Department of Otorhinolaryngology Head and Neck Surgery, Faculty of Medicine Udayana University/ Sanglah General Hospital, Denpasar
Corresponding Author E-mail: wulan_tht@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2443
Abstract
Nasal irrigation with Dewandaru leaf extract can reduce inflammatory cell infiltration in the nasal mucosa of rats, and is effective in reducing RA symptoms and has an effect no different from topical corticosteroids. This study aims to prove whether nasal irrigation with ethanolic extract of Dewandaru leaves can reduce the infiltration of allergic inflammatory mediators in the nasal mucosa of male white rats of Wistar strain suffering from RA after ovalbumin nasal spray was induced, by assessing IL-4 levels, TSLP expression and H4 receptor expression. The study was conducted with a randomized post test only control group design, using an animal model of allergic rhinitis, involving 34 male white rats of the Wistar strain, divided into 2, namely: 17 treatment groups and 17 control groups. After 4 weeks of exposure, termination and sampling of rat nasal mucosa was performed. Statistical analysis using the independent sample T test showed that the levels of IL-4 in the nasal mucosa of rats and the expression of H4 receptors on the nasal mucosa of rats were significantly lower than in the control group (p<0.05). Meanwhile, the TSLP expression of the nasal mucosa of rats in the treatment group (2.0154) was not significantly different from the control group (2.1891) with p=0.478, possibly associated with less severe damage to the epithelial barrier cells of the nasal mucosa of TSLP-producing in the group control. The ethanolic extract of Dewandaru leaves has the ability to suppress IL-4 cytokine levels through inhibition of the activity of RH4, so it can be used as a supporting therapy for nasal irrigation that can suppress allergic inflammatory mediators in the nasal mucosa of rats.
Keywords
Allergic Rhinitis; Dewandaru Extract; Nasal Irrigation; IL-4; RH4; TSLP
Download this article as:| Copy the following to cite this article: Sutanegara S. W. D. The Effect of Nasal Irrigation with Dewandaru (Eugenia Uniflora L) Leaves Ethanol Extract on Il-4 Levels, Thymic Stromal Lymphopoiethin (TSLP) Expression and Expression of H4 Mucosa Receptors of Wistar Rats with Allergic Rhinitis. Biomed Pharmacol J 2022;15(2). |
| Copy the following to cite this URL: Sutanegara S. W. D. The Effect of Nasal Irrigation with Dewandaru (Eugenia Uniflora L) Leaves Ethanol Extract on Il-4 Levels, Thymic Stromal Lymphopoiethin (TSLP) Expression and Expression of H4 Mucosa Receptors of Wistar Rats with Allergic Rhinitis. Biomed Pharmacol J 2022;15(2). Available from: https://bit.ly/3NS7iCE |
Introduction
Dewandaru leaves (Eugenia uniflora L.) contain flavonoids, saponins, myricetin, quercetin, kaempferol, and tannins.1,2 H4 receptors can interact with quercetin.3 Reynertson et al. found that of the 17 species Eugenia studied, and made extracts of Dewandaru leaves contained myricetin 100%, quercetin 71%, and kaempferol 24%.4
Administration of intranasal irrigation with a rough extract of Dewandaru leaves in male white rats Wistar strain induced into allergic rhinitis had lower inflammatory cell infiltration compared to the allergic rhinitis-induced group without being given intranasal irrigation. The use of intranasal irrigation will reduce the systemic effects of the medication.5
The rough extract of 100mg/kgBB doses or Dewandaru leaves or 200mg/kgBB doses are effective in lowering symptoms of allergic rhinitis and has effects not dissing from topical corticosteroids.6 Based on the above explanation, the author wants to know the effect of nasal irrigation with ethanol extract of Dewandaru leaves (Eugenia uniflora L) towards IL-4 levels, TSLP expression as well as H4 receptor expression in the nasal mucosa of Wistar strain male white rats suffering from AR after previously being induced with ovalbumin nasal spray.
Material and Methods
This study was a purely experimental study aimed at proving the impact of the ethanolic extract of Dewandaru leaves as nasal irrigation fluid to reduce IL-4 levels and TSLP expression and to reduce H4 receptor expression in the nasal mucosa of Wistar strain white male rats. This research was conducted with the design randomize post-test only control group design.
The determination of the sample was done randomly simply in the following way:
From the population of Wistar strain white male rats the samples selected were those that meet the inclusion criteria.
From samples that have met the inclusion criteria taken randomly as many as 17 samples of Wistar strain white male rats.
The 17 samples selected were then divided into two groups.
Wistar strain white male rats with a weight of 300 grams adapted to the cage environment for 1 week, then grouped into 2 groups. Group I (AR/control), group II (AR irrigated with ethanol extract of Dewandaru leaves dose of 20 mg per 100ml NaCl 0.9%).
Prior to treatment, the Wistar strain white male rats were sensitized with an ovalbumin preparation of 100μg/0.1mL each rat intraperitoneally, then from day 14 induced with 25μl ovalbumin 2% in saline 0.9% (total 50μl for both nostrils) once in a day intranasally using micropipettes for 14 days starting on days 15 to 30.
An assessment of the clinical state of the nasal condition of the rats was conducted at the beginning of day 14 using a nasal score conducted by 2 observers. After being adapted for 10 minutes, then
the rats were observed for symptoms on the nasal such as sneezing, itching, and runny nose for 10 minutes. The score is rated 0-3 points. AR scores were recorded on days 15, 16, 18, 20, 22, 25, and 30. AR in a rat is said to occur well when the score reaches 5 points.
At the end of the study (day 30), surgery was performed with a transpalatal approach to take a sample of the nasal mucosa. Previously animals testing to be given anesthesia xylazine (5mg / KgBB, im) and ketamine (200mg / KgBB, im). Anesthesia is intended to provide comfort and no pain in animals testing when euthanized, by first praying that good karma of experimental animal get a decent reward from God. After the animals testing was terminated, the surgical field was cleaned, followed by an incision starting in the right buccal area until it reached the left buccal area. Incision deepened, tracing the base of the cavum nasal towards the posterior to the os vomer, the cutting of the os vomer and the cavum of the right and left rice is lifted completely. Cavum nasal is cut into two parts in the septum area for ELISA and IHK examination. The rest of the unused organs will be destroyed by being buried in the ground.
Examination of IL-4 levels uses nasal mucosal tissue and is performed by ELISA method. After dissection, the sample is stored in a cooling room with a temperature of minus 600C in the Pharmacology and Therapy section of FK UNUD until all sample counts are met. The tissue sample is then extracted and mixed with a phosphate buffer (Phosphate-buffered saline/ PBS) at a ratio of 30% tissue to 70% PBS fluid, the mixture is then centrifuged at a speed of 5000 rpm for 5 minutes to obtain serum as an inspection material using rat IL-4 Fine Test ELISA kit. This examination is used to determine serum (quantitative) ANP levels at invitro. The principle of this ELISA Kit is sandwich-ELISA. Microplate ELISA coated by capture antibody and biotin-conjugated antibody is used as a detection antibody. Standard, test sample and biotin-conjugated antibody are added to well, are further washed with a washing buffer. Horseradish peroxidase-streptavidin is added and unbound conjugates are washed with a washing buffer. Substrate 3,3′,5,5′-Tetramethylbenzidine used to visualize enzymatic reactions Horseradish peroxidase-streptavidin is catalyzed by Horseradish peroxidase-streptavidin to produce a blue color that turns yellow after adding acidic stop solution. The detected yellow density is proportional to the number of target samples captured. The concentration of IL-4 in the sample is then determined by comparing the optical density/OD in the sample with the standard curve.
Immunohistochemistry examination of TSLP expression and R-H4 expression using nasal mucosal tissue with painting Hematoxylin Eosin and examined tissue histopathologically in the Integrated Animal Laboratory FK UNUD.
Embedding. After dissection, the sample is fixated in solution formaldehyde 10% for 1-7 days. It is further soaked in 70% ethanol for at least 24 hours followed by 80% ethanol for 2 hours. It is being soaked in 90% and 95% ethanol respectively for 30 minutes, respectively. After that soak 3 times in absolute ethanol for 30 minutes each in a different bottle. Soaked in xylol twice each for 30 minutes. The next process is carried out in an incubator with a temperature of 56 – 580C soaked in xylol, paraffin 3 times then continued with embedding by dipping nasal mucous tissue in liquid paraffin that has been poured into the container. After a while, paraffin will solidify and nasal mucosal tissue is in the paraffin block.
Object glass coating. Object glasses are marked with misery and soaked in 70% alcohol for a minimum overnight. It is drained and avoided dust. Dipped for 30 seconds in 0.05% warm gelatin dissolved in aquabidest. 0.5% gelatin as much as 100ml is used for 100 glasses of objects. It is dried in an enclosed space. Object glassescan be used in 2 days.
Manufacture of Nasal Mucous Tissue Preparation. The nasal mucosal tissue on the paraffin block as the result of embedding is inserted into the block holder microtome and set the alignment of the surface, cut with a microtome knife and placed it horizontally. Cutting begins by setting the thickness of the slice above 10 um. A good cut will result in a ribbon-like cut shape. Slices are taken with tweezers and put in water (room temperature) to open the folds that may occur in the preparation. The slices are moved with a stemmed needle into warm water (38-400C) to straighten the fine wrinkles. The perfectly outstretched slices are taken with an object-glass. Selected pieces are dried and placed on a hotplate (38-400C) until dry. Next, the preparation is stored in incubator temperature 38 – 400C for 24 hours.
TSLP expression and R-H4 expression. Preparations are dyed in xylol twice, graded alcohol (100%, 90%, 80%, 70%, 30%) and aquadest respectively. It is washed in a 7.4 pH PBS for 3×5 minutes, then soaked in 3% hydrogen peroxide / H2O2 (in DI water) for 5–10 minutes. Washed in a 7.4 pH PBS for 3×5 minutes. Soaked in 3% H202 (in DI water) for 5-10 minutes. Washed in 1% PBS pH 7.4 for 30 minutes. Soaked in 1% PBS for 5-10 minutes. Soaked in 1% BSA in PBS for 10-30 minutes at room temperature. Washed in a 7.4 pH PBS for 3×5 minutes. Added antibody primary Anti-Mouse Polyclonal RH-4 or TSLP overnight at 40C. Washed in a 7.4 pH PBS for 3×5 minutes. Added secondary antibody universal anti-mouse polymer HRP for 1 hour at room temperature. Washed in a 7.4 pH PBS for 3×5 minutes. Added Chromogen DAB (3.3 diaminobenzidine tetrahydrochloride) for 3×5 minutes. Performed counterstain with hematoxylin for 5 minutes at room temperature. Washed in the aquadest for 3×5 minutes. Performed mounting with entellen. The observation was done using a microscope at 100x magnification.
Statistical analysis of data obtained using the statistics program SPSS for Windows version 24.0
Result and Discussion
This experimental study involved 34 samples of Wistar strain male white rats with allergic rhinitis, divided into two groups, with each control group of 17 rats and a treatment group of 17 rats. All rats as samples were obtained from FK UNUD Integrated Biomedical Laboratory, and the study was conducted by applying the principle of 3R (replacement, reduction, refinement). This research process was carried out based on the certificate of Ethical Clearance issued by the Ethical Clearance Commission FK UNUD/RSUP Sanglah No. 62/UN14.2.2.VII.14/LT/2021 with no.protokol 2020.03.1.1183. Before being treated, Wistar strain male white rats, weight around 300 grams adapted in a cage environment for 1 week. During the adaptation process rats are fed rat and drinking water refill ad libitum and treated in accordance with the principle of 5F (Freedom), namely: free from thirst and hunger, free from pain, free from fear, free from discomfort, and free to express their natural behavior.
After the adaptation process, sensitization with an ovalbumin preparation of 100μg / 0.1mL per mouse intraperitoneally for 14 days was done, ranging from day 1 to day 14 as many as 7 injections. Then the rats were grouped into 2 groups, Group K (Control) of AR rats induced with ovalbumin 25μl ovalbumin intranasal once a day, group P (Treatment) of AR rats induced with ovalbumin 25μl ovalbumin intranasal once a day continued nasal irrigation with ethanol extract of Dewandaru leaves 20 mg /100ml NaCl 0.9% once a day, starting on the 15th to 30th day. An assessment of the clinical state of the nasal condition of the rats was conducted at the beginning of day 14 using a nasal score conducted by 2 observers. After being adapted for 10 minutes, then the rats’ symptoms on the nose such as sneezing, itching, and runny nose were observed for 10 minutes. The score is rated 0-3 points. AR scores were recorded on days 15, 16, 18, 20, 22, 25, and 30.
From the results of observations in both group K and group P, on the 15th day obtained a nasal score of more than 5. But in group P there was a decrease in nasal scores starting on day 18 in contrast to group K which still showed nasal scores above 5 until day 30. On the 30th day, nasal mucosa was taken in all animals testing after first injecting analgesia xylazine (5mg / KgBB) and ketamine (200mg / KgBB) intramuscularly.
This study is a purely experimental study aimed at proving the effect of 20mg/100ml NaCl 20mg/100ml NaCl ethanol extract as nasal irrigation fluids that can decrease IL-4 levels, decrease the expression of Thymic Stromal Lymphopoietin (TSLP) and decrease the expression of H4 receptors nasal mucosa of Wistar strain male white rats. This research was conducted using the randomize post-test only control group design.
The normality test in this study used the Kolmogorov Smirnov Test, which is an effective and valid normality testing method used for small samples. In this study, the study subjects numbered 17 rats for each group so normality testing using Kolmogorov Smirnov was perfect for the study.
Based on the data normality test using Kolmogoro Smirnov at a 95% confidence interval (=0.05), it showed normally distributed data, so it continued with parametric statistical tests, using the test independent sample T-test to determine the difference in IL-4 levels, TSLP expression, H4 receptor expression between groups that are not paired and have no association, as well as the number of samples of < 30 tails. As for the results obtained:
Levels of IL-4 in nasal mucosa of Wistar strain male white rats suffering from allergic rhinitis after induced ovalbumin nasal spray
Based on the results of the independent sample T-test, this study showed levels of IL-4 in nasal mucosa of Wistar strain male white rat who suffered from AR induced ovalbumin nasal spray, after nasal irrigation with ethanol extract of Dewandaru leaves (Eugenia uniflora L) 20mg/100ml NaCl 0.9% significantly lower (124.4588 + 34.35153 μg/300mg) compared to control (193.0518 + 80.29365 μg/300mg) with p<0.05
Table 1: Results of T-test levels of IL-4 in nasal mucosa of rat.
| Group | N | Mean
(µg/300mg) |
p |
| Control | 17 | 193.0518 + 80.29365 | 0.003* |
| Treatment | 17 | 124.4588 + 34.35153 |
*Significant with p < 0.05
Expression of TSLP in nasal mucosa of Wistar strain male white rat suffering from allergic rhinitis after induced ovalbumin nasal spray
In this study, TSLP expression was obtained in the nasal mucosa of Wistar strain white male rats who suffered from AR induced ovalbumin nasal spray, after nasal irrigation with ethanol extract of Dewandaru leaves (Eugenia uniflora L) 20mg/100ml NaCl 0.9% in the treatment group (2.0154 + .72816) lower than the controls (2.1891+ .68123). However, after statistical analysis using the test independent sample T-test showed insignificant results where p = 0.478.
Table 2: T-test results of TSLP expression of rat’s nasal mucosa.
| Group | N | Mean
(*per-absorbent) |
p |
| Control | 17 | 2.1891 + .68123 | 0.478* |
| Treatment | 17 | 2.0154 + .72816 |
*Insignificant with p>0.05
Expression of H4 receptor in nasal mucosa of Wistar strain white male rat suffering from allergic rhinitis after induced ovalbumin nasal spray
After analyzing the data using an independent sample T-test, the result showed the expression of H4R in the nasal mucosa of Wistar strain white male rat who suffered from AR induced ovalbumin nasal spray, after nasal irrigation with ethanol extract of Dewandaru leaves (Eugenia uniflora L) 20mg/100ml NaCl 0.9% (1.8333 + .57751) significantly with a p = 0.033 lower than control (2.3078 + .65873) with a confidence interval of 95% and the level of meaning p < 0.05.
Table 3: T-test results of H4R expression in rat’s nasal mucosa.
| Group | N | Mean
(*per-absorbent) |
p |
| Control | 17 | 2.3078 + .65873 | 0.033 |
| Treatment | 17 | 1.8333 + .57751 |
*Significant with p<0.05
Results of T-test of IL-4 levels in nasal mucosa of research sample
In this study it was obtained levels of IL-4 in nasal mucosa of Wistar strain male white rats who suffered from AR induced ovalbumin nasal spray, after nasal irrigation with ethanol extract of Dewandaru leaves (Eugenia uniflora L) 20mg/100ml NaCl 0.9% (124.4588 + 34.35153 μg/300mg) is significantly lower (p = 0.003) lower than control (193.0518 + 80.29365 μg/300mg) (p < 0.05).
Several similar studies have had an inhibition effect on STAT6 activation that correlates well with the inhibition of the cell’s response to the cytokine IL-4. Research has found that quercetin and kaempferol effectively suppressed the development of IgE-related allergic inflammation in rats’ intestinal cell models. Flavonols are also known to suppress the activation of extra signal protein kinase regulation in IgE-specific OVA and in chemokine release. Flavonols also have an effect on IL-4, IL-13, and CD40 ligand expression by basophils.7,8,9
Velickovic’s research, et al. about the antiallergic effects on flavones such as luteolin and apigenin are known to suppress CD40 ligand expression by basophils. Flavones significantly decrease the number of cells on the surface and expression of the protein γc, where it is known that γc may be the target molecule at the inhibition by flavones on the IL-4 signal.7
Chronic inflammation in asthma is one of the factors affecting the severity, exacerbation, and remodeling of the airways characterized by increased apoptosis of the epithelium of the bronchioles. Interleukin (IL)-4 is one of the type Th2 cytokines that are a sign of inflammatory processes. Runiawan, et al. examined the relationship between IL-4 levels and the incidence of apoptosis of bronchioles and asthmatic bronchioles and proved that increased levels of IL_4 affect the increased apoptosis of bronchioles.10
Results of TSLP expression in the nasal mucosa of the research sample
In the study, it was obtained the expression of TSLP in nasal mucosa of Wistar strain male white rats who suffered from AR induced ovalbumin nasal spray, after being irrigated nasal with ethanol extract of Dewandaru leaves (Eugenia uniflora L) 20mg/100ml NaCl 0.9% in the sample lower than control. But from the results of statistical analysis obtained insignificant data (p > 0.05).
The role of TSLP in the pathogenesis of allergic rhinitis has not been extensively studied. TSLP is an interleukin (IL)-7-like cytokine that triggers dendritic cells and mast cells to induce an inflammatory response to Th2. The expression of TSLP is primarily performed by epithelial cells (ECs) and Epidermal Keratinocytes (KCs). In several recent human studies, TSLP has been linked to asthma pathogenesis, allergic rhinitis, nasal polyps, atopic dermatitis, and eosinophilic esophagitis. Invitro studies and murine studies have been conducted with small samples of TSLP expression in allergic rhinitis. There are currently no genetic association studies of TSLP and allergic rhinitis.
According to Toshiro Takai, TSLP is produced in response to environmental and endogenous factors. TSLP is expressed primarily by epithelial cells and keratinocyte cells in the epidermal barrier, epithelium, submucosal skin, airways, and eyes. From the results of nasal mucosal histopathology of rats in this study, the condition of mucocutaneous junction in the control group was still in good condition after 14 days of study. The level of mucosa damage that occurs is still minimal, so the possibility of this causing TSLP production is not too much. (As seen in Figure 1).
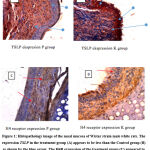 |
Figure 1: Histopathology image of the nasal mucosa of Wistar strain male white rats. The expression TSLP in the treatment group. |
Activation of identifying receptors in airway epithelial cells will trigger the release of various cytokines, chemokines, antimicrobial peptides, lipid mediators, nitric oxide, and reactive oxygen species. These inflammatory mediators will result in various consequences including the withdrawal of leukocytes to the airway, regulation of airway tone and secretion, as well as induction of antimicrobial and antiviral activity. The release of epithelial cytokines especially IL-25, IL-33, and thymic stromal lymphopoietin (TSLP) is a key process that initiates the type 2 immune response and allergic inflammatory environment in asthma. IL-25, IL-33, and TSLP produced by epithelial cells will specifically induce inflammatory cell influenza as well as dendritic cell (DC) activation and mobilization. Dendritic cells are specific immune cells that use the MHC class 2 system to mediate the response of helper T cells to foreign proteins such as aeroallergens. Dendritic cells are also necessary for the differentiation of naïve T cells into T helpers including Th2 cells. Immature dendritic cells which are affected by epithelial cell signals will move from the bone marrow to the airway. Once in the mucosa of the airway, dendritic cells will be located between epithelial cells, forming close bonds and maintaining the integrity of the epithelial barrier. Epithelial cytokines especially TSLP will cause DC mobilization to the local KGB, where DC will activate naïve CD4+ T cells into IL-4-competent states. These IL-4-competent T cells will migrate from the KGB to zone B-cell, where they differentiate into follicular helper (TFH) T cells, which will then go into circulation and complete maturation as Th2 cells.
Results of T-test expression of H4R in nasal mucosa of research sample
The results showed H4R expression in the nasal mucosa of Wistar strain male white rats suffering from AR induced ovalbumin nasal spray, after nasal irrigation with ethanol extract of Dewandaru leaves (Eugenia uniflora L) 20mg/100ml NaCl 0.9% significantly lower than control (p < 0.05).
Histamine is an important mediator in inflammation due to allergies released in the respiratory tract, but treatment with antihistamines today has not shown effective results in controlling allergy symptoms. Thus, the discovery of histamine receptor H4 triggered the re-emergence of new research in the field of allergic response in the airways.
H4 (H4R) receptors are expressed by immunologically relevant tissues, such as the spleen, thymus, mast cells, and leukocytes, such as eosinophils. H4R is mainly expressed in eosinophils, T cells, dendritic cells, basophils, and mast cells. It is a type of cell that is closely involved with the development and preservation of allergic responses. These receptors have been shown to mediate mast cells, eosinophils, and dendritic cell chemotaxis and can modulate cytokine production of dendritic cells and T cells.
H4R in low concentrations is able to induce eosinophil chemotaxis. Histamine quickly induces shape changes in eosinophils and increases the response to chemokines, this effect can be blocked by H4 receptor antagonists but not by other histamine receptor antagonists, additional RH4 agonists induce shape changes in eosinophils with histamine-like properties.11
Similar research was conducted by Moelyono, et al. in 2011 on the levels of quercetin in Jawer Kotok leaf extract and examined its interaction with histamine receptor H4 to determine its anti-inflammatory activity. The results showed the phytochemical content of Jawer Kotok leaf extract, quercetin, are able to interact with H4R through the formation of hydrogen bonds with Lys158 (2,006 Å) and Glu182 (2,048 Å), and van der Waals interactions with Trp90, Leu91, Asp94, Tyr95, Phe168, Thr178, Ser179, Tyr319, Phe344, and Tyr340. Therefore, quercetin has the opportunity to be used as an antagonist of the histamine receptor H4.3
Invitro research conducted by Paul, et al., as published in Journal of Immunology, 2006, reported on studies using models of allergic rats that experienced H4R deficiency in therapy with specific H4R antagonists showing suppression of H4R activity in dendritic cells resulting in decreased chemokines and cytokine production, among others IL-4, as well as inhibiting its ability to activate the response of Th2 cells. This suggests that H4R may trigger an allergic response in the airway through the T cell activation pathway. This study underscores the importance of histamine’s role, particularly H4R, in the immune system, providing evidence for the usefulness of H4R antagonists in the treatment of allergic diseases.
Histamine, a chemical mediator, plays an important role in the appearance of rhinitis symptoms in the nose, but the location of histamine receptors in the mucosa of the human nose is unknown. Naonubu et al, performed a nasal mucosa immunohistochemistry examination to find out the location of the histamine receptor subtype (H1R, H2R, H3R & H4R). His research shows that in the mucosa of the human nose, H1R is localized primarily in the epithelium, blood vessels, and nerves, H2R is localized primarily in the epithelium and glands, as well as H3R and H4R localized primarily in the nasal concha-inferior nerve endings.
An in vivo study, using histamine receptor antagonist H4, JNJ7777120, in an allergic rat model. Observations were done to symptoms of allergic rhinitis (sneezing & scratching), total serum measurements of Ig E, and cytokine levels (IL-4) in rats’ nasal fluids. The results showed at certain doses it was shown to lower symptoms of allergic rhinitis, decreased serum total Ig E and decreased levels of IL-4 nasal fluids of rats. Further mentioned that the study showed a link of H4R in the incidence of allergic rhinitis and its role in allergic rhinitis pathogens. In conclusion, it is said that histamine receptor antagonist H4 can be used as an allergy treatment that also has an effect as an immunomodulator.12
Conclusion
The ethanolic extract of Dewandaru leaves has the ability to suppress IL-4 cytokine levels through inhibition of the activity of RH4, so it can be used as a supporting therapy for nasal irrigation that can suppress allergic inflammatory mediators in the nasal mucosa of rats.
Acknowledgment
The authors would like to thank ORL-HNS Department of Sanglah General Hospital for the support.
Conflict of Interest
There is no conflict of interest.
Funding Sources
There is no funding source.
References
- Suhendi, A., Sjahid, L.R., Hanwar, D. 2011. Isolasi dan Identifikasi Flavonoid dari Daun Dewandaru (Eugenia uniflora L.). Pharmacon, 12:2:73-81.
CrossRef - Daniel, G. and Krishnakumari, S. 2015. Screening of Eugenia uniflora (L.) Leaves in Various Solvents for Qualitative Phytochemical constituents. Int J Pharm Bio Sci, 6(1):1008-1015.
- Moelyono Moektiwardoyo, dkk, 2011. Penentuan kuersetin dari ekstrak metanol daun jawer katok dan studi in siliconya pada reseptor histamin H4. Dalam Majalah Farmasi Indonesia, vol. 22(3), hal. 191– 196.
- Reynertson, K.A., Basile, M.J., and Kennelly, E.J. 2005.Antioxidant Potential of Seven Myrtaceous Fruits. Ethnobotany Research & Applications, 3:025-035.
CrossRef - Anggraini, Sutanegara, S.W.D., Saputra, K.A.D. 2019. Pengaruh cuci hidung dengan daun dewandaru (Eugenia uniflora L) terhadap infiltrasi sel inflamasi pada mukosa hidung tikus wistar yang menderita rinitis alergi. Intisari Sains Medis 10(3): 772-776. DOI: 10.15562/ ism.v10i3.452
CrossRef - Fredlina, U.D.F., Sutanegara, S.W.D., Astawa, I.N.M, 2018. Efektivitas Ekstrak Kasar Daun Dewandaru (Eugenia Uniflora L.) Terhadap Kadar Imunoglobulin E Pada Tikus Wistar Yang Menderita Rinitis Alergi. Thesis. FK Udayana, Denpasar
- Velickovic, T.C., Gavrovic-Jankulovic, M. 2014. Phytochemical and Hypersensitivity Disorders. Food Allergens, Food Microbiology and Food Safety. New York: Springer. 155-173.
CrossRef - Tanaka, T., Higa, S., Hirano,T., Kotani, M., Matsumoto, M., Fujita, A., and Kawase, I. 2003. Flavonoids as potential anti-allergic substances. Curr. Med. Chem.- Anti-Inflammatory & Anti-Allergy Agents, 2:57-65.
CrossRef - Tanaka, T., Hirano, T., Higa, S., Arimitsu, J., Kawai, M. 2006. Allergic Disease and Flavonoids. Foods Food Ingredients J. Japan, 211(71)
- Runiawan Condro S, HMS Chandra Kusuma, Teguh Wahju S. 2010. Peran Ekspresi Interleukin (IL)-4 dalam Apoptosis Epitel Bronkiolus Mencit Asma. Jurnal Kedokteran Brawijaya, Vol. 26, No. 2.
CrossRef - Iwan J.P. de Esch, Robin L. Thurmond, Aldo Jongejan and Rob Leurs. 2005. The histamine H4 receptor as a new therapeutic target for inflammation, TRENDS in Pharmacological Sciences Vol.26 No.9 September.
- Yuji Takahashi a, Yoto Kagawa a, Kana Izawa a, Rie Ono a, Masaaki Akagi b, Chiaki Kamei , 2009, Effect of histamine H4 receptor antagonist on allergic rhinitis in mice. International Immunopharmacology, Elsevier. 734-738
CrossRef









