Rupesh S. Parati* , Ponvijaya M. Yadav
, Ponvijaya M. Yadav , Vijayshree S. Gokhale
, Vijayshree S. Gokhale and Atiullah Imran Malik
and Atiullah Imran Malik
Department of Medicine, Dr. D. Y. Patil Medical College, Hospital and Research Centre, Dr. D. Y. Patil Vidyapeeth, Pune, India.
Corresponding Author E-mail:rupeshparati@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2517
Abstract
Background: Monoclonal immunoglobulin deposition disease (MIDD) is a rare and sporadic phenomenon often manifesting in individuals who are in their 5th – 6th decade of life. MIDD along with restrictive cardiomyopathy and Renal AL amyloidosis as in the present case is also an unaccustomed phenomenon. Often, the patient presents with unusual symptoms and is more prone to misdiagnosis. The congo red (-) deposition of the monoclonal light chain can be frequently noted in multiple organs including the heart and kidney. The light chain deposition can be either isolated lambda or gamma chain and they are classified accordingly. While serum electrophoresis at an early presentation may reveal underlying monoclonal gammopathy, the immunofluorescence and histopathological examination of renal biopsy remain the mainstay diagnostic tool. Objective: The case study was done because of its uniqueness in the usage of a novel strategy in the treatment of MIDD. Results: In the current case, a 52-year-old male presented with chest pain and NYHA grade 3 dyspnea for 8 days along with bilateral lower limb pitting oedema since 3 months. In certain prevailing situations, a patient can be misdiagnosed with ischemic heart disease or acute on chronic renal failure. However, detailed history and time-bound investigation will act as a safeguard. Conclusion: When patients have no co-morbidities and no addictions, and the patient’s investigation reveals heavy proteinuria, the rheumatological and haematological etiologies must be ruled out to establish a final diagnosis for timely treatment. As in our case, the rheumatological work-up was negative but we found an M band spike on serum electrophoresis, which expedited our search for underlying plasma-cell dyscrasia.
Keywords
AL Renal Amyloidosis; Bortezomib; Dexamethasone; Monoclonal Immunoglobulin Deposition Disease; Restrictive Cardiomyopathy
Download this article as:| Copy the following to cite this article: Parati R. S, Yadav P. M, Gokhale V. S, Malik A. I. A Case of Monoclonal Immunoglobulin Deposition Disease – on Bortezomib regimen, A Novel strategy in Multisystemic Involvement. Biomed Pharmacol J 2022;15(3). |
| Copy the following to cite this URL: Parati R. S, Yadav P. M, Gokhale V. S, Malik A. I. A Case of Monoclonal Immunoglobulin Deposition Disease – on Bortezomib regimen, A Novel strategy in Multisystemic Involvement. Biomed Pharmacol J 2022;15(3). Available from: https://bit.ly/3Tf5FlF |
Case Presentation
Herein we report a case of a 52-year-old male, Hindu, vendor by occupation who was brought to ER with complaint of chest pain and dyspnea of NYHA grade 3 in the last 8 days and bilateral pitting oedema of lower limb lasting for 3 months. The patient is non-diabetic, non-hypertensive and didn’t have any comorbidity or addiction or history of any drug intake. The patient didn’t have any significant family history. On examination, the patient appeared to be pale with raised JVP, however, his BP was 130/70 mm of Hg and his pulse rate was 102/min with 100% saturation. The patient had no lymph node enlargement, clubbing or cyanosis. There was also no history of any joint pain, rash, or weight loss. ECG was suggestive of ST depression in lead I, II, III, and avF along with low voltage complexes. Upon emergency 2D echocardiography, it was revealed that there was a Global left ventricular (LV) hypokinesia, L-V systolic function Ejection fraction of 50%, moderate concentric LV hypertrophy, trivial atrial regurgitation (AR), Moderate mitral regurgitation (MR) with grade 2 diastolic dysfunction (DD). The patient was later subjected to laboratory investigations as given below.
Table 1: Complete Blood Count parameters before and after treatment.
| Parameter | Pre-Treatment | Post Treatment |
| Hemoglobin | 9.8 g/dl | 11g/dl |
| WBC count | 19400 | 11000 |
| Absolute Eosinophil | 8% | 3% |
| Platelet | 1.68 lakh | 2.72 lakh |
| PCV | 29% | 31% |
| RBC | 3.6 | 4.7 |
| MCV | 81 fl | 86 fl |
| MCHC | 33.6 g/dl | 34.8 g/dl |
| RDW | 17.3 % | 19% |
| Neutrophil | 82% | 79% |
Table 2: Other blood parameters before and after treatment.
| Parameter | Pre-Treatment | Post-Treatment |
| Total bilirubin | 0.23 | 0.22 |
| ALP | 566 | 110 |
| Creatinine | 2.42 | 1.9 |
| Urea | 37 | 42 |
| Na | 133 | 134 |
| K | 4.9 | 4.3 |
| UPCR | 14.99 | 9 |
| Sr Calcium | 6 | 8 |
| Sr ionized calcium | 1.09 | 4mg/dl |
| Corrected calcium | 7.4 | 9 |
| Total protein | 5.7 | 5.8 |
| Albumin | 2.3 | 2.6 |
| Uric acid | 5.9 | 5.5 |
| Urine routine | 2+ proteinuria but no cast | Traces |
| Trop i | 80 | 26 |
| CKMB | 54 | 40 |
The patient was referred to a haemato-oncologist and medical oncologist, who advised bone-marrow aspiration and biopsy, Serum Free-light chain, Beta-2 Micro-globulin levels, Renal Biopsy, and abdominal fat pad biopsy.
 |
Figure 1: Serum-Electrophoresis report |
Bone marrow Aspiration – Biopsy revealed Hyper-Cellular Marrow M: E ratio of 1:1, Micro-normoblasts, erythroid hyperplasia with myeloid and lymphoid series within normal limit. However, eosinophils were slightly elevated to 5 % along with Plasma Cell 7-8%.
Wherein the serum-protein electrophoresis was suggestive of the sharp distortion in the gamma region .
Renal Biopsy with Immuno-Fluorescence revealed 13 Glomeruli which revealed deposits of eosinophilic hyaline material within a mesangium. While few glomeruli showed silver positive and congo negative nodules while SAA by IHC was negative. The immune-Fluorescence for IgG, IgA, IgM, C3, C1q and Kappa were negative. However, glomeruli showed intense lambda positive staining. These findings were consistent with monoclonal immunoglobulin deposition disease.
Table 3: Findings of Renal biopsy under Ligh microscopy (LM) & DIF; and Abdominal fat pad biopsy.
|
Serum |
Free Kappa light chain | 146 (increased) | Ratio of Kappa & Lambda – 1.49 |
| Free Lambda light chain | 97 (Increased) | ||
| Beta 2- Micro-globulin | 13348 ng/ml | ||
| Renal Biopsy under LM and DIF | · Mesangial and subendothelial aggregates of randomly oriented fibrillary structures measuring about 9-12 nm in diameter. (Mean fibril diameter 10.5 nm).
· No evidence of immune complex type electron-dense deposits in GBM or mesangial areas. · The ultra-structure featured in the context of LM and DIF is consistent with Renal amyloid fibril deposition |
||
| Abdominal fat pad biopsy | No amyloid deposition seen | ||
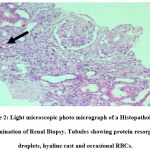 |
Figure 2: Light microscopic photo micrograph of a Histopathological examination of Renal Biopsy. Tubules showing protein resorption droplets, hyaline cast and occasional RBCs. |
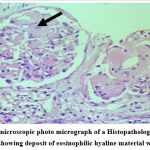 |
Figure 3: Light microscopic photo micrograph of a Histopathological examination of Renal Biopsy showing deposit of eosinophilic hyaline material within mesangium. |
After the availability of all the investigational data, the patient was subjected to an X-ray of Skull, spine and bilateral lower limb. But no lytic bone lesion was found. The data at our disposal divided our differentials into viz; Multiple Myeloma, Mono-clonal gammopathy of undermine significance and Monoclonal immunoglobulin deposition disease with AL Renal Amyloidosis. The data didn’t support the Multiple myeloma as the differential as it did not fulfil the criteria for the same. While MGUS as a differential was also ruled out as the patient had anaemia and renal insufficiency. However, we considered the MIDD as the final diagnosis as the patient had renal insufficiency, cardiomyopathy, heavy proteinuria, elevated serum free light chain and biopsy suggesting few glomeruli showing silver positive and congo red negative nodule with lambda light chain deposition in GBM. While light microscopy revealed aggregates of random fibrillar deposition in the mesangial and subendothelial regions which were consistent with renal amyloidosis. In our case, we could not establish the restrictive cardiomyopathy on cardiac MRI as the patient had already developed renal insufficiency and the use of contrast in cardiac MRI would accelerate the underlying progressive renal insufficiency. However, the 2D echocardiography supports the possibility of restrictive cardiomyopathy in this patient.
The patient was later referred to a nephrologist, onco-physician and haemato-oncologist and was advised Inj. Bortezomib 2 mg in 100 ml NS over 10 minutes and Inj. Dexamethasone 40 mg IV over 30 minutes also commonly known as BD regimen on Day 1-Day 8-Day 15-Day 22 (1st Cycle) followed by maintenance. Currently, the patient is on the bi-weekly regimen of bortezomib and dexamethasone in similar doses and is on the regular follow-up with improvement in the haematological and renal parameters as compared to the baseline. The patient is also on supportive medications like tablet torsemide, aspirin, and rosuvastatin considering the risk of underlying cardiomyopathy and risk of failure.
Discussion
We herein report the case of Monoclonal immunoglobulin deposition disease with lambda light chain deposition and biopsy-proven AL type renal amyloidosis with restrictive cardio-myopathy. MIDD is a rare form of plasma cell dyscrasia, often characterised by aggregates of the deposits of either light chain or heavy chain in a linear fashion along the glomerular basement membrane.1
The incidence of MIDD in the western population is estimated to be 1 per million with the median age group of 56 to 64-year.2,3 Among the renal biopsies conducted above the age of 18 years, only 0.33% were found to be MIDD, substantiating that the incidence of MIDD is quite variable. Often, Multiple myeloma is regarded as a cause of MIDD, while in our case the scenario was contrasting.
Cohen C, Royer B, Javaugue V et al1, in their study substantiated that nephrotic syndrome can be encountered as an initial presentation of MIDD while it only comprises 16 % of the patient who presented with glomerular symptoms but in our case the patient presented with cardiac failure which is an unusual presentation of MIDD.
With detailed investigation, we surmised that the patient is non-diabetic and non-hypertensive, which compelled us to subject the patient for further evaluation of proteinuria. However, RBC in the urine was never encountered during the multiple urine analysis, which in fact is an uncustomary phenomenon if the patient presents with the glomerular symptoms. Following the detailed examination and evaluation by a nephrologist, the biopsy was performed which showed silver positive and congo red negative nodules in the basement membrane while Light microscopy emanated at the conclusion of AL Renal amyloidosis.
With such clinical presentation, with unexplained anaemia, renal insufficiency and cardiomyopathy, where we didn’t confront any underlying comorbidity, the biopsy should be promptly performed for evaluation of monoclonal gammopathy and amyloidosis as we did in our case. While in our case the abdominal fat pad biopsy didn’t substantiate any amyloid deposition.
A study performed by Nasr et al at Mayo clinic which enrolled 64 subjects of MIDD, among which 51 had LCD(light chain disease) while LCD and HCD (heavy chain disease) was encountered in 6 of them each respectively. However, isolated HCD was noted in only 7 subjects. In this study group, the mean age was 56 years, while in our case the age of the patent was 52 years. All the patients were subjected to serum and urine electrophoresis and it was regarded that 67 per cent had Free light chain deposition. Hence, it has culminated that, serum electrophoresis/ immunofixation should be considered in the patients above the age of 50 years old with unexplained renal insufficiency as in our case.1,5
Said S, J Cooper C et al, also reported a case in the year 2014, and in their literature concluded that 80% had hypertension while more than 90 per cent had an underlying renal dysfunction while only 16 per cent required dialysis during the presentation.5,6 While in our case, the patient presented with heart failure and restrictive cardiomyopathy and renal insufficiency.
A dedicated retrospective case series of 20 subjects with MIDD (Biopsy proven) with AL Renal Amyloidosis from France conducted by A Ramonatxo, R Garcia etal,7 concluded that at the time of initial diagnosis of MIDD, 95% i.e.; 19 subjects had a history of hypertension while 3 had atrial fibrillation, 3 had developed grade 3 to 4 NYHA dyspnoea. While in our case, the patient didn’t have hypertension, however, he developed NYHA grade 3 dyspnoea. In the said studied group, 40% had Low/Micro voltage patterns and pseudo-Q waves each and 2D Echocardiography revealed diastolic dysfunction in all the subjects, while 38 % showed global longitudinal strain pattern and just 1 subject had depressed LVEF. While a similar pattern of low voltage ECG was noted in our patient, with additional changes of ST depression in lead I, II, III, aVF was noted and 2D echocardiography substantiated Global L-V Hypokinesia, L-V systolic function Ejection fraction of 50%, moderate concentric LVH, trivial AR, Moderate mitral regurgitation with grade 2 diastolic dysfunction. In the said study, 10 subjects who underwent gadolinium-enhanced cardiac MRI, and none showed cardiac enhancement. However, overall features from ECG and echocardiography substantiated the pattern close with the characteristic feature of amyloidosis of heart cum restrictive pattern. Taking this result into the account, and the risk vs benefit of Gadolinium Enhanced Cardiac MRI in our patient, who already developed renal insufficiency and with the impending risk of gadolinium-induced renal injury, we deferred cardiac MRI.7
A study conducted by Gokden N, Barlogie B et al8 which included 46 subjects concluded that 24 had kappa LCD and 16 had lambda LCD along GBM and/or TBM. The study has also concluded that isolated light microscopy alone can be suspected but isn’t the diagnostic tool for LCD disease. While comparing Immunofluorescence with light microscopy, former is the more sensitive than LM for the absolute diagnosis of LCDD/MIDD. While in our case we performed both investigations and found that there were deposits of eosinophilic hyaline material within a mesangium and a few glomeruli showing silver positive and congo negative nodules. While on immune-Fluorescence IgG, IgA, IgM, C3, C1q and Kappa were negative and glomeruli showed intense lambda positive staining.
With all investigations at our disposal, the patient was referred to Haemato-oncologist, medical-oncologist and nephrologist, and with their expert opinion, the patient was started on Inj Bortezomib 2MG in 100 ml NS over 10 minutes and Inj. Dexamethasone 40 MG IV over 30 minutes regimen on D1-D8-D15-D22 (1st Cycle). Currently, the patient is on the bi-weekly regimen of bortezomib and dexamethasone in similar doses and is on the regular follow-up with improvement in the haematological and renal parameters as compared to the baseline. The patient is also on supportive medications like tablet torsemide, aspirin, and rosuvastatin considering the risk of underlying cardiomyopathy and risk of failure.
A French-based retrospective study conducted by Camille Cohen, Bruno Royer et al 2015, all the 49 study subjects with MIDD were administered Inj Bortezomib, a novel anti-myeloma drug and Inj Dexamethasone. The study concluded that Bortezomib and dexamethasone combination improved the overall renal outcome and haematological response.1
While in 2017 a case reported by Noto, R., Kamiura, N., Ono, Y. et al substantiated that BD regimen is an effective strategy in a case of MIDD.9
Another case series published by Efstathios et al in 2009 also supported the fact that BD regimen should be considered the preferred and initial treatment modality in patients with light chain deposition disease (MIDD).10
Lee H, Duggan P et al also reported a case of monoclonal gammopathy of renal significance who was initiated on a cyclophosphamide-based BD regimen. Contrary to our case where we administered the BD regimen. They also opined that the treatment modality for MIDD/MGRS can be onerous when there is no crystal clear evidence of dysproteinaemia and clonal cells in the bone marrow. While in our case the bone marrow didn’t reveal much information except plasma cells of 7-8% and slight eosinophilia but there was significant proteinuria. While considering the Pros-Cons of Cyclophosphamide in such a patient with significant renal involvement and with the consensus of a Haemato-oncologist and nephrologist, we decided to initiate a patient on BD regimen. The patient currently is on a maintenance dose as mentioned earlier with improved haematological and renal parameters.
Charaf E, Iskandar SB et al, in their case report and literature concluded that the Bortezomib can be used as a treatment modality in a patient with cardiac amyloidosis. As in our case, we couldn’t establish the cardiac amyloidosis and restrictive cardiomyopathy as a biopsy wasn’t performed while taking into account the study published by A Ramonatxo, R Garcia et al7. While 2D echocardiography and electrocardiography findings were consistent with restrictive cardiomyopathy in our patient, we noted the improvement in Echocardiography after 2 months of successful treatment with BD regimen. Currently, the patient is on a Bi-weekly regimen of BD and regular follow up.
Conclusion
MIDD/LCDD is an infrequent and scarce form of disease entity, mainly encountered in middle-aged individuals. The patient may present with any conventional symptoms, like Bilateral lower limb swelling, anuria, hematuria, dyspnea, chest pain etc. Detailed history taking is of paramount importance. Those patients who are non-diabetic and non-hypertensive and who presented with proteinuria must be thoroughly investigated to rule out plasma cell dyscrasia and monoclonal gammopathy. Prompt diagnosis and treatment are cardinal to prevent the progression of the disease to ESRD.
Early diagnosis with swift commencement with bortezomib-based regimens i.e. clone-targeted chemotherapy, is presently the most coherent plan in MIDD.13, 14
Acknowledgement
Authors acknowledge the department of pathology for their support.
Conflict of Interest
Authors have declared that no competing interests exist.
Funding Sources
There is no funding source.
References
- Cohen C, Royer B, Javaugue V, Szalat R, El Karoui K, Caulier A, Knebelmann B, Jaccard A, Chevret S, Touchard G, Fermand JP, Arnulf B, Bridoux F. Bortezomib produces high hematological response rates with prolonged renal survival in monoclonal immunoglobulin deposition disease. Kidney Int. 2015 Nov;88(5):1135-43. doi: 10.1038/ki.2015.201. Epub 2015 Jul 15. PMID: 26176826.
CrossRef - Jean-Paul Fermand, Frank Bridoux, Angela Dispenzieri, Arnaud Jaccard, Robert A. Kyle, Nelson Leung, Giampaolo Merlini; Monoclonal gammopathy of clinical significance: a novel concept with therapeutic implications. Blood 2018; 132 (14): 1478–1485. doi: https://doi.org/10.1182/blood-2018-04-839480
CrossRef - Dysproteinemias and Glomerular Disease Nelson Leung, Maria E. Drosou, Samih H. Nasr CJASN Jan 2018, 13 (1) 128-139; DOI: 10.2215/CJN.00560117
CrossRef - Ronco P, Plaisier E, Mougenot B, Aucouturier P. Immunoglobulin light (heavy)-chain deposition disease: from molecular medicine to pathophysiology-driven therapy. Clin J Am Soc Nephrol. 2006 Nov;1(6):1342-50. doi: 10.2215/CJN.01730506. Epub 2006 Oct 11. PMID: 17699367.
CrossRef - Nasr SH, Valeri AM, Cornell LD et al. Renal monoclonal immunoglobulin deposition disease: a report of 64 patients from a single institution. Clin J Am Soc Nephrol 2012; 7: 231–239.
CrossRef - Said S, J Cooper C, C Nwosu A, E Bilbao J, T Hernandez G. Hypertension, renal failure, and edema in a 38-year-old man: light chain deposition disease; a case report and review of the literature. J Nephropathol. 2014;3(2):63-8. doi: 10.12860/jnp.2014.14. Epub 2014 Apr 1. PMID: 24772399; PMCID: PMC3999586.
- A Ramonatxo, R Garcia, F Joly, B Degand, N Bidegain, C Bouleti, L Christiaens, S Levesque, E Desport, F Bridoux, Randall-type monoclonal immunoglobulin deposition disease: description of cardiac involvement,European Heart Journal, Volume 41, Issue Supplement_2, November 2020, ehaa946.2140, https://doi.org/10.1093/ehjci/ehaa946.2140
CrossRef - Gokden N, Barlogie B, Liapis H. Morphologic heterogeneity of renal light-chain deposition disease. Ultrastruct Pathol. 2008 Jan-Feb;32(1):17-24. doi: 10.1080/01913120701854002. PMID: 18300034.
CrossRef - Noto, R., Kamiura, N., Ono, Y. et al.Successful treatment with bortezomib and dexamethasone for proliferative glomerulonephritis with monoclonal IgG deposits in multiple myeloma: a case report. BMC Nephrol 18, 127 (2017). https://doi.org/10.1186/s12882-017-0524-7.
CrossRef - Efstathios Kastritis, Magdalini Migkou, Maria Gavriatopoulou, Panos Zirogiannis, Valsamakis Hadjikonstantinou, Meletios A. Dimopoulos. Treatment of light chain deposition disease with bortezomib and dexamethasone. Haematologica 2009;94(2):300-302; https://doi.org/10.3324/haematol.13548.
CrossRef - Lee, H., Duggan, P., Neri, E. P., Tay, J. and Zepeda, V. J. (2019) “Bortezomib maintenance for the treatment of Monoclonal Gammopathy of Renal Significance”,Mediterranean Journal of Hematology and Infectious Diseases, 11(1), p. e2019007. doi: 10.4084/mjhid.2019.007.
CrossRef - Charaf E, Iskandar SB, Blevins A, Abi-Saleh B, Fahrig S. Cardiac amyloidosis responding to bortezomib: case report and review of literature.Curr Cardiol Rev. 2009;5(3):228-236. doi:10.2174/157340309788970360.
CrossRef - Cohen C, Joly F, Sibille A, Javaugue V, Desport E, Goujon J-M, et al. Randall-Type Monoclonal Immunoglobulin Deposition Disease: New Insights into the Pathogenesis, Diagnosis and Management. Diagnostics 2021;11:420. https://doi.org/10.3390/diagnostics11030420
CrossRef - Nelson Leung, Maria E. Drosouand Samih H. Nasr. Dysproteinemias and Glomerular Disease CJASN January 2018, 13 (1) 128-139; DOI: https://doi.org/10.2215/CJN.00560117
CrossRef








