Manuscript accepted on :31-08-2022
Published online on: 15-09-2022
Plagiarism Check: Yes
Reviewed by: Dr. Swastika Maity, Dr. Cherry Bansal
Second Review by: Dr. Sumayah Faruq, Dr. Shabana Khatoon
Final Approval by: Dr. Jihan Seid Hussein
Begum Fauziyah* , Asna Defi Batrisyia
, Asna Defi Batrisyia , Alif Firman Firdausy
, Alif Firman Firdausy , Burhan Ma’arif
, Burhan Ma’arif and Siti Maimunah
and Siti Maimunah
Department of Pharmacy, Maulana Malik Ibrahim State Islamic University, Malang, Indonesia.
Corresponding Author E-mail: fauziyah@farmasi.uin-malang.ac.id
DOI : https://dx.doi.org/10.13005/bpj/2510
Abstract
Dates (Phoenix dactylifera L.) are a unique fruit because they have many health benefits. Ajwa dates are one of the dates that many people are interested in for ingestion. Making date-infused water or nabeez water is one technique to ingest dates as exemplified by the Prophet SAW and is believed to have many benefits for digestion. As a result, there has never been any research on the analysis of active compounds from nabeez water, particularly Ajwa dates themselves, so the goal of this study was to determine the content of active compounds in nabeez water as well as the total content based on qualitative and quantitative analysis. The nabeez water from dates was tested qualitatively and quantitatively using phytochemical tests and UV-Visible Spectrophotometry. The results show that the nabeez water contains active ingredients of flavonoids, saponins, alkaloids, and tannins, according to qualitative analytical results. The quantitative analysis showed that nabeez water has a total concentration of flavonoid compounds of 5.749 g/mL and a concentration of tannin compounds of 52.934 g/mL.
Keywords
Active compounds; Nabeez water; Phytochemical test; Qualitative; Quantitative; Spectrophotometry
Download this article as:| Copy the following to cite this article: Fauziyah B, Batrisyia A. D, Firdausy A. F, Ma’arif B, Maimunah S. Analysis of Bioactive Compounds in Nabeez Water. Biomed Pharmacol J 2022;15(3). |
| Copy the following to cite this URL: Fauziyah B, Batrisyia A. D, Firdausy A. F, Ma’arif B, Maimunah S. Analysis of Bioactive Compounds in Nabeez Water. Biomed Pharmacol J 2022;15(3). Available from: https://bit.ly/3UcPtBL |
Introduction
Dates (Phoenix dactylifera L.) are a popular food in Islamic countries and are a staple in the Middle East. Date palms are one of the many types of palm trees that belong to the Arecaceae family, which includes over 2.500 species and 200 genera 1. The date palm is the most significant cultivated plant in North Africa and the Middle East, and it is one of the world’s oldest cultivated plants 2. This is based on the nutritional value and benefits of dates, which are well-known around the world for their high-quality nutrients and health-promoting characteristics 1,3.
According to prior research, dates contain flavonoids, carotenoids, glucose, fructose, sucrose, protein, ascorbic acid, phenolic acid, potassium (K), magnesium (Mg), phosphorus (P), sodium (Na), and iron (Fe) 4-6. Dates also known to have antioxidants, antihyperlipidemic, antimutagenic, anti-inflammatory, hepatoprotective, and nephroprotective properties, making them beneficial as an immunomodulator 7-10.
Dates have a unique feature in that they are one of 20 fruits mentioned in the Qur’an. One of the Islamic teachings for its believers is to consume all products that are permissible (halal) and good (thayyib) according to the relevant specifically the Quran and the Hadith 11. Dates are in great demand because they have many health benefits and are halal food. The Ajwa type, sometimes known as Prophet dates, is widely grown in Madinah and is also commonly found in Indonesia. Prophet Muhammad strongly advises that this sort of date be consumed every day because it has a compound that is beneficial for the health of the body 12,13
The Prophet Muhammad demonstrated one method of digesting dates by soaking the dates flesh in water in a closed container overnight and drinking the soaked water the next morning. The outcome of this immersion is known as Nabeez water. It is well known that Nabeez water is beneficial for removing acid from the stomach, avoiding flatulence, neutralizing uric acid, launching the digestive system, and serving as a detox element because it works to remove toxins from the body 14-17.
Based on the various benefits of Nabeez water, and the absence of studies discussing the analysis of compounds in Nabeez water, particularly from Ajwa dates themselves, it can be used as an opportunity for research related to Nabeez water. The purpose of this research was to determine the content of the compounds in Nabeez water based on qualitative and quantitative analysis using the UV-Vis spectrophotometry.
Material and methods
Material Preparation
The dates were purchased from a store that sells ajwa type dates and then the dates were separated from seeds. 5 g of date flesh was soaked in 100 mL of water in a closed container and allowed to stand for 18 hours at room temperature (± 25 C), and then filtered to get Nabeez water.
Qualitative Analysis of Nabeez Water
Flavonoid Test
5 mL of Nabeez water was added to a sterilized test tube, then 0.05 mg of magnesium (Mg) powder and 1 mL of concentrated hydrogen chloride (HCl) 37% were added, and shaken vigorously. The positive result of flavonoids are indicated by the change of the original color of nabeez water (muddy brown) to red, yellow, or nge in nabeez water 18,19.
Saponin Test
1 mL of Nabeez water was placed in a test tube, followed by 10 mL of hot water, cooled, and vigorously shaken for 10 seconds. Positive results are obtained if the foam is formed which persists for not less than 1 minute as high as 10 cm, or if 1 drop of 2N HCl is added, the foam does not disappear, indicating the presence of saponins 20,21.
Alkaloid Test
Add 1 mL of Nabeez water into a test tube, then add 5 mL of 2 N HCl and heat it in a water bath for 2 minutes. Then added 3 drops of Dragendrof LP reagent (Sigma-Aldrich, Germany). A positive result if a yellow-orange to brick-red precipitate is formed indicates the presence of alkaloids 21,22.
Tannin Test
Take 2 mL of Nabeez water and then heated for ± 5 minutes. Followed by adding a few drops of 1% ferric chloride (III) (FeCl3). Positive results are indicated by the formation of a greenish-brown or blue-black color in the solution 22,23 .
Quantitative Analysis of Nabeez Water
Total Flavonoid Content Test
In the process of making the standard curve, 10 mg of standard quercetin was weighed and put in a 10 mL volumetric flask and dissolved with methanol: aquadest (8:2). Pipette the solution as much as 0.5 mL and then dissolve it with 10 mL of distilled water to obtain a concentration of 50 ppm. Furthermore, 0 mL; 0.3 mL; 0.5 mL; 0.7 mL; and 0.9 mL of the 50 ppm solution was pipetted to obtain concentration 0 ppm; 3 ppm; 5 ppm; 7 ppm; and 9 ppm. Next, the absorbance was measured at a wavelength of 430 nm. In addition, the supporting reagent was made by weighing 0.1 g of acetate and 0.06 g of Plumbum(II) oxide (PbO). Then put into a beaker glass and dissolved in 10 mL of distilled water, stirring until dissolved and a cloudy solution is formed.
The total flavonoid content was determined by putting 0.05 g/mL with an immersion time of 18 hours into as much as 0.2 mL, put in a beaker glass, and dissolved with 5 mL of methanol, sonicated for 30 minutes. Then 0.1 mL of support reagent was added and centrifuged for 10 minutes at 3500 rpm. 1 mL of this solution was pipetted and 1 mL of 2% aluminium chloride (AlCl3) was added. Samples were incubated at room temperature for 30 minutes. After incubation, the absorbance of the samples was measured using a UV-Vis spectrophotometer at a wavelength of 430 nm. The sample absorbance calculation was repeated three times for each analysis so that the average absorbance value was obtained.
Total Tannin Content Test
In the process of making a standard curve, 10 mg of standard gallic acid was weighed, then put in a 10 mL volumetric flask and dissolved with methanol: aquadest (8:2). Pipette the solution as much as 0.5 mL and then dissolve it with 10 mL of distilled water to obtain a concentration of 50 ppm. Furthermore, 0 mL; 0.3 mL; 0.5 mL; 0.7 mL; and 0.9 mL of the 50 ppm solution were pipetted to obtain concentration of 0 ppm; 3 ppm; 5 ppm; 7 ppm; and 9 ppm. Next, the absorbance was measured at a wavelength of 765 nm. In addition, the supporting reagent was made by weighing 0.1 grams of Pb acetate and 0.06 grams of PbO. Then put into a beaker glass and dissolved in 10 mL of distilled water, stirring until dissolved and a cloudy solution is formed.
The total tanin content was determined by putting 0.05 g/mL with an immersion time of 18 hours as much as 0.1 mL, put in a beaker glass, and dissolving it with 5 mL of methanol. Then sonicated for 30 minutes. Then 0.1 mL of support reagent was added and centrifuged for 10 minutes at a speed of 3500 rpm. From the sample solution, 1 mL of Folin-Ciocalteu reagent (Sigma-Aldrich, Germany) was added, shaken, and allowed to stand for 5 minutes. Then 2 mL of 15% Na2CO3 solution was added, shaken until homogeneous, and allowed to stand for 5 minutes. Next, demineralized water was added to the exact volume of 10 mL, homogenized, and allowed to stand for 90 minutes. Then the absorbance was observed at a maximum wavelength of 765 nm. The tannin content was obtained from the average absorbance value for three repetitions of the analysis.
Data Analysis
Analysis of the data on the results of the qualitative test will be compared with the standard of qualitative testing based on the literature. Then the results of the quantitative test will be obtained in the form of primary data obtained from the absorbance of the standard solution. Then a calibration curve is made to obtain a linear regression equation. The calculation of the total content of the compound was obtained by substituting it into the linear regression equation y = ax + b which was obtained by comparing the calibration curve, and the calculation results were expressed in units of g/mL.
Results and Discussion
Nabeez water with a concentration of 0.05 g/mL was utilized in this investigation as the test sample. To eliminate sample selection problems and to verify that the correct dates were utilized, the dates were recognized and identified as raw materials before performing Nabeez water Nabeez water is made using the maceration extraction method, in which the dates’ flesh is immersed in a water-based solvent 24.
Before testing for quantitative analysis, the qualitative analysis was used to determine the presence of secondary metabolites in Nabeez water. After being provided reagents for the flavonoid, saponin, alkaloid, and tannin components, this qualitative examination took the form of a phytochemical test, which looked for any organoleptic alterations (color, precipitate formation, foam development) 25-26. Table 1 summarizes the findings of the qualitative analysis.
According to the results of the qualitative test above, the Nabeez water contains active substances such as flavonoids, saponins, alkaloids, and tannins. A change in the hue of the sample to yellow showed the presence of flavonoid molecules (Figure 1). The combination of Mg and HCl is intended to decrease the benzopyron core in the flavonoid structure, allowing flavilium salts to form 19,21,27. The equation for the reduction of active flavonoid compounds by Mg and HCl is:
Flavonoid Compound + Mg2+ + HCl → Orange Color + other products
Table 1: Nabeez Water Qualitative Analysis Results.
| Identification of Compounds | Reagent | Positive Indication | Conclusion |
| Flavonoids | Mg + HCl | Formation of red, yellow, or orange color | Positive |
| Saponins | Aquadest + 2N HCl | Foam is formed | Positive |
| Alkaloids | Dragendrof LP | A yellow-orange to brick-red precipitate is formed | Positive |
| Tannins | FeCl3 | Formation of greenish-brown or blue-black color | Positive |
 |
Figure 1: Flavonoid Compounds Qualitative Test Results. (a) Before administration of reagents; (b) After administration of reagents. |
The qualitative examination of Nabeez water revealed that saponins were also present, as evidenced by the development of foam when the sample was mixed with HCl and shaken (Figure 2). Saponins froth when shaken because hydrophilic groups connect to water while hydrophobic groups bind to air. The addition of HCl solution increases the polarity of the combination, making the interaction of the hydrophilic groups of saponins with water more stable, as well as the foam generated. The foam is formed when the polar groups face outward and the nonpolar groups face inward in the micelle structure 21,28.
 |
Figure 2: Saponin Compounds Qualitative Test Results. (a) Before administration of reagents; (b) After administration of reagents. |
A qualitative test on nabeez dates water showed positive results containing alkaloid compounds with Dragendrof LP reagent indicated by the presence of orange-yellow precipitate (Figure 3). Because the alkaloids are alkaline, before adding Dragendrof’s reagent, HCl was added first, so the extraction was carried out with a solvent containing acid. When the Dragendroff’s reagent is added, bismuth nitrate dissolves in HCl so that the bismuth salt is not hydrolyzed to bismuth ions (BiO+) as the reaction equation:
Bi3++ H2O → BiO++ 2H+
 |
Figure 3:Alkaloid Compounds Qualitative Test Results. (a) Before administration of reagents; (b) After administration of reagent. |
The Bi3+ ion from reacting bismuth nitrate with KI forms a black precipitate of bismuth(III) iodide, which dissolves in excess KI to form potassium tetraiodobismuthate. The use of nitrogen in this test aims to form a coordinate covalent bond with K+ as the metal ion 21,28. The following is an approximate the equation for the reaction of alkaloids with Dragendroff’s reagent:
Alkaloids Compounds + K[BiI4] → Precipitated Potassium Alkaloids + Orange Color
The color solution changed hue to greenish-brown after adding FeCl3 solution to Nabeez water indicating that the water contained tannins (Figure 4). This implies that the added FeCl3 solution will react with one of the hydroxyl groups in the tannin molecule to generate a complex compound with Fe3+ ions due to the presence of phenolic compounds in the form of tannins in the water sample of Nabeez dates. To identify phenolic substances such as tannins, the FeCl3 reagent is commonly used 21,29.
 |
Figure 4: Results of Qualitative Test of Tannin Compounds. (a) Before administration of reagents; (b) After administration of reagents. |
Meanwhile, quantitative analysis was carried out to determine the total levels of active compounds contained in the Nabeez water dates using the UV-Vis spectrophotometry method. The total levels of compounds tested were the total levels of flavonoid compounds and tannin compounds based on the standard curve for each compound. In determining the total flavonoid content using the results of the standard curve values. The standard curve obtained with the linear regression equation is y = 0.079x – 0.0063 and the value of r squared is 0.9986 (Figure 5).
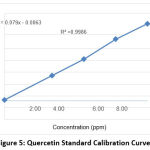 |
Figure 5: Quercetin Standard Calibration Curve. |
The total levels of flavonoid compounds can be determined using the test parameters of total quercetin equivalent levels and the result of the regression equation obtained. The flavonoid concentration of the Nabeez water was 5.749 g/mL on average (Table 2). Flavonoid molecules contain antioxidant and diuretic properties, as well as anticholesterol properties 30,31.
Table 2: Results of Determination of Total Flavonoid Levels in Nabeez Dates Water.
|
Replication |
Absorbance (y) |
Initial Total Flavonoid Level (µg/mL) | Total Flavonoid Level (µg/mL) | Percent Content of Total Flavonoid (%) |
| 1
2 3 |
0.012±0.001
0.010 ± 0.000 0.012±0.001 |
0.225
0.206 0.232 |
5,858
5,365 6,023 |
0.0006
0.0005 0.0006 |
In the quantitative test for the determination of tannin levels, the regression equation is obtained, namely y = 0.1673x + 0.0116 and the value of r squared is 0.9971 (Figure 6). The average content of tannins contained in the Nabeez water with the total test parameter of gallic acid equivalent level was 52,934 g/mL (Table 3). Tannin compounds are astringents that are useful as antidiarrheal, hemostasis, and anti-inflammatory, especially in the oral mucosa, as well as antiseptics due to the presence of phenolic groups 30,32
Table 3: Results of Determination of Total Tannin Levels in Nabeez Dates Water.
|
Replication |
Absorbance (y) |
Initial Total Tannin Content
(µg/mL) |
Total Tannin Content
(µg/mL) |
Percent Content of Total Tannins (%) |
| 1
2 3 |
0.186 ± 0.000
0.187±0.001 0.181 ± 0.001 |
1.048
1.051 1.015 |
53.443
53.596 51.763 |
0.0053
0.0054 0.0052 |
The Nabeez water of the Ajwa type were discovered to include flavonoid chemicals, saponins, alkaloids, and tannins, according to the results of qualitative and quantitative analyses. According to Hamad et al (2015) 4 study, the level of flavonoid components in 100 g of dry weight extract of Ajwa dates was 27.8 g/g. The levels of active compounds in the water samples of Nabeez water are lower than the levels of active compounds in the extracts of dates, according to these findings. This is possible because the Nabeez water dates is only made from the results of soaking the flesh of the dates (water extract of dates fruit) so that the total levels of active compounds are lower.
Conclusion
Based on the results of the research that has been done, it can be concluded that the Nabeez water contains active compounds of flavonoids, saponins, alkaloids, and tannins. Nabeez water have an average level of total flavonoids with quercetin equivalent test parameters of 5.749 g/mL and average levels of total tannins with gallic acid equivalent test parameters of 52,934 g/mL. As for supporting this research, it is necessary to analyze the content of other compounds in water nabeez dates, because there is still little research related to water nabeez dates, it is important to know the overall content of any compound in Nabeez and can be used as a reference to see the potential of compounds for health..
Conflict of Interest
All authors declare there is no conflict of interest in this manuscript.
Funding Sources
There are no funding sources.
References
- Al-Alawi RA, Al-Mashiqri JH, Al-Nadabi, JSM, Al-Shihi BI, and Baqi Y. Date Palm Tree (Phoenix dactylifera L.): Natural Products and Therapeutic Options. Frontiers in Plant Science. 2017; 8: 845.
CrossRef - Chao CT., & Krueger RR. The date palm (Phoenix dactylifera L.): overview of biology, uses, and cultivation. HortScience. 2007; 42(5): 1077-1082
CrossRef - Parvin S, Easmin D, Sheikh A, Biswas M, Sharma SCD, Jahan Md GS, Islam Md A, Roy N, Shovon MS. Nutritional Analysis of Date Fruits (Phoenix dactylifera L.) in Perspective of Bangladesh. American Journal of Life Sciences. 2015;3(4):274-278.
CrossRef - Hamad I, Abd Elgawad H, Jaoni SA, Zinta G, Asard H, Hassan A, Hegab M, Hagagy N, Selim S. Metabolic Analysis of Various Date Palm Fruit (Phoenix dactylifera L.) Cultivars from Saudi Arabia to Assess Their Nutritional Quality. Molecules. 2015;20(8):13620-13641.
CrossRef - Imo C, Uhegbu FO, Arowora KA, Ezeonu CS, Opara IJ, Nwaogwugwu CJ, and Anigbo CJ. Chemical composition of Cyperus esculentus nut and Phoenix dactylifera fruit. African Journal of Biotechnology. 2018; 18(19): 408-415.
CrossRef - Samad MA, et al. Antibacterial Properties and Effects of Fruit Chilling and Extract Storage on Antioxidant Activity, Total Phenolic and Anthocyanin Content of Four Date Palm (Phoenix dactylifera) Cultivars. Molecules. 2016; 21(419): 1-14.
CrossRef - Abdillah M, Nazilah NRK, and Agustina E. Identification of Active Compounds in Methanol Extract of Ajwa Dates Flesh (Phoenix dactylvera L.). In: Proceedings of the 2017 National Seminar III; Malang, April 29, 2017. Malang. Biology Education Study Program-FKIP. 2017; 69-74.
- Hariadi B, and Widodo A. Effect of Date Fruit Extract (Phoenix dactylifera L.) Ajwa Variety on NO Levels in Balb/C Mice Infected with Salmonella typhimurium. Diponegoro Medical Journal. 2018; 7(2): 751–61.
- Nurmeilis FI, and Susanti O. Test of Immunomodulator Effects of Dates Sari (Phoenix dactylifera) on Nonspecific and Specific Immune Responses in Male Mice (Mus musculus). Journal of MedikaIslamika. 2014; 11(1): 201-208.
- Rosnizar EK, Ramli IM, and Muliani F. Test of Immunostimulant Effects of Dates Fruit (Phoenix dactylifera) on Male Mice (Mus musculus) Balb/C Strain. In: Proceedings of the 2015 Biotic National Seminar. 2015; 292-297.
- Anggraini I, Dewanti DS. The Effect of Halal Foods Awareness on Purchase Decision with Religiosity as a Moderating Variable. Journal of Economics Research and Social Sciences. 2020; 4(1) 17-29
CrossRef - Fibonacci A. Antioxidant Activity of Nabeez Water from Ajwa Palm Date Fruits (Phoenix dactylifera L) as a Favorite Drink of the Prophet Muhammad SAW. Journal of Physics: Conference Serie. 2020; 1-8.
CrossRef - Ali SA, Parveen N., & Ali AS. Links between the Prophet Muhammad (PBUH) recommended foods and disease management: A review in the light of modern superfoods. International journal of health sciences. 2018; 12(2): 61.
- Muzaifa M, Lubis YM, and M Arifullah. Study of Making Infused Water from Dates (Phoenix dactylifera) with the Addition of Lime (Citrus aurantiifolia). Indonesian Journal of Agricultural Technology and Industry. 2019; 11(02): 84–89.
CrossRef - Naheda Tibb An-Nabawi, The Medicine of Holi Prophet. Science, Health and Wellness. 2013; 94-104.
- Omar SR, and Omar SN. Reviving the Authenticity of Prophetic (Sunnah) Drinks in Beverage Industry in Malaysia: A Review. Journal of Fatwa Management and Research. 2018; 505-520.
CrossRef - Putri EBP, Putri FK, and Suliha S. Comparison of Flavonoid and Vitamin C Levels in Infused Water Goji Berry (Lyciumbarbarum) and Water Nabeez Dates (Phoenix dactylifera L.). Medical Technology and Public Health Journal. 2020; 4(1): 32-37.
CrossRef - Hasma H, and Winda W. Identification of Secondary Metabolite Compounds Ethanol Extract of Kepok Banana Peel (Musa paradisiaca L) by TLC Method. Manarang Health Journal. 2019; 5(2): 125-131.
CrossRef - Wahid AR, and Safwan S. Phytochemical Screening of Secondary Metabolite Compounds Against Fracture Twig Plant Extract (Euphorbia tirucalli L.). Pharmacy Barns: Journal of Pharmaceutical Sciences. 2020; 1(1): 24-27.
CrossRef - Putra IWDP, Dharmayudha AAGO, and Sudimartini LM. Identification of Chemical Compounds of Moringa Leaf Ethanol Extract (Moringa oleifera L) in Bali. Indonesia MedicusVeterinus. 2016; 5(5): 464-473.
- Handayani TW, Yusuf Y, and Tandi J. Qualitative and Quantitative Analysis of Secondary Metabolites of Moringa Seed Extract (Moringa oleifera Lam.) with UV-Vis Spectrophotometric Method. COVALEN: Journal of Chemical Research. 2020; 6(3): 230-238.
CrossRef - Dewi NP. Qualitative and Quantitative Test of Secondary Metabolites Ethanol Extract of Awar-Awar Leaves (Ficus septicaBurm. F) with UV-Vis Spectrophotometer Method. Acta HolisticaPharmaciana. 2020; 2(1): 16-24.
- Ergina E, Nuryanti S, and Pursitasari ID. Qualitative Test of Secondary Metabolic Compounds on Palado (Agave angustifolia) Leaves Extracted with Water and Ethanol Solvents. Journal of Academic Chemistry. 2014; 3(3): 165-172.
- Endarini LH. Modul : Farmakognosi dan Fitokimia. Jakarta: Kementerian Kesehatan Republik Indonesia, 2016.
- Vifta RL, and Advistasari YD. Phytochemical Screening, Characterization, and Determination of Total Flavonoid Content of Extracts and Fractions of Parijoto Fruit (Medinilla speciosa B.). In Proceedings of the Unimus National Seminar. 2018; 1: 8-14.
- Al Hazmi GG, and Harijono H. Effect of Drying and Maceration Time with Ethanol and Hexane Double Solvents on Bioactive Compounds of Putri Palm Seed Flesh (Veitchiamerillii). Journal of Food and Agroindustry. 2019; 7(2): 13-23.
CrossRef - Baud GS, Sangi MS, and Koleangan HS. Analysis of Secondary Metabolite Compounds and Toxicity Test of Ethanol Extract of Fractured Plant Stem (Euphorbia tirucalli L.) with Brine Shrimp Lethality Test (BSLT) method. Scientific Journal of Science. 2014; 14(2): 106-112.
CrossRef - Simaremare, ES. Phytochemical Screening of Itchy Leaf Ethanol Extract (Laportea decumana (Roxb.) Wedd). PHARMACY: Indonesian Pharmacy Journal (Pharmaceutical Journal of Indonesia). 2014; 11(1).
- Marlinda M, Sangi MS and Wuntu AD. Analysis of Secondary Metabolite Compounds and Toxicity Test of Ethanol Extract of Avocado Seeds (Persea americana Mill.). Journal of Mathematics and Natural Sciences. 2012; 1(1): 24-28.
CrossRef - Hanani E. Phytochemical Analysis. Jakarta: ECG Medicine Book, 2014.
- Azizah DN, Kumolowati E, and Faramayuda F. Determination of Flavonoid Levels by AlCl3 Method in Methanol Extract of Cocoa Fruit Peel (Theobroma cacao L.). Kartika: Scientific Journal of Pharmacy. 2014; 2(2): 33-37.
CrossRef - Amelia FR. Determination of Tannin Types and Determination of Tannin Content from Young Bungur Fruit (Lagerstroemia speciosa Pers.) Spectrophotometry and Permanganometry. CALYPTRA. 2016;4(2): 1-2.







