Jargalsaikhan Gombodorj1,2* , Enkhjargal Bayarsaikhan3
, Enkhjargal Bayarsaikhan3 , Chimedragchaa Chimedtseren2
, Chimedragchaa Chimedtseren2 , Uuganbayar Baatartsogt2
, Uuganbayar Baatartsogt2 , Baigali Gansukh2 and Seesregdorj Surenjid1
, Baigali Gansukh2 and Seesregdorj Surenjid1
1International School of Mongolian Medicine, Mongolian National University of Medical Sciences, Ulaanbaatar, Mongolia.
2Institute of Traditional Medicine and Technology, Ulaanbaatar, Mongolia.
3National cancer center of Mongolia, Ulaanbaatar, Mongolia.
Corresponding Author E-mail:saihan_j@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2507
Abstract
Objectives: To investigate the anti-inflammatory activity of Khurtsiin deed-6 in migraine and neurodegeneration rat models. Methods: This study uses nitroglycerin induced migraine was model and alcohol exposed neurodegeneration model in Wistar rats to evaluate anti-inflammatory effect of Khurtsiin deed-6 at the 3 different doses of 50, 100and 150 mg/kg, orally. After anesthesia, the brains were removed, then trigeminal nucleus caudal is and hippocampus region isolated from fresh brain. And followed by protein and RNA extraction. Interleukin-1β expression was analyzed by real time polymerase chain reaction. Results: Nitroglycerin induced rat migraine model had increased Interleukin-1β expression in brain TNC area (p<0.001). It has been decreased dramatically after treatment of Khurtsiin deed-6 by doses of 50mg/kg, 100mg/kg and 150mg/kg treatment in brain trigeminal nucleus caudal is region (p<0.01) comparing ibuprofen treated group. Moreover alcohol exposed neurodegeneration rat model had observed increased Interleukin-1β expression in brain Hp area (p<0.001). But Khurtsiin deed-6 doses of 50mg/kg, 100mg/kg and 150mg/kg treatment reduced neuroinflammatory cytokines IL1β expression in hippocampus region (p<0.01, p<0.001) of alcoholic rat models comparing pyridoxamine administrated animals. Conclusion: The present finding indicates that Khurtsiin deed-6shows anti-inflammatory activity decreasing the level of Interleukin -1β cytokine in nitroglycerin induced migraine and alcohol exposed neurodegeneration rat models
Keywords
Hippocampus Region; Interleukin 1β; Khurtsiin deed-6; Migraine; Neurodegeneration; Nucleus Caudalis; Trigeminal
Download this article as:| Copy the following to cite this article: Gombodorj J, Bayarsaikhan E, Chimedtseren C, Baatartsogt U, Gansukh B, Surenjid S. An Analysis of Khurtsiin Deed-6 Compound’s Effect on IL-1β Expression in Nitroglycerin Induced Migraine and Alcohol Exposed Neurodegeneration Model in Rat. Biomed Pharmacol J 2022;15(3). |
| Copy the following to cite this URL: Gombodorj J, Bayarsaikhan E, Chimedtseren C, Baatartsogt U, Gansukh B, Surenjid S. An Analysis of Khurtsiin Deed-6 Compound’s Effect on IL-1β Expression in Nitroglycerin Induced Migraine and Alcohol Exposed Neurodegeneration Model in Rat. Biomed Pharmacol J 2022;15(3). Available from: https://bit.ly/3RaJMC2 |
Introduction
Headache (migraine) is the sixth most prevalent illness in the world and is one of the leading causes of disability by becoming one of the individual and society’s pressures. 1 Considering the statistics of the health sector of Mongolia, migraine and other types of headaches among nervous system diseases reached 26.5% in 2019, which showed that it is 5.2% higher than the 10-year average.
In the Mongolian Traditional Medicine’s sourcebook, traditional compound Khurtsiin deed-6 (KhD-6) is used for the treatment of colic caused by wind and blood disorder (neuralgia), headache caused by blood and bile disorder, headache due to the fever caused by bacteria, headache from gland disease, and leucoma.2 The KhD-6 is composed of Terminalia chebula Retz, Carthamus tinctorius L, Odontites rubra /Baumg/ Pers, Saussurea lappa L., Commiphorae mukul and Moschus moschiferus.3
It was determined that KhD-6 contained biologically active substances such as, gallic acid Retention factor (Rf)=0.27, costunoloids Rf=0.71, saflomin A Rf=0.21, E-guggulsterone Rf=0.5, apigenin Rf=0.46, and as a polyphenolic compound- flavonoids and essential oil.4 The other studies have shown that these biologically active substances had an effects of reducing inflammatory cytokines, inhibiting microglial activity, apoptosis, and protecting nerves.5-8
The neuroinflammation induces vascular permeability, leukocytes infiltration, activation of glial cells, and release of inflammatory mediators.9 During migraine progression the neuroinflammation is related to the activation of glia in the hypothalamus. The activation of glia in the hypothalamus induces the trigeminal nucleus caudalis (TNC) and trigeminocervical complex (TCC). The researchers continue to prove that this establishes the sensitization and due to the release of calcitonin gene-related peptide and inflammatory cytokines it activates pain.10 Inflammation occurs easily during the brain ischemia and injuries. If it is sustained for long periods of time, then the neurodegenerative diseases such as Alzheimer and Parkinson start to develop. Therefore, the alcohol induced neuroinflammation is mediated by the increase in interleukin (IL)-1β, proinflammatory cytokines. It was studied that the increased IL-1β in the brain during the alcohol induced neuroinflammation innate immune response.11 However, there isn’t enough evidence related to alcohol how it affects the brain at cellular and molecular levels. It has been suggested that alcohol alters the neurotrophic and neuroimmune signaling which result in neurodegeneration and leads to the loss of executive function.12
In traditional medicine for diseases that are comparable with migraine KhD-6 is prescribed widely.
Within the scope of our research we have studied the effect of KhD-6 on nitroglycerin induced migraine and neurodegeneration associated with alcoholism. Specifying the IL-1β in our study is considered significant based on the researcher’s experimental evidence determining that inflammation is an important factor in neurodegeneration, and the IL-1 cytokine is crucial mediator as well as it shows rapid response to neuronal injury.13
Materials and Methods
Traditional Mongolian medicine KhD-6(internal serial number 220220) was provided by Institute of Traditional Medicine and Technology of Mongolia and ibuprofen (Batch No: F20091215MO02268, Shijiazhuang, P.R. China) was purchased from local pharmacy. Nitroglycerin (NTG; Batch No: F140119CP03590.) infusion was obtained from hospital pharmacy.
Migraine inducing rat method
In this study eighteen male Wistar rats (healthy, weighing 250-300g) were randomly selected from the animal house of Research center under the normal condition with a (20±2C) temperature, as well as with standard animal diet. The experimental animals were divided into 6 groups each consists 3 animals: normal group, control NTG group, KhD-6 50, 100 and 150 mg/kg, and Ibuprofen (IBU)85 mg/kg group. According to the methodology of Xiao- Fan Zhang (2017) et al. NTG induced migraine the rats were treated with KhD-6 by oral injection at doses 50, 100 and 150 mg/kg and ibuprofen at doses of 85 mg/kg respectively for 10 consecutive days.14 One hour later after taking their last medicine the rats except normal group received NTG 10 mg/kg subcutaneous injections. It was observed that after the injection rats were scratching heads and shaking their body. Animals were observed for 4 hours and sacrificed under anesthesia, brain were removed and Trigeminal nucleus caudalis (TNCs) were carefully dissected out and stored at -80 0C for later use. TNCs region’s level of IL-1β was assessed by real time polymerase chain reaction (RT-PCR) method.
Neurodegeneration inducing method
Thirty six male Wistar rats (healthy, weighing 250-300g) were randomly selected from the animal house of Research center under the normal condition with a (20±2C) temperature, as well as with standard animal diet. The experimental animals were divided into 6 groups each consists 3 animals: normal group, control group, 3 different doses of Khd-6 (50 mg/kg, 100 mg/kg and 150 mg/kg) and Pyridoxamine group. According to the methodology of Fulton Crew2 (2008), rats administered 25% of 5ml ethanol (ETOH) by oral injection and followed by KhD-6 (50 mg/kg, 100 mg/kg and 150 mg/kg) and injected orally after 1 hour for 10 days. The positive control group was treated by 1g/l Pyridoxamine in drinking water for 10 consecutive days with ethanol.15
Therefore, we were conducted 6 groups of rat models (normal group, control group, KhD-6 treated group by 3 different doses 50 mg/kg, 100 mg/kg, 150 mg/kg, and pyridoxamine treated group). First alcoholic rat models were generated by daily oral injection of 25% of 5ml ETOH for 10 days and followed by treatment from 11th day (KhD-6 dose of 50 mg/kg, 100 mg/kg, 150 mg/kg) for 10 consecutive days. Moreover 1g/l Pyridoxamine administrated in drinking water.
After completed the treatment animals were sacrificed under anesthesia, brain was removed and Hippocampus (HP) regions were isolated and stored at -80 0C for later use
Polymerase chain reaction method
Experiment animals were anesthetized with pentobarbital and perfused transcardially with 400 ml of phosphate-buffered saline (PBS). TNC and Hps were carefully dissected out and stored at -80C for later use. Total RNA was purified using an RNeasy Mini Kit (Qiagen GmbH, Hilden, Germany, cat num: 74106 ) and its yield and purity were assessed by Nanodrop 200C (Thermo Fisher Scientific Inc). cDNA was synthesized using the QuantiTect Reverse Transcription Kit (Qiagen GmbH, Hilden, Germany, cat. num: 205314. RT-PCR was performed using syber green kit (LightCycler 480 SYBR green I master, Roche Diagnostics, USA, cat.num: 04707516001) in a LightCyler 480(I) real time PCR detection system. The PCR was performed under the following conditions: denatured for 3 min at 94C followed by amplification step for 45 cycles of 10 s at 95C, 25 s at 56C, 10 s at 72C, and 10 s at 40C. The specific primers was used in this study are follows:
Table 1: Sequence of primers used in this study.
| Primer name | Sequence |
| IL-1β-F | 3’-TTGGGCCTCAAAGGAAAGAAT-5 |
| IL-1β-R | 3’-TGCTTGTGAGGTGCTGATGTA-5’ |
| GAPDH-F | 3’-CGT GAT CGAGGGCTGTTG G-5 |
| GAPDH-R | 3’-CTGCTTCAGTTG GCC TTT CG-5 |
Statistical analysis
Graphpad Prism-8 software was used for statistical analysis. The data were expressed as mean ±SE (n=54). Data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test, and probabilities of p <0.05 were considered as statistically significant.
Ethical Statement
The Research Ethics Committee of the Mongolian National University of Medical Sciences approved the study protocol (2015/11-20).
Results
The effect of KhD-6 on nitroglycerin induced migraine:
As shown in Figure 1. Compared with the normal group it was detected that nitroglycerin induced migraine model’s IL-1β expression was increased 88.5% by RT-PCR method, which indicates that the migraine model is formed (p˂0.001).
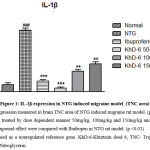 |
Figure 1: IL-1β expression in NTG induced migraine model. (TNC area). |
According to the figure 1 the expression of IL-1β was significantly decreased in KhD-6 dose of 50 mg/kg (0.49±0.15) by 94.4% (p<0.001), 100 mg/kg (2.86±0.28)by 67.2%(p<0.001), and KhD-6dose of 150 mg/kg (3.95±0.29)by 54.6% (p<0.01)respectively compared with control group (8.71±0.34), and in Ibuprofen dose of 85 mg/kg (1.50±0.16) by 82.7% was significantly decreased as well (p<0.01).
The effect of KhD-6on alcohol induced neurodegenerative model:
As shown in Figure 2 Compared with the normal group it was detected that neurodegenerative model associated with alcohol’s IL-1β expression was increased 95.2% by RT-PCR method, which indicates that the neurodegenerative model is formed (p˂0.001).
According to the figure 2A the expression of IL-1β was significantly decreased in ETOH/KhD-6group dose of 50 mg/kg (3.44±0.14) by 83.4% (p<0.01) compared with control group (20.78±0.24). Moreover the expression of IL-1β was statistically difference in ETOH/KhD-6dose of 100 mg/kg and 150 mg/kg (p>0.05).
According to the figure 2Bafter neurodegenerative model being formed the expression of IL-1β was significantly decreased in ETOH+KhD-6group dose of 50 mg/kg (3.26±0.14) by 84.3%, 100 mg/kg (0.86±0.01) by 95.8% and 150 mg/kg (0.49±0.01) by 97.6% respectively (p˂0.001, p<0.01). And in Pyridoxamine dose of 1gr/l (3.94±0.01) by 81% was significantly decreased as well (p<0.01).
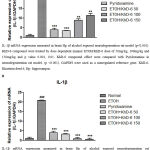 |
Figure 2: IL-1β expression in alcohol exposed neurodegeneration model. (Hp region). |
Discussion
According to the research, among the neuroinflammation model the most proven model was the detection of IL-1β in central nervous system’s microglia.16 The recent experiments are still proving that inflammation is playing pivotal role in neurodegeneration and the IL-1 (interleukin-1) cytokine is the main mediator. The IL-1 is expressed quickly in response to neuronal injury. Inflammation occurs easily during the brain insults and trauma. If it is sustained for long periods of time, then the neurodegenerative diseases such as Alzheimer, Parkinson and multiple sclerosis starts to develop.13 And also, neuroinflammation associated with alcohol is mediated by IL-1β proinflammatory cytokines. Experimental evidences indicate that alcohol induced inflammatory activation leads to increased IL-1β in the brain.11
In order to determine the effect of KhD-6 in neuroinflammation especially in IL1 β expression, we have conducted research on nitroglycerin induced migraine model treated with KhD-6 dose dependently and Ibuprofen treated as a comparative medicinal drug group. 14 Also, we were selected alcoholic model for neurodegenerative disease to evaluate IL1 β expression. Pyridoxamine was treated as a comparative medicinal drug group.17
During the migraine pathogenesis the levels of IL-1β, IL-6 and TNF-α were significantly higher.6 Evidences are showing that IL-1β contributes to migraine pathogenesis, for example in internal jugular blood the level of IL-1β was increased during continuous and occasional migraine.18
It has been reported that Valproate was used for treatment in two groups and the expression level of IL-1β was evaluated in nitroglycerin-induced migraine model. The expression level of IL-1β was significantly increased (***p<0.001) and was decreased in two treatment group of Valproate (###p<0.001) in nitroglycerin-induced migraine model.19 Therefore, the NOD-like receptor protein 3 (NLRP3) and IL-1β in microglia in the TNC was evaluated during nitroglycerin-induced chronic and acute migraine. During hyperalgesia both the levels of NLRP3 and IL-1β were increased, which shows the activation of inflammation response.20 Researchers Mami Noda et al., explained the neuroprotective role of bradykinin (BK) because of the attenuation of pro-inflammatory cytokine release from activated microglia, and during the lipopolysaccharide-induced model BK (100 nmol/L) affected the IL-1β level by decreasing it 48%, which showed inhibition of pro-inflammatory cytokine IL-1β release.21
In order to determine the effects of prevention and treatment in neurodegenerative model associated with alcohol, KhD-6 was administered with ethanol and the ethanol was given solely to form a disease model. ETOH/KhD-650 mg/kg group’s level of IL-1β was decreased (p<0.001), treatment group of ETOH+KhD-650 mg/kg, 100 mg/kg and 150 mg/kg were decreased respectively (p<0.001) . Moreover, group of KhD-650 mg/kg, 100 mg/kg and 150 mg/kg the level of IL-1β were decreased in nitroglycerin-induced migraine model (p<0.01, p<0.001).
Conclusion
It can be concluded that the treatment with KhD-6compound exertedanti-inflammatory effect through reduction of IL-1β level of the brain in migraine and neurodegeneration models. Our result showed that KhD-6 inhibits neuroinflammation in TNC area, which found significant decrease in IL-1β. Moreover, KhD-6 compound has an effect of decreasing IL-1β expression in Hp region.
Acknowledgement
The authors would like to show our gratitude to the Research Center, Institute of Traditional Medicine and Technology for providing the laboratory to conduct our experimental research.
Conflict of Interest
The authors state no conflict of interest.
Funding Sources
Hospital internal research funding support.
References
- G. Tsagaankhuu, Neurology, 2th ed. Ulaanbaatar, 2011.
- Luvsanchoimbol, “‘Jedun ninor’ Traditional Medical source book,” “Mongol Printing” printing house, Ulaanbaatar, p. 16, 1993.
- D. B. C. Ch, A reference book of traditional drug and medicinal materials, 2th ed. Ulaanbaatar, Mongolia: Jicom press, 2015.
- C. C. Myadagbadam U, Purevsuren S, Norovnyam R, “Results of the quality and safety parameters of the Khurtsiin deed-6 traditional medicinr.” Ulaanbaatar, Mongolia, 2021.
CrossRef - D. B. Reddy and P. Reddanna, “Chebulagic acid (CA) attenuates LPS-induced inflammation by suppressing NF-κB and MAPK activation in RAW 264.7 macrophages,” Biochem. Biophys. Res. Commun., vol. 381, no. 1, pp. 112–117, 2009, doi: 10.1016/j.bbrc.2009.02.022.
CrossRef - C. C. Wang et al., “Protective effect of dried safflower petal aqueous extract and its main constituent, carthamus yellow, against lipopolysaccharide-induced inflammation in RAW264.7 macrophages,” J. Sci. Food Agric., vol. 91, no. 2, pp. 218–225, 2011, doi: 10.1002/jsfa.4172.
CrossRef - S. G. Lee and H. Kang, “Saussurea lappa Clarke extract exhibits potent antioxidant effect and attenuates neuroinflammatory responses in lipopolysaccharide-stimulated microglial cells,” Trop. J. Pharm. Res., vol. 19, no. 9, pp. 1911–1917, 2020, doi: 10.4314/tjpr.v19i9.16.
CrossRef - R. Ragavi and S. A. Surendran, “Commiphora mukul: An overview,” Res. J. Pharm. Technol., vol. 11, no. 7, pp. 3205–3208, 2018, doi: 10.5958/0974-360X.2018.00589.9.
CrossRef - G. Y. Ji RR, Xu ZZ, “Emerging targets in neuroinflammation-driven chronic pain.,” Nat Rev Drug Discov, vol. 13(7), pp. 533–48, 2014, doi: 10.1038/nrd4334.
CrossRef - O. ML, “Sensitization and ongoing activation in the trigeminal nucleus caudalis No Title,” Pain, vol. 155(7), pp. 1181–1182, 2014, doi: 10.1016/j.pain.2014.04.001.
CrossRef - D. Lippai, S. Bala, J. Petrasek, T. Csak, I. Levin, and E. A. Kurt-jones, “Alcohol-induced IL-1β in the brain is mediated by NLRP3 / ASC inflammasome activation that amplifies neuroinflammation,” vol. 94, no. July, pp. 171–182, 2013, doi: 10.1189/jlb.1212659.
CrossRef - R. P. Vetreno and F. T. Crews, Current hypotheses on the mechanisms of alcoholism, 1st ed., vol. 125. Elsevier B.V., 2014.
CrossRef - A. Simi, N. Tsakiri, P. Wang, and N. J. Rothwell, “Interleukin-1 and inflammatory neurodegeneration,” Biochem. Soc. Trans., vol. 35, no. 5, pp. 1122–1126, 2007, doi: 10.1042/BST0351122.
CrossRef - X. F. Zhang et al., “Analgesia effect of baicalein against NTG-induced migraine in rats,” Biomed. Pharmacother., vol. 90, pp. 116–121, 2017, doi: 10.1016/j.biopha.2017.03.052.
CrossRef - F. T. Crews, “Alcohol-related neurodegeneration and recovery: mechanisms from animal models.,” Alcohol Res. Health, vol. 31, no. 4, pp. 377–88, 2008, [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/23584011%0Ahttp://www.pubmedcentral.nih.gov/ articlerender.fcgi?artid=PMC3860462.
- A. De Corato et al., “Trigeminal satellite cells express functional calcitonin gene-related peptide receptors , whose activation enhances interleukin-1 β pro-in fl ammatory effects,” J. Neuroimmunol., vol. 237, no. 1–2, pp. 39–46, 2011, doi: 10.1016/j.jneuroim.2011.05.013.
CrossRef - Byun K, Bayarsaikhan D, Bayarsaikhan E, Son M, Oh S, Lee J, Son HI, Won MH, Kim SU, Song BJ, Lee B. Microglial AGE-albumin is critical in promoting alcohol-induced neurodegeneration in rats and humans. PLoS One. 2014 Aug 20;9(8):e104699. doi: 10.1371/journal.pone.0104699. PMID: 25140518; PMCID: PMC4139297.
CrossRef - P. Sarchielli et al., “Proinflammatory Cytokines , Adhesion Molecules , and Lymphocyte Integrin Expression in the Internal Jugular Blood of Migraine Patients Without Aura Assessed Ictally,” vol. 4, pp. 200–207, 2006, doi: 10.1111/j.1526-4610.2006.00337.x.
CrossRef - L. Yi et al., “Valproate Plays a Protective Role against Migraine by Inhibiting Protein Kinase C Signalling in Nitroglycerin-treated Mice,” Basic Clin. Pharmacol. Toxicol., vol. 122, no. 3, pp. 310–316, 2018, doi: 10.1111/bcpt.12915.
CrossRef - W. He et al., “Microglial NLRP3 inflammasome activation mediates IL-1β release and contributes to central sensitization in a recurrent nitroglycerin-induced migraine model,” J. Neuroinflammation, vol. 16, no. 1, pp. 1–17, 2019, doi: 10.1186/s12974-019-1459-7.
CrossRef - M. Noda et al., “Neuroprotective role of bradykinin because of the attenuation of pro-inflammatory cytokine release from activated microglia,” J. Neurochem., vol. 101, no. 2, pp. 397–410, 2007, doi: 10.1111/j.1471-4159.2006.04339.x.
CrossRef







