtained for the plant was evaporated under ceiling fan and in a water bath until dried.
GC-MS analysis
The GCMS analysis was conducted at the Indian institute of Madras (IITM) Chennai. 2uL aliquot was injected into a fisons GC8000 series GC coupled to a MD800 MS with quadrapole mass analyzer (fision instrument, Milano, Italy). The chromatography was performed by using the DB5-MS column. Injection temperature was 230°C. Helium flow was 1mL/min. After a 5 min solvent delay time at 70°C; the oven temperature was increased at 5°C/min to 310°C, 1min isocratic, and cooled to 70°C, followed by the additional 5min delay. The ion trace integration was done using the mass lab find target method for the characteristic fragment of assigned peaks.
Identification of Components
Interpretation of mass spectrum GCMS was conducted. The molecular weight, molecular formula, and the number of hits used to identify the name of the compounds from IITM were recorded.
Ligand Preparation
The compounds reported in Gas Chromatography and Mass Spectrum (GCMS) analysis of ethanolic extract leaf of Carica papaya were used in the present study. The structures of the 21 compounds (ligands) were retrieved. The structure of the compounds were drawn using ChemSketch and the structure was obtained in the form of (.sk2). This was further converted to (.pdb) format using the Chimer tool. Once the ligands were obtained, each of the compound energy minimised using the same tool.
Protein preparation
The target protein and ligand molecules were retrieved separately and prepared before performing molecular docking analysis. The proteins were obtained in the PDB format from the Protein Data Bank (http://www.rcsb.org/). The protein and their corresponding PDB IDs are tabulated in Table. The protein molecules were obtained in the PDB format was first cured and further energy minimised.
Binding Site prediction
The binding site plays a major role in compound activity. Hence, the binding site for each of the protein molecule was obtained from Metapocket server. This server provides us with three best active site pockets. For our docking analysis, we use the pocket with maximum number of amino acids.
Docking Analysis
Molecular docking continues to hold a great promise in the field of computer based drug design which screens small molecules by orienting and scoring them in binding site of a protein. Protein and ligand molecules were used as starting structures for the docking analysis. Molecular Docking analysis was performed using Autodock 4.2 tools [6]. Prior to the docking analysis, the protein molecules and the ligand molecules were energy minimized using Chimera tools. The energy minimised molecules were used as the starting structures for molecular docking. For all the protein molecules, the binding pocket was identified sing MetaPocketserver. The grid box was set around the active site for perfect binding of the ligands. All the ligands were torsion-fixed, the grid was maintained using autogrid and rigid docking for all the protein molecules were performed using autodock with the Lamarckian algorithm. As a result of docking, a cluster of docked complexes were obtained. The best docked structure with the minimum binding energy was used for further analysis.
Result
GC-MS analysis were performed to find out different compounds present in the plants sample and a total of 21 compounds were identified in the ethanolic extract of Carica papaya leaves, which includes the active principles with their retention time, molecular formula, molecular weight are presented in (Table 1 and Fig. 1). On further study of each compound, it was found that they individually have its own biological importance. These phytocompounds were treated as ligands and it is docked with target protein PI3CKA and BCL2.
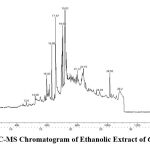 |
Figure 1: GC-MS Chromatogram of Ethanolic Extract of Carica papaya. |
Table 1: GCMS analysis of Ethanolic Extract of Carica papaya.
| Sl. No | RT | Name of the compound | Molecular Formulae | Molecular Weight | Peak Area % |
| 1 | 22.15 | Coumarine3-(2,4-dinitrophenol) | C6H4N2O5 | 184.11 | 1.72 |
| 2 | 18.05 | Alpha-lardeine | C36H60O30 | 141.5 | 4.54 |
| 3 | 16.02 | 3,7-Dimethyl-6-nonen-1-ol acetate | C13H24O2 | 212.3 | 1.52 |
| 4 | 16.85 | Dodecanoicacid, 10-methyl-, methyl ester | C14H28O2 | 228.37 | 2.16 |
| 5 | 17.47 | Hexadecanoicacid, ethyl ester | C18H36O2 | 284.47 | 2.87 |
| 6 | 18.63 | Phytol | C20H40O | 296.5 | 1.63 |
| 7 | 19.03 | 9,12-Octadecadienoic acid, ethyl ester | C20H36O2 | 308.5 | 11.27 |
| 8 | 21.2 | 5,8-Octadecadienoic acid, methyl ester | C19H36O2 | 294.5 g/mol | 8.32 |
| 9 | 13.04 | Flavone | C15H10O2 | 222.24 | 2.76 |
| 10 | 28.02 | Androst-4-en-3-one,17-methoxy, 3-methoxime | C20H30O2 | 302.5 | 7.15 |
| 11 | 24.08 | 2-methylbutyl 4-(4-pentylphenyl)benzoate | C23H30O2 | 338.5 | 5.08 |
| 12 | 12.6 | Delta- Iraldeine | C14H22O | 206.32 | 3.59 |
| 13 | 26.52 | 2,6,10,14,18-Pentamethyl-2,6,10,14,18-eicosapentaene | C25H42 | 342.6 | 16.52 |
| 14 | 14.57 | Dodecyl acrylate | C15H28O2 | 240 | 1.63 |
| 15 | 16.0 | δ-Thionodecalactone | C10H18OS | 186.32 | 1.23 |
| 16 | 19.32 | Neodecanoic acid | C10H20O2 | 174.25 | 2.76 |
| 17 | 21.92 | Dasycarpidan-1-methanol, acetate(ester) |
C20H26N2O2 |
326.4 | 2.87 |
| 18
|
14.12 | 4-(2,4,4-Trimethyl-cyclohexa-1,5-dienyl)-but-3-en-2-one | C13H18O | 190.28 | 4.99 |
| 19 | 32.68 | 2-Monolinoleoylglycerol trimethylsilyl ether | C27H54O4Si2 | 498 | 10.83 |
| 20 | 11.49 | Ethyl cholate | C26H44O5 | 436 | 15.06 |
| 21 | 12.58 | 11-Eicosenoic acid | C20H38O2 | 310 | 24.32 |
In Silico docking analysis
The Insilico docking analysis was performed by molecular docking using “Argus Lab” software to find a suitable antagonistic ligand from the plant compound for the tumor progressive proteins PIK3CA, BCL2. The obtained 12 compounds identified in the GC-MS analysis, were selected for the molecular docking in the “Argus Lab server” to obtain the best antagonistic ligand in terms of interactions between the ligand identified from the ethanolic extract of Carica papaya leaf by GC-Ms analysis and the proteins such as PIK3CA, BCL2. Based on the docking score and glide energy Table [2] the compounds which exhibit lowest binding energy were selected to identify the mode of binding of the ligands with the particular protein, in terms of the amino acid interaction were studied. The doxorubicin the anticancer drug was used as a positive control in the present study.
Table 2: Docking score values of selected Phytoligands of Carica papaya with PIK3CA and BCL2.
| S. No | Compound Name | Binding energy expressed in (kJ mol-1) | |
| PIK3CA | BCL2 | ||
| 1 | 2,6,10,14,18-Pentamethyl-eicosapentaene | -6.11 | -3.51 |
| 2 | Alpha-lardeine | -5.81 | -3.50 |
| 3 | 5,8-Octadecadienoic acid, methyl ester | -5.09 | -4.07 |
| 4 | 2-methylbutyl4,39;-pentyl-4-biphenylcarboxylate | -4.65 | -4.92 |
| 5 | Androst-4-en-3-one,17-methoxy,3-methoxime | -3.73 | -2.82 |
| 6 | Hexadecanoic acid,ethyl ester | -3.72 | -2.78 |
| 7 | 9,12-Octadecadienoic acid, ethyl ester | -7.13 | -3.90 |
| 8 | Coumarine 3-(2,4-dinitrophenol) | -3.24 | -2.42 |
| 9 | 3,7-Dimethyl-6-nonen-1-ol acetate | -4.20 | -3.89 |
| 10 | Phytol | -3.28 | -3.07 |
| 11 | Dodecanoic acid, 10-methyl-, methyl ester | -3.54 | -3.03 |
| 12 | Flavone | -7.0 | -4.89 |
Table 3: Interaction with the proteins PIK3CA and BCL2 with phytochemicals of EECP.
| S.No | Compound | Amino acid binding site PIK3CA protein | Amino acid binding site BCL2 protein | ||
| H bonding sites | Hydrophobic contact sites | H bonding sites | Hydrophobic contact sites | ||
| 1 | Phytol | 4 | 21 | 3 | 13 |
| Lys160,
Lys 277, Ser9, Asp293 |
Glu230, Thr213, Met229, Ala234, Phe439, Tyr231, Leu158, Phe443, Gly159, Gly161, Arg6, Thr7, Glu279, Thr8, Glu236, Asn280, Lys181, Met282, Val166, Thr292, Ala179
|
Lys62, Gly31,
His47, |
Val30, Cys28, Cys44, Gly22, Phe98, Ile9, Ala17, Phe5, Tyr21, Leu2, Gly29, Tyr51, Asp48, | ||
| 2 | Hexadecanoic acid, ethyl ester
|
3 | 15 | 3 | 10 |
| Phe5,
His47, Gly29 |
Asp48, Val30, Cys28, Val3, Leu2, Ala18, His6, Ala17, Ala94, Ile9, Tyr21, Phe 98,
Gly22, Cys44, His27 |
Asp300,
Tyr62, Glu233 |
Leu162, Leu165, Hie101, Trp58, Asn298, Arg195, Ash197, Ala198, His201, Thr163 | ||
| 3 | Coumarine 3-(2,4-dinitrophenol)
|
3 | 13 | 6 | 17 |
| Lys 62,
His47, Gly31 |
Asp48, Tyr51, Gly29, Leu2, Tyr21, Phe5, Ala17,
Ile9, Phe98, Gly22, Cys44, Cys28, Val30 |
His299, Tyr62,
Asp300, Glu233, Ile235, Tyr151 |
Trp58, Arg195, Asp197, Gly306, Phe256, Asn298, Ser199, Ala198, Lys200, Val234, His201, His305, Trp59, His101, Leu162, Thr163, Leu165 | ||
| 4 | Dodecanoic acid, 10-methyl-, methyl ester
|
1 | 18 | 2 | 14 |
| Ala232 | Gly164, Lys160, Gly159, Lys165, Glu279, Asn280, Val166, Leu158, Phe439,
Ala179, Met282, Tyr231, Thr292, Asp293, Lys181, Gly161, Leu183, Phe163
|
Thr213,
Ala232 |
Gly159, Ala179, Asp293, Met229, Thr292, Lys290, Glu230, Met282,
Tyr231, Phe439, Asn233, Gly235, Leu 158, Val166 |
||
| 5 | Androst-4-en-3-one,17-methoxy, 3-methoxime
|
2 | 14 | 2 | 16 |
| Thr213,
Ala232 |
Lys290, Glu230, Met282, Tyr231, Phe439, Asn233, Gly235, Leu158, Val166, Gly159, Ala179, Asp293, Met229, Thr292 | Lys181,
Ala232 |
Thr292, Phe294, Glu200, Asp293, Leu204, Met229, Thr213, Glu230, Ala179, Tyr231, Leu158, Gly235, Phe439, Glu236, Val166, Met282 | ||
| 6 | Doxorubicin | 2 | 19 | 1 | 16 |
| Tyr 836
Val 851 |
|||||
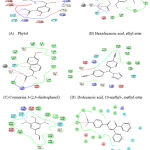 |
Figure 2: Amino acid Interaction of PIK3CA protein with specific ligands. |
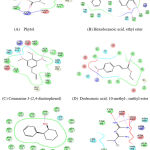 |
Figure 3: Amino acid Interaction of BCL2 protein with specific ligands. |
In the present study out of the 12 ligands docked against the tumor progressive proteins PIK3CA and BCl-2, five ligands namely phytol, Coumarin 3-(2,4-dinitrophenol), Hexadecanoic acid, ethyl ester, Dodecanoic acid, 10-methyl-, methyl ester and Androst-4-en-3-one, 17-methoxy, 3-methoxime showed lowest gliding energy. These five ligands were further analyzed for their inhibitory potential of the tumor progressive proteins PIK3CA and BCl-2 in terms of their interactions with the amino acid residues present in the active site. Table 2 and Fig. 2 & Fig.3 clearly explains the intermolecular interactions existing between the molecules of phytochemical and the amino acids of the particular protein. It is observed that in all the proteins, number of hydrophobic interactions are more and the hydrophilic interactions are very less.
Discussion
In spite of the availability of advanced diagnosis and treatment the cancer chemotherapy still remains a challenging one to the mankind. So, there has been always an increased interest for researchers to develop a new anticancer drug with high potency against the cancer cells 7. According to8 the selective growth inhibitory activity of the various plant extracts is due to the phytochemicals that can act in different pathways to arrest the cancer cell growth. With the above scenario the present investigation is undertaken to study the anti-cancer activity of Ethanolic extract of Carica papaya leaves (EECP) in Human Ovarian cancer PA-1 cell line 9 have reported the traditional use of the Papaya leaves extract by Australian aboriginal people for its anti-cancer activity.
The natural antioxidants present in vegetables and fruits are of much importance as they protect the human body from the oxidative stress and diseases 10. These holistic plants derived herbal medicines can be utilized for research to obtain a successful plant derived drug for the treatment of cancer since the modern synthetic cancer treatment The P53 is a transcription factor and a tumor suppressor which plays an important role in cell cycle progression and cell death by arresting the cell cycle at G1 phase by triggering apoptosis 11. According to 12 in ovarian cancer it is found to be highly mutated leading to lower survival rates, higher relapse rates and increased resistance to treatment in the form of chemotherapy and radiation. In the normal cells upon accumulation of damaged DNA p53 gets activated and trigger another transcriptional factor p21, by directly binding to p21/CDKN1Apromoter which in turn inhibit cyclin D dependent-kinases required for cell cycle progression 13. PTEN is a tumor suppressor gene also named as phosphatase and tensin homolog deleted on chromosome 10 is a negative regulator phosphatidylinositol-3-kinase (PI3K)/AKT signaling pathway to control the growth and survival 14. Although the functions of several genes and proteins are become altered in cancer cells, we analyzed the up regulation of these three genes by the Carica papaya leaf extract treatment in the PA-1 ovarian cancer cell lines. The presence of alkaloids such as carpaine, pseudocarpaine, piperideine, dehydrocarpaine I and II act as a potent anti tumor agent as stated by Okwu 15. According to Huang 16 the flavonoids present in the extract are potent free radical scavengers which protect the cells from oxidative injury caused by several carcinogens and exert strong anticancer activity.
The complicated apoptosis process is strictly balanced by many proteins and any disturbance in this critical down streaming process will lead to uncontrolled proliferation, as observed in the cancer cells. So, from the therapeutic standpoint, correcting the over expression of the altered proteins to induce apoptosis is the main goal of cancer therapy. In this context the selected phytochemicals from the EECP extract were docked against few tumor promoting proteins such as PIK3CA, BCL 2 and their inhibitory activity in terms of amino acid interaction were studied. Recently, occurrence of somatic mutation in the gene coding for phosphatidylinositol 3-kinase (PI3K) catalytic subunit, PIK3CA is observed in almost all kinds of human cancers. Phosphatidylinositol-3-Kinase (PIK3CA), is an important enzyme needed for cell survival, differentiation and proliferation 17. Many researchers have identified the dysregulation of the PI3K pathway in several human cancers, such as breast, colorectal cancer and hematologic malignancies 18. So, it will be of great value in finding out the suitable antagonistic ligand which can inhibit the Phosphatidylinositol-3-Kinase (PIK3CA) thereby inhibiting the tumor progression.
B-cell lymphoma-2 (BCL-2) belongs to a family of proteins which along with the pro apoptotic proteins namely Bax and Bak trigger the intrinsic apoptotic pathway through the permeabilizing outer mitochondrial membrane and activating the special enzymes namely cysteine proteases collectively called as caspases cascade. But this apoptotic signal gets neutralized in the presence of abnormally higher expression of Bcl-2 proteins in the cancer cell and thereby prevents their death by blocking the activation of caspases 19. According to several studies there exist a correlation between elevated Bcl-2 expression and poor prognosis in different types of cancer including breast, prostate, small cell lung, colorectal 20 and bladder cancers 21. With the above context BCL-2 interfering strategy may be considered as a successful therapeutic approach to trigger the apoptosis in the cancer cells. Based on the docking studies three ligands namely Hexadecanoic acid, ethyl ester.
In the present investigation there was a selective inhibition of PIK3CA, BCL 2 proteins by the phyto constituents phytol and Dodecanoic acid, 10-methyl-, methyl ester. Phytol is considered as a diterpene alcohol, a metabolic product of chlorophyll 22 abundantly present in leaves. Phytol (3,7,11,15-tetramethylhexadec-2-en-1-ol) is a diterpene, a member of the group of branched-chain unsaturated alcohols. It is the product of chlorophyll metabolism in plants; hence, phytol is abundantly available in nature23 have showed the breast cancer inhibitory activity of the purified compound phytol from Gracilaria edulis. The polyunsaturated phytol side chain in tocotrienols has got the inhibitory effect on breast tumour induced by 17b-estradiol epoxide-carcinogenesis was stated by Yu 24. In our present investigation through molecular docking studies it is observed phytol acts as a potential antagonistic ligand for the proteins PIK3CA, BCL 2 in terms of the inhibition of the amino acid residues. This is supported by the work done by 25, where they have proved that the phytol exhibits apoptosis in Gastric Adenocarcinoma AGS Cells by down regulating the PIK3ca pathway as well as Bcl-2 proteins Dodecanoic acid, 10-methyl-, methyl esteris commonly called as lauric acid is a fatty acid present in plants as well as mothers milk. Initially Shin 26 have demonstrated the apoptotic potential of this fatty acid in destroying the prostate cancer cells by ROS generation and interference with the Akt-mTOR signalling. The studies showed by Rosamarial 27 also proves the apoptotic effects of lauric acid in breast and endometrial cancer cells.
Conclusion
In our present investigation considerable inhibition of PIK3CA and Bcl2 proteins by the antagonistic ligand Dodecanoic acid, 10-methyl-, methyl ester was observed leading to the conclusion that the anti- proliferative activity of our plant extract may be due to the cumulative effects of these phytoconstituents.
Acknowledgement
I would like to acknowledge Dr. M.G.R Educational and Research Institute (University), Maduravoyal, Chennai for providing us the necessary facilities to carry out this.
Conflicts of interest
There are no conflicts of interest.
Funding Sources
There is no funding sources.
References
- Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev. 2007;33(1):9-23.
CrossRef - Gao N, Flynn DC, Zhang Z, Zhong XS, Walker V, Liu KJ, Shi X, Jiang BH. 2004. The G1 cell cycle progression and the expression of G1 cyclins are regulated by PI3K/AKT/mTOR/p70S6K1 signaling in human ovarian cancer cells. Am J Physiol Cell Physiol. 2004Aug;287(2):C281-91.
- Korsmayer SJ: BcI-2: an antidote to programmed cell death. Cancer Surveys 1992, 15:105-118.
- Mirza MU, Ikram N. Integrated computational approach for virtual hit identification against Ebola viral proteins VP35 and VP40. Int J Mol Sci. 2016;17(11): p. E1748.
- Bin X, Tao JS, Wei-Dong H and Fucheng Z: Expression of cyclooxygenace -2 in colorectal cancer and its clinical significance. World journal of gastroenterol 2005; 8: 1105-1108.
- Morris GM, Huey R, Lindstrom W, Michel FS. AutoDock4 and AutoDockTools4: Automated Docking With Selective Receptor Flexibility J Comput Chem. 2009 Dec;30(16):2785-91.
- Nelson, B 2013, ‘Targeting a murky cancer reservoir’, Cancer Cytopathology, vol. 121, no. 2, pp. 55-56
- Pandey, S, Walpole, C, Cabot, PJ, Shaw, PN, Batra, J & Hewavitharana, AK 2017, ‘Selective anti-proliferative activities of Carica papaya leaf juice extracts against prostate cancer’, Biomedicine & Pharmacotherapy, vol. 89, pp. 515-523
- Nguyen, TTT, Shaw, PN, Parat, MO & Hewavitharana, AK 2012, ‘Anticancer activity of Carica papaya: A review’, Molecular Nutrition & Food Research, vol. 57, no. 1, pp. 153-164
- Ali, S, Kasoju, S, Luthra, T, Singh, A, Sharanabasava, H & Sahu, A 2008, ‘Indian medicinal herbs as sources of antioxidants’, Food Research International, vol. 41, no. 1, 1-15
- Milner, BJ, Allan, LA, Eccles, DM, Kitchener, C, Leonard, RCF, Kelly, KF et al. 1993, ‘p53 mutation is a common genetic event in ovarian carcinoma’, Cancer Research, vol. 53, no. 1, pp. 2128-2132
- Petitjean, A, Mathe, E, Kato, S, Ishioka, C, Tavtigian, SV, Hainaut, P & Olivier, M 2007, ‘Impact of mutant p53 functional properties onTP53mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database’, Human Mutation, vol. 28, no. 6, pp. 622-629
- Quaas, M, Müller, G & Engeland, K 2012, ‘p53 can repress transcription of cell cycle genes through a p21WAF1/CIP1-dependent switch from MMB to DREAM protein complex binding at CHR promoter elements’, Cell Cycle, vol. 11, no. 24, pp. 4661-4672
- Downes, CP, Ross, S, Maccario, H, Perera N, Davidson L & Leslie, NR 2007 ‘Stimulation of PI 3-kinase signaling via inhibition of the tumor suppressor phosphatase, PTEN’, Advances Enzyme Regulation, vol. 47, no. 1, pp. 184-194
- Okwu, DE & Ekeke, O 2003, ‘Phytochemical screening and mineral composition of chewing sticks in South Eastern Nigeria’. Global Journal of Pure and Applied Sciences, vol. 9, no. 2, pp. 53-57
- Huang, WY, Cai, YZ & Zhang, Y 2009, ‘Natural phenolic compounds from medicinal herbs and dietary plants: potential use for cancer prevention’, Nutrition and Cancer, vol. 6, no. 2, pp. 1-20
- Cantley, LC 2002, ‘The phosphoinositide 3-kinase pathway’, Science, vol. 296, no. 5, 1655-1657
- Asati, V, Mahapatra, D & Bharti, S 2016, ‘PI3K/Akt/mTOR and Ras/Raf/MEK/ERK Signaling Pathways Inhibitors as Anticancer Agents: Structural and Pharmacological Perspectives’, European Journal Medical Chemistry , vol. 47, no. 13, 314-341
- Chinnaiyan, AM, Chaudhary, D, Rourke, K & Dixit, VM 1997, ‘Role of CED-4 in the activation of CED-3’, Nature, 388, no. 4, pp. 728-729
- Sinicrope, FA, Hart, J, Michelassi, F & Lee, JJ 1995, ‘Prognostic value of bcl-2 oncoprotein expression in stage II colon carcinoma’, Clinical Cancer Research: an official Journal of the American Association for Cancer Research, vol. 5, no. 10, pp. 1103-1110
- Gazzaniga, P, Gradilone, A, Vercillo, R, Gandini, O, Silvestri, I, Napolitano, M et al. 1996, ‘bcl-2/bax mRNA expression ratio as prognostic factor in low-grade urinary bladder cancer’, International Journal of Cancer, vol. 69, no. 2, pp. 100-104
- McGinty, D, Letizia, CS & Api, AM 2010, ‘Fragrance material review on phytol’, Food and Chemical Toxicology, vol. 48, pp. 59-63
- Sheeja, L, Lakshmi, D, Shruthi, BK & Sajidha Parveen, K 2016, ‘Anticancer activity of phytol purified from Gracilaria edulis against human breast cancer cell line (MCF-7)’, International Journal of Current Science, 19, no. 4, pp. 36-46
- Yu, FL, Gapor, A & Bender, W 2005, ‘Evidence for the preventive effect of the polyunsaturated phytol side chain in tocotrienols on 17β-estradiol epoxidation’, Cancer Detection and Prevention, vol. 29, no. 4, 383-388
- Song, Y & Cho, SK 2015, ‘Phytol Induces Apoptosis and ROS-Mediated Protective Autophagy in Human Gastric Adenocarcinoma AGS Cells’, Biochemistry & Analytical Biochemistry, vol. 04, no. 04, p. 08
- Shin, S, Jing, K, Jeong, S, Kim, N, Song, KS & Heo, JY 2013 ‘Biochemistry and Cell Biology Breaking the cycle: the role of omega-3 polyunsaturated fatty acids in inflammation-driven cancers’, Biochemistry and Cell Biology, 3, no. 3, pp. 35-45
- Rosamaria L, Anna, S & Francesca, C 2017 ‘The lauric acid-activated signaling prompts apoptosis in cancer cells, Cell Death Discovery, vol. 3, no.1, p. 17063.







