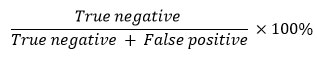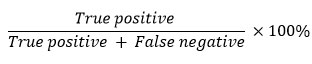Burhan Ma’arif1 , Fariza Amanatul Sholihah1, Anisah Mahardiani1
, Fariza Amanatul Sholihah1, Anisah Mahardiani1 , Begum Fauziyah1
, Begum Fauziyah1 , Denis Mery Mirza2 and Mangestuti Agil3*
, Denis Mery Mirza2 and Mangestuti Agil3*
1Department of Pharmacy, Faculty of Medical and Health Science, Maulana Malik Ibrahim State Islamic University, Malang-65144, Indonesia
2Department of Pharmacy, Faculty of Medicine, Islamic University of Malang, Malang-65144, Indonesia
3Department of Pharmaceutical Science, Faculty of Pharmacy, Universitas Airlangga, Surabaya-60115, Indonesia
Corresponding Author E-mail: mmangestuti@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2439
Abstract
Estrogen deficiency can contribute to osteoporosis in postmenopausal women. Phytoestrogens are becoming more widely recognized as potential estrogen replacement therapy. The administration of phytoestrogens can cause bone formation, which is marked by an increase in Runx2 expression in osteoblast cells and can be seen using western blot and immunohistochemistry approaches. This review aimed to compare the detection methods of Runx2 in phytoestrogen-induced bone tissue using western blots and immunohistochemistry. Selectivity, sensitivity, processing time, and cost-effectiveness were the parameters that were compared. This review was done by identifying articles in several databases (Google Scholar, PubMed, and Science Direct). The process of selecting the articles used the PRISMA guidelines to create a flowchart with inclusion and exclusion study criteria. Meta-synthesis was done to analyze, identify, and interpret all of the data in the articles systematically. 70 articles in total were obtained from the selection process, with 21 articles being relevant to the topic. The result shows that the selectivity and sensitivity of western blot for detecting Runx2 on tissue were 93.5–100%, respectively, whereas immunohistochemistry selectivity and sensitivity were 45–99.5%, respectively. Compared to immunohistochemistry, western blot can save up to 57.26%. Immunohistochemistry takes 46 hours to process, while Western blot takes 25 hours and 20 minutes. In comparison to immunohistochemistry, the western blot is more selective, sensitive, rapid and affordable for detecting Runx2 in bone tissue.
Keywords
Immunohistochemistry; Phytoestrogens; Runx2; Western Blot
Download this article as:| Copy the following to cite this article: Ma’arif B, Sholihah F. A, Mahardiani A, Fauziyah B, Mirza D. M, Agil M. Runt-Related Transcription Factor 2 (Runx2) Measurement in Phytoestrogen-Induced Bone: A Comparison of Western Blot and Immunohistochemistry Methods. Biomed Pharmacol J 2022;15(2). |
| Copy the following to cite this URL: Ma’arif B, Sholihah F. A, Mahardiani A, Fauziyah B, Mirza D. M, Agil M. Runt-Related Transcription Factor 2 (Runx2) Measurement in Phytoestrogen-Induced Bone: A Comparison of Western Blot and Immunohistochemistry Methods. Biomed Pharmacol J 2022;15(2). Available from: https://bit.ly/3tStd4E |
Introduction
Estrogen deficiency or decreased estrogen production in postmenopausal women is common, and it can occur spontaneously as women get older1. Estrogen deficiency can enhance the activity of osteoclasts, resulting in increased bone resorption, as well as blocking the development of osteoblast precursors, preventing bone growth. Osteoporosis can be caused by an imbalance in the bone remodeling process2, 3. Osteoporosis is defined by a reduction in bone mineral density (BMD) and damage to the microarchitecture of bone tissue, both of which increase the risk of fracture4, 5.
According to statistics from the International Osteoporosis Foundation, more than 200 million people worldwide suffered from osteoporosis in 20166. According to Amelia’s research7, the prevalence of osteoporosis in Indonesia is 18-36% in women and 20-27% in males under the age of 70. Women account for 53.6% of those above the age of 70, while males account for 38%. Furthermore, one in every four Indonesian women between the ages of 50 and 80 is at danger of getting osteoporosis. In Indonesia, women are four times more likely than males to develop osteoporosis. The prevalence of osteoporosis in women increases with age, according to these findings. It’s possible that this is linked to estrogen deficiency in postmenopausal women8.
Hormone replacement therapy (HRT) can be used to replace estrogen loss in postmenopausal women. Long-term use of HRT, on the other hand, has the potential to be carcinogenic (an increase in endometrial cancer and breast cancer), embolic, and even cause stroke, all of which can result in mortality9-11. The results of research into the use of natural components to replace the function of estrogen with minimal negative effects have been published, and the findings will lead to the usage of phytoestrogens12.
Phytoestrogens are described as compounds with a structure similar to 17-estradiol or compounds that have a high affinity for the estrogen receptor and can have a therapeutic effect similar to estrogen13-16. Phytoestrogens have been shown to increase osteoblastic activity while inhibiting the development of osteoclasts17. The phytoestrogens in the n-hexane extract and the fraction resulting from the separation of Marsilea crenata leaves could promote the differentiation of MC3T3-E1 osteoblast cells, according to Ma’arif et al18. Daidzein, a phytoestrogen, can boost OCT1 osteoblast cell proliferation and differentiation19. The phytoestrogen puerarin can promote osteogenesis in MC3T3-E1 osteoblast cells, according to Wang et al20. An increase in runt-related transcription factor 2 (Runx2), a transcription factor involved in osteoblast cell development, can be linked to increased bone production by phytoestrogens21.
Western blot (WB) and immunohistochemistry (IHC) approaches can be used to examine Runx2 bone tissue. Both of these approaches use antigen-antibody interactions to detect proteins22-24. The advantages and disadvantages of immunohistochemistry and western blot procedures can be seen based on numerous parameters such as selectivity, sensitivity, processing time, and cost-efficiency. These four factors must be taken into account when choosing protein observation methods. A method must be selective in order to be able to distinguish the target protein among other components in the sample carefully and thoroughly25. The technology adopted must also be sensitive enough to detect protein at very low concentrations26. The time and expense of developing a method must also be taken into account in order to develop a method that is both effective and efficient. So, it is intended that this comparative literature analysis will provide information on the best approach to observe Runx2 depending on the parameters that have been specified.
Materials and Methods
Materials
Criteria of collecting data
Inclusion criteria used to select the articles were: (i) Studies from original articles that referred to the effects of phytoestrogens on bone formation; (ii) Studies that measured Runx2 using immunohistochemistry or western blot; (iii) Publication from 2010 until 2021; (iv) Publication using English and Indonesian language; and (v) Articles that are full text or can be fully accessed.
The exclusion criteria applied to each article were: (i) Original article that published before 2010, (ii) Article in another language besides English and Indonesian; and (iii) article that was not in full text or could not be fully accessed.
Collecting strategy and article selection
Articles were searched using PubMed, Google Scholar, and Science Direct databases with phytoestrogens, western blot, immunohistochemistry, runx2, bone, tissue as the keywords. Articles were checked for the suitability of their titles and abstracts with the research topic. In addition, it was also checked for the possibility of duplication of articles among the databases used. Then the feasibility test was carried out by reading the entire text in the selected articles based on predetermined criteria. In addition, the selected articles are full text and open access script with the theme of the study related to the effect of phytoestrogens on the expression of Runx2 in bone tissue by using immunohistochemistry or western blot methods. Relevant articles are taken as primary data27, 28.
Methods
The method for searching and selecting research articles is illustrated using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline 27, 29, 30. Based on the PRISMA guideline with database and keywords, 70 articles were obtained, but only 21 articles had relevant topics, all of which were published within the last 10 years (Figure 1). From this quality assessment, 12 articles were obtained from Google Scholar, 3 articles from PubMed, and 6 articles from Science Direct (Figure 2).
Data Analysis
Articles data obtained from the PRISMA diagram were then analyzed qualitatively. Qualitative data analysis is used to describe and compare the results of research articles found. 21 Articles were used to compare immunohistochemical and western blot methods through Runx2 expression. This comparison covers aspects of numerous parameters such as selectivity, sensitivity, processing time, and cost-efficiency.
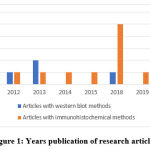 |
Figure 1: Years publication of research articles. |
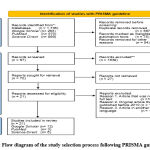 |
Figure 2: Flow diagram of the study selection process following PRISMA guideline 31 |
Result and Discussion
In Vivo Study of Phytoestrogen Effects on Bones
Bone tissue is composed of three main cells, namely osteoblasts, osteoclasts, and osteocytes. The cells that have a major role in bone formation are osteoblasts. These cells differentiate from mesenchymal stem cells, mediating bone formation32. Osteoblast differentiation is due to the commitment of osteogenic precursors from mesenchymal stem cells, followed by differentiation into pre-osteoblasts, immature osteoblasts, and mature osteoblasts33. Runx2, which is a transcription factor for osteoblast differentiation, is expressed by mesenchymal stem cells during early embryonic development and acts as a major regulator of cells leading to osteoblasts34. Thus, Runx2 is considered as one of the earliest and most specific markers during the process of osteogenesis35.
Runx2 observations were made on ovariectomized rats and mice36-38. Osteoporosis in either ovariectomized rats or mice showned many pathophysiological features similar to postmenopausal osteoporosis in humans, so either ovariectomized rats or mice can be good osteoporosis models38. Ovariectomy induces estrogen deficiency in experimental animals, which results in osteoporosis. Experimental animals that were ovariectomized were shown to have decreased expression of Runx2, which resulted in decreased bone formation36, 39.
Experimental animals with estrogen deficiency were given phytoestrogen therapy. Then, the effect of these phytoestrogens on bone formation was analyzed by measuring the expression of Runx2. Phytoestrogen compounds were given in various doses and intervals of administration, which can be seen in Table 1. In this study, positive control (estrogen) and negative control (untreated) controls were also used as comparisons. The effect of giving phytoestrogens on Runx2 expression can also be seen in Table 1.
Table 1: Effects of Phytoestrogens on Runx2 Expression
| No. | Phyto-estrogen | Methods | Doses | Administra-tion Interval | Increased Runx2 Activity | Signs of Bone Formation | Citation |
| 1. | Glabrene | WB | 25; 50; and 100 mg/kg | 13 weeks | ü | Increased expression of Runx2, catenin, Lrp‐5, Osx, OPG and BGP | 36 |
| 2. | Phenolic Acid | WB | 100 g | 14-40 days | ü | Increased expression of ALP, osteocalcin, and Runx2 | 47 |
| 3. | Resveratrol | WB | 50; 100; and 200 mg/day | 12 weeks | ü | Decreased expression of NF-κB and increased expression of Smad7, BMP-2, and Runx2. | 38 |
| 4. | Icariin | WB | 600 mg/kg | 12 weeks | ü | Increased expression of Runx2 and OPG. | 42 |
| 5. | Genistein | WB | 250mg/kg | 8 weeks | ü | Increased expression of Runx2 and osteocalcin. | 50 |
| 6. | β-Ecdysterone | WB | 5 or 10 mg/kg | 5 weeks | ü | Increased expression of Runx2 and BMP-2 | 51 |
| 7. | Isoflavones
contained in soy protein isolate (SPI) |
WB | – | 2 weeks | ü | Decreased expression of caveolin-1, and Increased expression of BMP-2 sitosol, Smad and Runx2 | 52 |
| 8. | Isoflavones contained in soy protein isolate (SPI) | WB | – | 2 weeks | ü | Increased expression of Osterix, Runx2 and β-catenin, also decreased expression of caveolin-1 | 53 |
| 9. | Emodin | IHC | 100 mg/kg | 3 months | ü | Increased expression of Runx2, osterix, type 1 collagen, osteocalcin, and ALP | 41 |
| 10. | Prunetin
|
IHC | 0,125 mg/kg and 0,25 mg/kg | 2 weeks | ü | Increased expression of Runx2 | 54 |
| 11. | Total flavonoids of Rhizoma drynariae (TFRD) | IHC | 20 mg/kg | 18 days | ü | Increased expression of Runx2 and BMP-2 | 40 |
|
12. |
Cinnamaldehyde | IHC | 25 mg/kg; 50 mg/kg; 75 mg/kg | 12 weeks | ü | Increased amount of osteoblas, Increased expression of ALP, Runx2, osteocalcin and type Iɑ1 collagen. also decreased amount of osteoklas | 55 |
| 13. | Isoformonetin
|
IHC | 1; 10; and 25 mg/kg | 12 weeks | ü | Increased expression of type 1 collagen, osteokalsin and Runx-2 | 56 |
| 14. | Kaempferol | IHC | 5.0 mg/kg | 6 days | ü | Increased of Runx2 through Wnt signaling pathway | 49 |
| 15. | Icaritin | IHC | 8 mg | 2 weeks | ü | Increased expression of Osterix and Runx2 | 57 |
| 16. | Notoginsenoside R1 | IHC | 100 μL | 4 weeks | ü | Increased expression of Runx2 and osteocalcin | 58 |
| 17. | Cinnamaldehyde | IHC | 50 mg/kg | 5 weeks | ü | Increased expression of Runx2 and ALP | 59 |
| 18. | Ginsenoside Rg1 (G-Rg1) | IHC | 0,1; 1; 10; 50; 100 μg/mL | 4, 8, and 12 weeks | ü | Increased expression of Runx2, osteocalcin, RANKL and OPG | 32 |
| 19. | Kaempferol | IHC | 5mM | 12 days | ü | Increased expression of Runx2 and osterix | 60 |
| 20. | Icariin | IHC | 25 mg/ kg | 12 weeks | ü | Increased expression of Runx2 and osterix | 37 |
| 21. | Icariin | IHC | 20mg/kg | 2 weeks and 3 months | ü | Increased expression of type 1 collagen, Runx2 and osteocalcin |
39 |
Based on Table 1, it can be seen that phytoestrogens can increase the expression of Runx2 in the bone tissue of experimental animals. Runx2 is a key factor required for osteogenic differentiation and bone development of mesenchymal stem cells40. Increased expression of Runx2 stimulates mesenchymal cells to differentiate into osteoblasts41. So that the increase in Runx2 expression is also an indication of an increase in bone formation.
Research by Liu et al36 showed that administration of glabrene from the roots of Glycyrrhiza glabra (licorice) to ovariectomized rats could significantly increase Runx2 expression compared to negative controls (p<0.05). According to research by Zhang et al38, resveratrol is a natural polyphenolic compound with a structure similar to the estrogen diethylstilbestrol and is found in grapes, peanuts, canapes, and so on. Resveratrol can competitively bind to the estrogen receptor and exert an estrogen-like effect. Administration of resveratrol to ovariectomized rats could significantly increase Runx2 expression compared to negative control (p<0.05). According to research by Zhang et al42, administration of icariin, which is the active ingredient of Herba Epimedii in ovariectomized rats, can significantly increase Runx2 expression compared to negative control (p<0.05). The results of immunohistochemical analysis also showed that administration of icariin at a dose of 25 mg/kg in ovariectomized rats could significantly increase Runx2 expression compared to negative controls (p<0.01)37.
All tested compounds on experimental animals with estrogen deficiency in Table 1 can replace the function of estrogen in binding to the estrogen receptor, although they have different structures. The relationship between chemical structure and biological activity can be done by associating certain functional groups with certain biological responses. Compounds with the same functional group will have the same activity43. In this study, examples of isoflavonoid compounds are glabrene, genistein, daidzein, equol, isoformonetin, and prunetin. Isoflavones are phytoestrogen compounds because they have a chemical structure similar to the hormone estrogen, namely 17β-estradiol. Isoflavone compounds are able to bind to estrogen receptors then provide physiological activity as an estrogen hormone44. The most important chemical group of isoflavones is the phenolic ring, which is a binding site on the estrogen receptor to provide estrogenic effects45. This reason causes isoflavone class of compounds to have an anti-osteoporosis effect marked by an increase in Runx2.
The other flavonoids, such as icariin, icaritin, and kaempferol, and the other phenolic compounds, such as phenolic acid and resveratrol (polyphenols), and the compounds from other groups, such as emodin (anthraquinone), notoginsenoside R1, ginsenoside Rg1 and –ecdysterone, also have estrogenic effects. This is because there is a hydroxyl group (OH) in these compounds, which is one of the requirements for the occurrence of estrogenic activity. Estrogenic effects will appear when it binds to estrogen receptors46. The binding of these compounds to the estrogen receptor in bone can cause an anti-osteoporosis effect, which is indicated by an increase in Runx2.
Increased expression of Runx2 by phytoestrogen compounds generally occurs through several signaling pathways. The phytoestrogens like glabrene, phenolic acid and kaempferol are involved in bone formation by inducing activation of the Wnt signaling pathway. Thus β -catenin penetrates the nucleus and encounters the transcription factor TCF/LEF to initiate expression of target genes, such as Runx2. The Wnt/β-catenin regulates Runx2 expression in mesenchymal cells, then controlling osteoblast differentiation and skeletal development. Activation of the Wnt signaling pathway also affects the increase in osteoblast-specific genes, such as ALP, Osx, BGP, and type I collagen to promote osteoblast differentiation and maturation36, 47, 48, 49.
The next signaling pathway is BMP-2, which is a major growth factor that promotes the differentiation of mesenchymal cells into osteoblasts or chondroblasts61. The phytoestrogens resveratrol and SPI increase Runx2 expression through activation of the BMP-2 signaling pathway, cause promoting mesenchymal cell proliferation and osteoblast differentiation38,52. BMP-2 shows osteogenic action by activating Smad1/5/8 signaling and regulating the transcription of osteogenic genes, including distal-less homeobox 5 (Dlx5), which is a key mediator of BMP-2-induced Runx2 expression59.
The increased expression of Runx2 was influenced by G-protein-coupled receptor 30 (GPR30) too. GPR30 is a membrane-bound ER whose role is to mediate the action of non-genomic estrogens by stimulating Cyclic adenosine 3,5-monophosphate (cAMP). The research of Khan et al54 demonstrated that prunin stimulates osteoblast proliferation and differentiation by specifically activating GPR30, which causes an increase in cAMP levels in osteoblasts. Furthermore, activation of cAMP-dependent Erk/MAP kinase will upregulate Runx2 protein, thereby inducing osteoblast cell differentiation and bone formation.
Besides regulating osteoblast differentiation, Runx2 also plays a role in regulating the expression of several osteoblastic genes such as type I collagen, osteopontin, osteocalcin, and bone sialoprotein, by binding to OSE262. Increased expression of collagen type 1 markers and osteocalcin also indicate increased osteoblastic activity. Type I collagen acts as the main bone matrix63. Osteocalcin plays an important role in the process of mineralization and calcium ion homeostasis, and is often used as a marker of osteoblast differentiation62. Runx2 can also affect ALP marker recognition. ALP plays an important role in enhancing bone mineralization by providing phosphate as a result of hydrolysis of pyrophosphate, an inhibitor of hydroxyapatite propagation64. Another transcription factor that also plays a role in bone formation is osterix (Osx).
Runx2 Observation Method
Western Blot
Western blot is a biochemical technique used to identify specific proteins in complex sample mixtures. This method was developed in 1979 and combines electrophoretic screening with immunoassays for semiquantitative protein assays65. By this technique, it is possible to detect a single protein from a sample, but also obtain molecular weight information about that protein66. In the western blot method, proteins are recognized by specific antibodies as bands at certain positions on the membrane. The position is calibrated as the molecular weight of the protein in kilo Daltons (kD). If the band appears at a position on the membrane too far from the expected position of the theoretical molecular mass of the protein in question, the band is often considered a nonspecific protein67.
The procedure for detecting Runx2 with western blot includes sample preparation, protein quantification, electrophoresis, electrical transfer, blocking, incubation of primary antibodies, washing, incubation of secondary antibodies, and detection using chemiluminescence techniques. Based on these steps, the western blot method uses an indirect method (Figure 3), because two different antibodies are used, namely primary antibodies and secondary antibodies. With the indirect method, the signal given is stronger because several secondary antibodies bind to each primary antibody29.
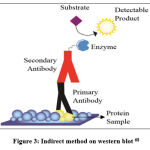 |
Figure 3: Indirect method on western blot 68 |
Immunohistochemistry
Immunohistochemistry (IHC) is a method used to determine the expression of biomarkers in tissues69. This technique basically identifies tissue constituents (antigens) through antigen-antibody interactions. The target antigen/protein in the tissue is recognized by antibodies that are highly specific to that antigen27, 70, 71. In addition, with this technique, the distribution and localization of biomarkers or differentially expressed proteins in different parts of biological tissues can be determined72.
The Runx2 detection procedure by immunohistochemistry method includes fixation, tissue implantation on slides, tissue cutting, deparaffinization, antigen retrieval, blocking, incubation of primary antibodies, washing, incubation of fluorescent secondary antibodies, washing, addition of chromogen substrate, washing, and detection using fluorescence microscopy. Based on these steps, the immunohistochemistry method used is an indirect method (Figure 4), because two different antibodies are used, namely primary and secondary antibodies.
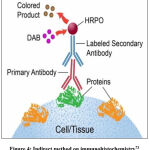 |
Figure 4: Indirect method on immunohistochemistry73 |
Parameter Comparison
Selectivity
Selectivity is the preferred method for binding its target protein among competing sample proteins in heterogeneous mixtures74. The selectivity of western blot and immunohistochemistry methods for detecting Runx2 protein in bone tissue can be affected by the use of specific antibodies. However, in western blot, that protein separation also affects the selectivity of the method75, 76.
The specific antibodies cause the target protein in the complex mixture to be found and bound selectively74. The selectivity of specific antibodies allows detection of target proteins in complex mixtures containing >100,000 different proteins76. The use of anti-Runx2 as a primary antibody to detect Runx2 protein in bone tissue is one of the factors in achieving the selectivity of western blot and immunohistochemistry methods. Anti-Runx2 will specifically bind to the Runx2 protein in bone tissue so that Runx2 can be detected. On the other hand, to increase the selectivity, the western blot and immunohistochemistry methods use blocking buffers which function to bind non-specific proteins to the membrane surface, so that the possibility of antibodies to bind the other proteins than the target protein is reduced74, 77.
The selectivity of western blot is also affected by the separation of proteins of different sizes by gel electrophoresis, the gel commonly used is sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The presence of SDS causes the protein to become negatively charged because it binds to the SDS anion. SDS that wraps around the polypeptide backbone causes protein denaturation. The negative charge is imparted by SDS to the polypeptide chain as long as to its length. Therefore, proteins can be separated according to their molecular weight76. The molecular weight of the identified protein can be determined using a known molecular weight standard77.
The determination of the selectivity value is based on the false positive and true negative values in the detection results. False positives are described as the number of samples with positive results in the western blot or IHC test, but negative in the standard method test. Meanwhile, true negative is described as the number of samples with negative results both in the test using the standard method and with the western blot or IHC method. The selectivity value is calculated by the following formula78-80.
Research by Arzouni et al81 showed that the western blot method was more selective in detecting antibodies to indicate bacterial infection than the immunofluorescence method. The research results of Liu et al82 that detected antibodies for infection by protozoan parasites using the western blot and indirect immunofluorescence antibody methods showed that western blot was more selective in detecting these antibodies than indirect immunofluorescence antibodies, with selectivity values of 100% and 91.4–95.8%, respectively. The results of the study from Jaskowski et al80 showed that the western blot method was more selective in detecting antibodies to markers of autoimmune disorders than indirect immunofluorescence antibodies, with selectivity values of 100% and 94.3%, respectively. According to research by Lindenmayer et al78, the western blot method is more selective in detecting antibodies that indicate bacterial infection than the indirect immunofluorescence antibody method, with selectivity values of 100% and 93.5%, respectively. Thus, western blot is a more selective method for detecting proteins (including Runx2) in bone tissue than immunohistochemistry.
Sensitivity
Sensitivity is described as the ability of a method to detect a certain amount of protein. Western blot is a method with very high sensitivity, and is more sensitive than the immunohistochemistry method73. This method can detect target proteins especially with low abundance (nano to pico gram) because of their high resolution capacity of gel electrophoresis and because of the high affinity of antibodies to their epitope76, 83-85. The final detection/amplification system of western blot also affects the sensitivity of the method83, 84. For example, the final detection method of chemiluminescence is more sensitive than fluorescence86.
The determination of the sensitivity value is based on the true positive and false negative values in the detection results. True positive is described as the number of samples with positive results, both in the test using the standard method and with the western blot or IHC method. Meanwhile, false negative is described as the number of samples with negative results in the western blot or IHC test, but positive in the standard method test. The sensitivity value is calculated by the following formula78–80.
Based on the research of Atehortúa et al87, western blot method is more sensitive than immunohistochemistry in detecting protein markers of muscular dystrophy, with sensitivity values of 100% and 99.5%, respectively. Na et al88 in their study also showed that the western blot method is more sensitive than immunohistochemistry in the detection of protein markers of muscular dystrophy. Arzouni et al81 showed that the western blot method is more sensitive in detecting antibodies that indicate the presence of bacterial infection than the immunofluorescence method. Basso et al89 showed in a study that detected antibodies for infection markers by parasitic protozoa using the western blot and indirect immunofluorescence antibody methods that western blot was more sensitive in detecting these antibodies than indirect immunofluorescence antibodies, with sensitivity values of 93.5% and 87.3%, respectively.
According to research by Lindenmayer et al78, the western blot method is more sensitive in detecting antibodies that indicate bacterial infection than the indirect immunofluorescence antibody method, with sensitivity values of 100% and 66.7%, respectively. Based on the research of Teysseire and Raoult79, the western blot method is more sensitive in detecting antibodies that indicate the presence of a pathogenic infection than the immunofluorescence method, with sensitivity values of 67% and 46%, respectively. Research results from Jensenius et al90 that detected various antigens based on antigen-antibody binding to determine the presence of infection by pathogens using western blot and indirect immunofluorescence antibody methods showed that western blot was more sensitive than indirect immunofluorescence antibody, with sensitivity values respectively being 100% and 45%. As a result, the western blot method is more sensitive than the immunohistochemistry/ immunofluorescence method.
Processing time
The processing time parameter describes how long the Runx2 observation process takes for each method. The total processing time was determined based on the time required for each step of the western blot and immunohistochemistry methods to detect Runx2 in primary data. Details of the processing time required for observations by the western blot method can be seen in Table 2, while the details of the processing time required for observations by the immunohistochemistry method can be seen in Table 3.
Table 2: Processing time of western blot
| No. | Step | Time required | Citation |
| 1. | Sample preparation | 2 hours 25 minutes | 36, 38, 91, 92 |
| 2. | Protein quantification | 2 hours | 93 |
| 3. | Elektrophoresis | 2 hours | 77, 94 |
| 4. | Transfer of proteins from gel to membrane | 1 hours 40 minutes | 77 |
| 5. | Immunodetection | ||
| Blocking (room temperature) | 2 hours | 36, 38 | |
| Primary antibody incubation (on 4°C) | 12 hours | 36, 38 | |
| Washing | 45 minutes | 38 | |
| Secondary antibody incubation (room temperature) | 2 hours | 38 | |
|
Total |
25 hours 20 minutes |
||
Table 3: Processing time immunohistochemistry
| No. | Step | Time required | Citation |
| 1. |
Sample preparation |
||
| Fixation | 24 hours | 95 | |
| Planting and cutting | 6 hours 15 minutes | 71, 72 | |
| Deparaffinization | 1 hour 5 minutes | 71 | |
| 2. | Antigen retrieval | 20 minutes | 41 |
| 3. | Blocking (room temperature) | 30 minutes | 95 |
| 4. | Primary antibody incubation (at 4°C) | 12 hours | 32, 37, 39, 56 |
| 5. | Washing | 15 minutes | 96 |
| 6. | Secondary antibody incubation (room temperature) | 1 hour | 54, 56 |
| 7. | Washing | 15 minutes | 95 |
| 8. | Addition of chromogen substrate | 5 minutes | 95 |
| 9. | Washing | 15 minutes | 95 |
| Total | 46 hours | ||
Based on Table 2, it can be seen that the observation of Runx2 expression by western blot method takes 25 hours and 20 minutes. Meanwhile, the observation of Runx2 expression by immunohistochemistry method took 46 hours. As a result, it can be concluded that the detection of Runx2 protein is faster by western blot method than immunohistochemistry.
Cost efficiency
The cost efficiency parameter describes how much is the cost used in the observations in each method. Based on the research of Atehortúa et al87, the western blot method is ±57.26% more cost-effective. The immunohistochemistry method requires a fluorescence microscope for observation. This microscope is more expensive than the observation instrument used in the western blot method, namely the chemiluminescence imager. The use of a chemiluminescence imager can save up to 68.89% of costs compared to using a fluorescence microscope96, 97. Thus, observations using the western blot method are more cost-efficient than the immunohistochemistry method.
Conclusion
Western blot and immunohistochemistry methods can be used to detect Runx2 in bone tissue induced by phytoestrogens, characterized by an increase in Runx2 expression. The western blot method is more selective, sensitive, requires less cost, and is faster than the immunohistochemistry method.
Authors’ Contribution
All of the authors participated actively in the research and writing of the article.
Conflict of Interest
This article’s author declares that there is no conflict of interest.
Funding Sources
There is no funding source.
References
- Andini, D.Hubungan lama menopause dengan kejadian disfungsi seksual pada wanita menopause di posyandu lansia wilayah kerja Puskesmas Panjang Bandar Lampung [skripsi].Bandar Lampung: Fakultas Kedokteran, Universitas Lampung. (2014)
- Ma’arif, B. Aktivitas ekstrak n-heksana dan fraksi hasil pemisahan daun Marsilea crenata terhadap diferensiasi sel preosteoblas MC3T3-E1 melalui Pengukuran alkaline phosphatase in vitro [tesis]. Surabaya: Fakultas Farmasi Universitas Airlangga. (2015).
- Sugiritama, W. dan Adiputra, N. Potensi antosianin dalam manajemen menopause. Jurnal Kesehatan Andalas, 8(1): 158-166 (2019).
CrossRef - Lerner, U.H. Bone remodeling in postmenopausal osteoporosis. Journal Dental Research, 85(7): 584-595 (2006)
CrossRef - Sihombing, I., Wangko, S., Kalangi, S.J.R. Peran estrogen pada remodeling tulang. Jurnal Biomedik. 4(3): 18-28 (2012).
CrossRef - Sözen, T., Özışık, L and Başaran, N.C. An overview and management of osteoporosis. European Journal of Rheumatol, 4(1): 46-56 (2016).
CrossRef - Amelia, W. Hubungan pengetahuan dan konsumsi susu pada wanita pralansia dengan upaya pencegahan osteoporosis di Baturaja tahun 2018. Jurnal ‘Aisyiyah Medika, 2: 47-56 (2018).
CrossRef - Kemenkes RI. Data dan Kondisi Penyakit Osteoporosis di Indonesia. Jakarta: Kemenkes RI (2015)
CrossRef - Bretler, D.M., Hansen, P.R., Lindhardsen, J., Andersson, C., Jensen, T.B., Rauns, J., Torp-Pedersen, C., Gislason, G.H. Hormone replacement therapy and risk of new onset atrial fibrillation after myocardial infarction-a nationwide cohort study. Plos ONE, 7(12): 1-7 (2012).
CrossRef - Lee, W.L., Tsui, KH., Seow, K.M., Cheng, M.H., Su, W.H., Chen, C.P., Wang, P.H. Hormone therapy for postmenopausal women and unanswered issue. Elsevier: Gynecology and Minimally Invasive Therapy, 2: 13-17 (2013).
CrossRef - Jantaratnotai, A.N., Utaisincharoen, B.P., Sanvarinda, A.P., Thampithak, C.A., Sanvarinda, Y. Phytoestrogens Mediated Anti-Inflammatory Effect Through Suppression Of IRF-1 and PSTAT 1 Expressions in Lipopolysaccharide-Activated Microglia. International Immunopharmacology, 17: 483–488 (2013).
CrossRef - Yang, T.S., Wang, S.Y., Yang, Y.C., Su, C.H., Lee, F.K., Chen, S.C., Tseng, C.Y., Jou, H.J., Huang, J.P. and Huang, K.E. Effects of standardized phytoestrogen on Taiwanese menopausal women. Elsevier : Taiwanese Journal of Obstetrics & Gynecology, 51(2): 229-235 (2012).
CrossRef - Cos, P., De Bruyne, T., Apers, S., Berghe, D.V., Pieters, L. and Vlietinck, A.J. Phytoestrogens: recent developments. Planta Medica, 69(7): 589-599 (2003).
CrossRef - Ososki, A.L. and Kennelly, E.J., Phytoestrogens: a review of the present state of research. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives, 17(8): 845-869 (2003).
CrossRef - Dweck, A.C. Isoflavones, phytohormones and phytosterols. Journal of Applied Cosmetology, 24(1): 17-33 (2006).
- Villiers, T.J. Bone health and osteoporosis in postmenopausal women. Elsevier: Best Practice & Research Clinical Obstetrics and Gynaecology, 23: 73-85 (2009).
CrossRef - Branca, F. Dietary phyto oestrogens and bone health. Proceeding of the Nutrition Society, 62(4): 877-887 (2003).
CrossRef - Ma’arif, B., Agil, M., Laswati, H. Alkaline phosphatase activity of Marsilea crenata extract and fractions as marker of MC3T3-E1 osteoblast cell differentiation. J Appl Pharm Sci, 8(3): 55–9. (2018).
- Hu, B., Yu, B., Tang, D., Li, S., Wu, Y. Daidzein promotes osteoblast proliferation and differentiation in OCT1 cells through stimulating the activation of BMP-2 / Smads pathway. Genetics and Molecular Research, 15(2): 1–10 (2016).
CrossRef - Wang, N., Wang, X., Cheng, W., Cao, H., Zhang, P., Qin, L. Puerarin promotes osteogenesis and inhibits adipogenesis in vitro. Chinese Medicine, 8(17): 1–12 (2013).
CrossRef - Komori, T. Regulation of proliferation, differentiation and functions of osteoblasts by Runx2. International Journal of Molecular Science, 20(7): 1694 (2019).
CrossRef - Kabiraj, A., Gupta, J., Khaitan, T and Bhattacharya, P.T. Principle and techniques of immunohistochemistry – A Review. Int J Biol Med Res, 6(3): 5204-5210 (2015).
- Matos, L.L.D., Trufelli, D.C., Matos, M.G.L.D and Pinhal, M.A.D.S. Immunohistochemistry as an important tool in biomarkers detection and clinical practice. Biomarker Insights, 5(5): 9-20 (2010).
CrossRef - Moore, C. Introduction to Western Blotting. Oxford: MorphoSys (2009).
- Petunjuk pelaksanaan validasi metode dan cara perhitungannya. Majalah Ilmu Kefarmasian, 1(3): 117–135 (2004).
CrossRef - Budiarti, A. dan Arifin, I. Optimasi dan validasi metode analisis sukrosa untuk menentukan keaslian madu perdagangan menggunakan kromatografi cair kinerja tinggi 1. Prosiding Seminar Nasional “Perkembangan Terbaru Pemanfaatan Herbal Sebagai Agen Preventif Pada Terapi Kanker”. Pp: 116–122. (2014).
- Snyder, H. Literature review as a research methodology: an overview and guidelines. Journal of Business Research, 104(July), 333–339 (2019).
CrossRef - Ma’arif, B., Suryanto, Muslikh, F. A., Suryadinata, B., Fauziyah, B. Systematic Review: Anti-Osteoporosis Potential Activities Of Phytoestrogen Compounds In Chrysophyllum cainito, Elaeis guineensis Jacq., Lannea acida Rich., Marsilea crenata Presl., and Medicago sativa L. Journal of Pharmaceutical Sciences and Community, 19(1): 41-52 (2022).
CrossRef - Selçuk, A. A. A guide for systematic reviews: PRISMA. Turkish Archives of Otorhinolaryngology, 57(1), 57–58 (2019).
CrossRef - Page, M.J., McKenzie, J.E., Bossuyt, P.M., Boutron. I., Hoffmann, T.C. and Mulrow, C.D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 372(71) : 1-9 (2021)
- PRISMA website: http://www.prisma-statement.org
- Li, X., Hong, S., Tan, C., Peng, B., Wu, Z. and Zhu, Y. Combined study on the action and mechanism of g-rg1/sr-cas bone substitute material for ossification and pro-vascularization. Materials Express, 10(2): 177–189 (2020).
CrossRef - Carbonare, L.D., Innamorati, G., and Valenti, M.T. Transcription factor Runx2 and its application to bone tissue engineering. Stem Cell Reviews and Reports, 8(3): 891–897 (2012).
CrossRef - Jonason, J.H., Xiao, G., Zhang, M., Xing, L. and Chen, D. Post-translational regulation of Runx2 in bone and cartilage. Journal of Dental Research, 88(8): 693–703 (20090.
CrossRef - Shakibaei, M., Shayan, P., Busch, F., Aldinger, C., Buhrmann, C., Lueders, C. and Mobasheri, A.. Resveratrol mediated modulation of sirt-1/Runx2 promotes osteogenic differentiation of mesenchymal stem cells: Potential role of Runx2 deacetylation. PLoS ONE, 7(4) (2012).
CrossRef - Liu, J., Deng, X., Liang, X. and Li, L. The phytoestrogen glabrene prevents osteoporosis in ovariectomized rats through upregulation of the canonical Wnt β‐catenin signaling pathway.pdf. J Biochem Mol Toxicol. 22653: 1–10 (2020).
CrossRef - Luo, Y., Zhang, Y., Miu, G., Zhang, Y. and Huang, Y. Icariin promotes osteoblast differentiation and inhibits adipogenesis of bone marrow stem cells through activation of Wnt β-catenin signaling in vivo and in vitro. Pharmazie, 73: 459–464 (2018).
- Zhang, Ye., Liu, M.W., He, Y., Deng, N., Chen, Y., Huang, J. and Xie, W. Protective effect of resveratrol on estrogen deficiency-induced osteoporosis though attenuating NADPH oxidase 4/nuclear factor kappa B pathway by increasing miR-92b-3p expression. International Journal of Immunopathology and Pharmacology, 34 (2020).
CrossRef - Bian, Q., Huang, J., Liu, S., Ning, Y., Yang, Z., Zhao, Y., Shen, Z. and Wang, Y. Different molecular targets of Icariin on bMSCs in CORT and OVX rats. Frontiers in Bioscience, 725: 1224–1236. (2012)
CrossRef - Yao, W., Zhang, H., Jiang, X., Mehmood, K., Iqbal, M., Li, A., Zhang, J., Wang, Y., Waqas, M., Shen, Y. and Li, J. Effect of total flavonoids of rhizoma drynariae on tibial dyschondroplasia by regulating BMP-2 and Runx2 expression in chickens. Frontiers in Pharmacology, 9: 1–10 (2018).
CrossRef - Yang, F., Yuan, P., Hao, Y.Q. and Lu, Z. M. Emodin enhances osteogenesis and inhibits adipogenesis. BMC Complementary and Alternative Medicine, 14(1): 1–9 (2014).
CrossRef - Zhang, Yong., Han, B., Wei, Y., Jing, J. and Li, J. Icariin promotes fracture healing in ovariectomized rats. Medical Science Monitor, 26: 1–8 (2020).
CrossRef - Cartika, H. Kimia Farmasi. Jakarta: Pusdik SDM Kesehatan (2016).
- Primiani, C.N. Potensi Tepung Tempe sebagai Estrogen Alami terhadap Uterus Mencit Premenopause. Jurnal Sains Dan Matematika, 1(2): 47–51 (2013).
- Herwana, E. Fenotip equol-producer dan hubungannya dengan asupan isoflavon dan kesehatan. Jurnal Biomedika Dan Kesehatan. 3(3): 159–165 (2020).
CrossRef - Ariyanti, H. dan Apriliana, E.Pengaruh Fitoestrogen terhadap Gejala Menopause. Majority. 5(5): 1–5 (2016).
CrossRef - Chen, J.R., Lazarenko, O.P., Wu, X., Kang, J., Blackburn, M.L., Shankar, K., Badger, T. M. and Ronis, M.J. Dietary‐induced serum phenolic acids promote bone growth via p38 MAPK β‐catenin canonical Wnt signaling.pdf. Journal of Bone and Mineral Research. 25(11): 2399–2411 (2010).
CrossRef - Haxaire, C., Haÿ, E. and Geoffroy, V. Runx2 controls bone resorption through the down-regulation of the Wnt pathway in osteoblasts. American Journal of Pathology. 186(6): 1598–1609 (2016).
CrossRef - Sharma, A.R. and Nam, J.S. Kaempferol stimulates WNT β-catenin signaling pathway to induce differentiation of osteoblasts. The Journal of Nutritional Biochemistry, 74: 108–228 (2019).
CrossRef - Yang, C.S., Mercer, K.E., Alund, A.W., Suva, L.J., Badger, T.M. and Ronis, M.J.J. Genistein supplementation increases bone turnover but does not prevent alcohol-induced bone loss in male mice. Experimental Biology and Medicine. 239(10): 1380–1389 (2014).
CrossRef - Tang, Y.H., Yue, Z.S., Xin, D.W., Zeng, L.R., Xiong, Z.F., Hu, Z.Q. and Xu, C.D. β Ecdysterone promotes autophagy and inhibits apoptosis in osteoporotic rats.pdf. Molecular Medicine Reports. 17: 1591–1598 (2018).
CrossRef - Zhang, J., Lazarenko, O. P., Wu, X., Tong, Y., Blackburn, M.L., Gomez-Acevedo, H., Shankar, K., Badger, T.M., Ronis, M.J.J. and Chen, J. R. Differential effects of short term feeding of a soy protein isolate diet and estrogen treatment on bone in the pre-pubertal rat. PLoS ONE. 7(4) (2012).
CrossRef - Zhang, J., Lazarenko, O.P., Blackburn, M.L., Badger, T.M., Ronis, M.J.J. and Chen, J.R.. Soy protein isolate down-regulates caveolin-1 expression to suppress osteoblastic cell senescence pathways. The FASEB Journal, 28(7): 3134–3145 (2014).
CrossRef - Khan, K., Pal, S., Yadav, M., Maurya, R., Trivedi, A.K., Sanyal, S. and Chattopadhyay, N. Prunetin signals via G-protein-coupled receptor, GPR30 (GPER1): Stimulation of adenylyl cyclase and cAMP-mediated activation of MAPK signaling induces Runx2 expression in osteoblasts to promote bone regeneration. Journal of Nutritional Biochemistry. 26(12): 1491–1501 (2015).
CrossRef - Wu, Z., Weng, S., Yan, D.., Xie, Z., Zhou, Q., Li, H., Bai, B., Boodhun, V., Shen, Z., Tang, J., Zhou, L., Tao, Z. and Yang, L. Administration of cinnamaldehyde promotes osteogenesis in ovariectomized rats and differentiation of osteoblast in vitro. Journal of Pharmacological Sciences. 138(1): 63–70 (2018).
CrossRef - Srivastavaa, K., Tyagi, A.M., Khan, K., Dixit, M., Lahiri, S., Kumar, A., Changkija, B., Khan, M.P., Nagar, G.K., Yadav, D.K., Maurya, R., Singh, S.P., Jain, G.K., Wahajuddin., Trivedi, R., Chattopadhyay, N. and Singh, D. Isoformononetin, a methoxydaidzein present in medicinal plants, reverses bone loss in osteopenic rats and exerts bone anabolic action by preventing osteoblast apoptosis. Phytomedicine, 20(6): 470–480 (2013).
CrossRef - Chen, S., Zheng, L., Zhang, J., Wu, H., Wang, N., Tong, W., Xu, J., Huang, L., Zhang, Y., Yang, Z., Lin, G., Wang, X. and Qin, L. A novel bone targeting delivery system carrying phytomolecule icaritin for prevention of steroid-associated osteonecrosis in rats. Bone. 106: 52–60 (2018).
CrossRef - Wang, L., Fu, H., Wang, W., Liu, Y., Li, X., Yang, J., Li, L., Wu, G. and Pan, Y. Notoginsenoside R1 functionalized gelatin hydrogels to promote reparative dentinogenesis. Acta Biomaterialia, 122(1): 160–171 (2020).
CrossRef - Wu, Z., Yan, D., Xie, Z., Weng, S., Zhou, Q., Li, H., Bai, B., Boodhun, V., Shen, Z., Tang, J. and Yang, L.. Combined treatment with cinnamaldehyde and PTH enhances the therapeutic effect on glucocorticoid-induced osteoporosis through inhibiting osteoclastogenesis and promoting osteoblastogenesis. Biochemical and Biophysical Research Communications. 505(3): 945–950 (2018).
CrossRef - Yang, L., Takai, H., Utsunomiya, T., Li, X., Li, Z., Wang, Z., Wang, S., Sasaki, Y., Yamamoto, H. and Ogata, Y. Kaempferol stimulates bone sialoprotein gene transcription and new bone formation. Journal of Cellular Biochemistry. 110(6): 1342–1355 (2010).
CrossRef - Young-Eun, C. and In-Sook, K. Zinc upregulates bone-specific transcription factor Runx2 expression via BMP-2 signaling and Smad-1 phosphorylation in osteoblasts. Journal of Nutrition and Health. 51(1): 23–30 (2018).
CrossRef - Jang, W.G., Kim, E.J., Kim, D.K., Ryoo, H.M., Lee, K.B., Kim, S.H., Choi, H.S. and Koh, J.T. BMP2 protein regulates osteocalcin expression via Runx2-mediated Atf6 gene transcription. Journal of Biological Chemistry. 287(2): 905–915 (2012).
CrossRef - Lubis, I., Rahardjo, R. dan Rahmat, M.M. Akibat aplikasi simvastatin pada proses penyembuhan tulang terhadap ekspresi kolagen tipe I (pada binatang coba tikus diabetes melitus tipe 1). Kedokteran Gigi. 6(4): 340–346 (2015).
- Weng, J.J. and Su, Y. Nuclear matrix-targeting of the osteogenic factor Runx2 is essential for its recognition and activation of the alkaline phosphatase gene. Biochimica et Biophysica Acta – General Subjects. 1830(3): 2839–2852 (2013).
CrossRef - Jin, S. and Kennedy, R.T.. New developments in western blot technology. Chinese Chemical Letters, 26(4): 416–418 (2015)
CrossRef - Gilda, J.E.., Ghosh, R., Cheah, J.X., West, T.M., Bodine, S.C. and Gomes, A.V. Western blotting inaccuracies with unverified antibodies: Need for a Western Blotting Minimal Reporting Standard (WBMRS). PLoS ONE. 10(8): 1–18 (2015).
CrossRef - Liu, X., Wang, Y., Yang, W., Guan, Z., Yu, W. and Liao, D.J. Protein multiplicity can lead to misconduct in western blotting and misinterpretation of immunohistochemical staining results, creating much conflicting data. Progress in Histochemistry and Cytochemistry. 51(3–4): 51–58 (2016).
CrossRef - Thermo scientific pierce western blotting handbook and troubleshooting guide. Thermofisher Scientific (2014)
- Hofman, F.M. and Taylor, C.R. Immunohistochemistry. Current Protocols in Immunology, 103: 1–26 (2013)
CrossRef - Nambiar, S.K., Haragannavar, V.C., Augustine, D., Rao, R.S. and Kumari, K. Immunohistochemistry: a brief review. Journal of Dental & Oro-Facial Research. 12(02) : 25–29 (2016)
- Ahmed, S.A., Suri, C., Palakurthi, N., and Panthala, V. Review article immunohistochemistry – a technical review. International Journal of Current Research. 9(6) : 52563–52567 (2017)
- Orakpoghenor, O., Avazi, D.O., Markus, T.P. and Olaolu, O.S. Immunogenetics : open access a short review of immunochemistry. Immunogenet Open Access. 3(1) : 1–6 (2018)
- Shojaeian, S., Lay, N.M. and Zarnani, A.H. Detection Systems in Immunohistochemistry. In Immunohistochemistry – The Ageless Biotechnology. IntechOpen (2018)
- Pillai-Kastoori, L., Heaton, S., Shiflett, S.D., Roberts, A.C., Solache, A. and Schutz-Geschwender, A.R. Antibody validation for western blot: by the user, for the user. Journal of Biological Chemistry. 295(4) : 926–939 (2019)
CrossRef - Akiyama, H., Imai, T., and Ebisawa, M. Japan Food Allergen Labeling Regulation-History and Evaluation. In Advances in Food and Nutrition Research. 62 (2011)
CrossRef - Ghosh, R., Gilda, J.E. and Gomes, A.V. Accuracy of western blots. Expert Review of Proteomics. 11(5) : 549–560 (2014)
CrossRef - Novus Biologicals. Western Blot Handbook. papers2://publication/uuid/57522661-60F6-4C2E-937A-FF24287C4939 (2011)
- Lindenmayer, J., Weber, M., Bryant, J., Marquez, E. and Onderdonk, A. Comparison of indirect immunofluorescent-antibody assay, enzyme-linked immunosorbent assay, and western immunoblot for the diagnosis of Lyme disease in dogs. Journal of Clinical Microbiology. 28(1) : 92–96 (1990)
CrossRef - Teysseire, N. and Raoult, D. Comparison of western immunoblotting and microimmunofluorescence for diagnosis of Mediterranean spotted fever. Journal of Clinical Microbiology. 30(2) : 455–460 (1992)
CrossRef - Jaskowski, T.D., Prince, H.E., Greer, R.W., Litwin, C.M. and Hill, H.R. Further comparisons of assays for detecting MAG IgM autoantibodies. Journal of Neuroimmunology. 187: 175–178 (2007)
CrossRef - Arzouni, J.P., Laveran, M., Beytout, J., Ramousse, O. and Raoult, D. Comparison of western blot and microimmunofluorescence as tools for lyme disease seroepidemiology. European Journal of Epidemiology. 9(3) : 269–273 (1993)
CrossRef - Liu, Q., Wang, Z.D.,Huang, S.Y. and Zhu, X.Q. Diagnosis of toxoplasmosis and typing of Toxoplasma gondii. Parasites and Vectors. 8(1) : 1–14 (2015)
CrossRef - Kurien, B.T., Dorri, Y., Dillon, S., Dsouza, A. and Scofield, R.H. An overview of western blotting for determining antibody specificities for immunohistochemistry. Methods in Molecular Biology. 717: 55–67 (2011)
CrossRef - Najafov, A. and Hoxhaj, G. Introduction. In Western Blotting Guru. London: Academic Press (2017)
CrossRef - Roy, J., Jain, N., Singh, G., Das, B. and Mallick, B. Small RNA proteome as disease biomarker: An incognito treasure of clinical utility. In AGO-Driven Non-Coding RNAs. 5. London: Academic Press (2019)
CrossRef - Kondo, Y., Higa, S., Iwasaki, T., Matsumoto, T., Maehara, K., Harada, A., Baba, Y., Fujita, M., Ohkawa, M. Sensitive detection of fluorescence in western blotting by merging images. PLoS ONE. 13(1) (2018)
CrossRef - Atehortúa, S.C., Lugo, L.H., Ceballos, M., Orozco, E., Castro, P.A., Arango, J.C. and Mateus, H.E. Cost-effectiveness analysis of diagnosis of duchenne/becker muscular dystrophy in colombia. Value in Health Regional Issues. 17: 1–6 (2018)
CrossRef - Na, S.J., Kim, W.J., Kim, S.M., Lee, K.O., Yoon, B. and Choi, Y.C. Clinical, immunohistochemical, western blot, and genetic analysis in dystrophinopathy. Journal of Clinical Neuroscience. 20(8) : 1099–1105 (2013)
CrossRef - Basso, W., Hartnack, S., Pardini, L., Maksimov, P., Koudela, B., Venturini, M.C., Schares, G., Sidler, X., Lewis, F.I., and Deplazes, P. Assessment of diagnostic accuracy of a commercial ELISA for the detection of Toxoplasma gondii infection in pigs compared with IFAT, TgSAG1-ELISA and Western blot, using a Bayesian latent class approach. International Journal for Parasitology. 43(7) : 565–570 (2013)
CrossRef - Jensenius, M., Fournier, P.E., Vene, S., Ringertz, S.H., Myrvang, B. and Raoult, D. Comparison of immunofluorescence, Western blotting, and cross-adsorption assays for diagnosis of African tick bite fever. Clinical and Diagnostic Laboratory Immunology. 11(4) : 786–788 (2004)
CrossRef - Western blot protocol dalam https://www.abcam.com/protocols/general-western-blot-protocol. Accessed on 2 June 2021 (2020)
- Taylor, S.C. and Posch, A. The design of a quantitative western blot experiment. BioMed Research International (2014)
CrossRef - Martínez-Flores, K., Salazar-Anzures, Á.T., Fernández-Torres, J., Pineda, C., Aguilar-González, C.A. and López-Reyes, A. Western blot: a tool in the biomedical field. Investigación en Discapacidad. 6(3) : 128-137 (2017)
- Mahmood, T. and Yang, P.C. Western blot: technique, theory, and trouble shooting. North American Journal of Medical Sciences. 4(9) : 429–434 (2012)
CrossRef - Kim, S.W., Roh, J. and Park, C.S. Immunohistochemistry for pathologists: Protocols, pitfalls, and tips. Journal of Pathology and Translational Medicine. 50(6) : 411–418 (2016)
CrossRef - BIO RAD VersaDoc MP 4000 Molecular Digital Imaging System dalam https://www.labx.com/item/bio-rad-versadoc-mp-4000-molecular-digitalimaging/1042 3683. Accessed on 2 July 2021.
- Leica DMI6000 CS Inverted Fluorescence w/ TCS SP5 Laser Scanning Confocal Microscope Pred SP8/STELLARIS – AV dalam https://www.labx.com/item/leica-dmi6000-cs-inverted-fluorescence-w-tcs-sp5-laser/13354470. Accessed on 2 July 2021.