Manuscript accepted on :11-03-2022
Published online on: 18-05-2022
Plagiarism Check: Yes
Reviewed by: Dr Rakesh Pandey
Second Review by: Dr. Daya Shankar Gautam
Final Approval by: Dr. Ian james martin
Enye Linus Anderson1* , Saka Olusola Stephen1
, Saka Olusola Stephen1 , Sajere Eric Oghenetega1, Komolafe Omobola Aderibigbe2
, Sajere Eric Oghenetega1, Komolafe Omobola Aderibigbe2 , Abijo Ayodeji Zabdiel3
, Abijo Ayodeji Zabdiel3 and Ige Mokolade Samson2
and Ige Mokolade Samson2
1Department of Anatomy, College of Medicine and Health Sciences, Afe Babalola University Ado Ekiti, Nigeria.
2Department of Anatomy and Cell Biology, College of Health Sciences, Obafemi Awolowo University Ile Ife, Nigeria.
3Department of Anatomy, Faculty of Basic Medical Sciences, Ladoke Akintola University of Technology, Ogbomoso, Nigeria.
Corresponding Author E-mail: enyelinus@abuad.edu.ng
DOI : https://dx.doi.org/10.13005/bpj/2450
Abstract
Caffeine is the major constituent found in coffee, tea, energy drinks, and chocolate bar among many others. Several studies have reported various effects of caffeine on the cardiovascular system, although there are inconsistencies in these findings. This study, based on these sought to investigate the role of Myristica fragrans on caffeine-induced cardiotoxicity in male Wistar rats. Twenty-five healthy Wistar rats, weighing 130-135 g were randomly assigned into groups (A-E) n=5 each. Group A received 2 mL/kg distilled water as placebo, group B was administered caffeine (40mg/kg), group C received Myristica fragrans only (200mg/kg), group D received caffeine (40mg/kg), and Myristica fragrans (100mg/kg), while group E received caffeine (40mg/kg) and Myristica fragrans (200mg/kg). The rats were orally-gavaged caffeine and Myristica fragrans with the aid of an oral cannula for 21 days. On the 22nd day after the last administration, rats were euthanized sacrificed and the heart tissues were obtained histological procedures. Percentage weight change was significantly decreased (p <0.05), and Lactate dehydrogenase (LDH) activity was significantly increased (p <0.05) in group B relative to the control group. The heart, relative heart weights, and cardiac troponin I were not significantly different (p=0.05) across all experimental groups relative to the control. Assessment of the cardiac histoarchitecture revealed diverse alterations in the caffeine-only group which were ameliorated by administration of 100 and 200 mg/kg Nutmeg extract in groups D and E respectively. Caffeine administration resulted in alteration in cardiac histoarchitecture with 100 and 200mg/kg Myristica fragrans ameliorating these alterations. Nutmeg could serve as a drug lead in the management of cardiac-related conditions.
Keywords
Ameliorated; Caffeine; Histoarchitecture; Myristica fragrans; Wistar rats
Download this article as:| Copy the following to cite this article: Anderson E. L, Stephen S. O, Oghenetega S. E, Aderibigbe K. O, Zabdiel A. A, Samson I. M. Myristica Fragrans (Nutmeg) Extract Ameliorated Caffeine-Induced Cardiotoxicity in Male Wistar Rats. Biomed Pharmacol J 2022;15(2). |
| Copy the following to cite this URL: Anderson E. L, Stephen S. O, Oghenetega S. E, Aderibigbe K. O, Zabdiel A. A, Samson I. M. Myristica Fragrans (Nutmeg) Extract Ameliorated Caffeine-Induced Cardiotoxicity in Male Wistar Rats. Biomed Pharmacol J 2022;15(2).Available from: https://bit.ly/38ysQox |
Introduction
Caffeine is still used in the form of coffee, beverages, soft drinks, and energy drinks1. Several studies on the effects of caffeine on the cardiovascular system have been conducted. These accounts, however, are contradictory1. Several mechanisms have been proposed to explain how caffeine causes toxicity in the cardiovascular system. Adenosine is a negative ionotropic and chronotropic substance that acts on certain receptors in the heart. Blocking cardiac adenosine receptors inhibits adenosine’s actions, resulting in tachycardia and arrhythmias via strong 1-receptor activity1. Caffeine has the capacity to produce adenosine antagonism and phosphodiesterase inhibition, interacting with the sympathetic nervous system and activating 1-receptors. As a result, there are positive inotropic and chronotropic effects, which account for increased heart rate and conductivity2. Caffeine increases intracellular cAMP and cyclic guanosine monophosphate (cGMP) via inhibiting non-specific phosphodiesterases, which influences heart contractility consequent to calcium release. The latter method may raise the risk of arrhythmias. Other caffeine’s mechanisms of action with indirect effects on the cardiovascular system have been reported, such as the stimulation of the sodium-potassium-ATPase, which is an integral membrane protein responsible for a decrease in the plasma levels of potassium and the ion’s transfer from the circulation to intracellular compartments, rendering the membrane potential more negative. This increases the likelihood of ventricular arrhythmias3
Caffeine has been linked to a lower ventricular fibrillation threshold in certain laboratory studies4, and as little as 50 mg of caffeine can cause tachycardia and agitation. Caffeine toxicity, like amphetamine poisoning, can result in convulsions, psychosis, cardiac arrhythmias, and, in rare cases, death5. Caffeine-related mortality from energy drinks was recorded, with electrocardiograms revealing that the patient had acute myocardial ischemia, most likely caused by caffeine-induced coronary vasospasm, which raises heart rate and has the potential to cause cardiac arrhythmias5. The pathogenesis of such coronary events is thought to entail enhanced platelet aggregation and decreased endothelial functionality5.
Myristica fragrans, a popular household spice, is derived from the tree Myristica fragrans, which grows in the Indonesian Banda Islands (commonly known as the Spice Islands)6. It has been proven to be beneficial as a heart and brain tonic, as well as in sexual and general debility. In Indonesia, Malaysia, England, and China6, it has been used to cure rheumatism. Externally, the essential oil is used to alleviate rheumatic symptoms, limb pains, general aches, and inflammation. In Malaysia and India, Myristica fragrans has been used to treat nervous symptoms and induce sleep due to its calming effect. Aphrodisiac action of Myristica fragrans 50 percent ethanolic extracts in male mice has also been found.
Extracts of Myristica fragrans and clove were discovered to stimulate male mice’s mounting behavior as well as considerably improve their mating performance7.
There is no data on the impact of simultaneous caffeine and Myristica fragrans ingestion on the heart. This study thus attempted to evaluate the potential ameliorative potentials of Myristica fragrans ethanol extract on caffeine-induced cardiotoxicity in male Wistar rats based on this premise.
Materials and Methods
Ethical Consideration
Ethical clearance for the study was sought and obtained from the local institutional committee. All experimental procedures for this study were per the guidelines of the institutional animal care and use committee (IACUC).
Chemicals, Drugs, and Reagents
Caffeine in its pure white form was acquired from (Sigma, Mo, USA) and used for the study. Elisa Kits for biochemical assay were procured from reliable sources. All other reagents utilized for the study were of analytical grade and purity.
Rat Care and Management
Twenty-five healthy male Wistar rats (Rattus norvegicus) of weight range 130 to 135 g were procured from the Animal Holdings of the Department of Anatomy, Afe Babalola University, Ado-Ekiti and were used for this research. The rats were housed in the Animal Holdings of Afe Babalola University, Ado-Ekiti. They were placed on a standard laboratory chow and also provided unrestricted access to clean water. The rats were maintained in natural day and night cycle and temperature and allowed to acclimatize for two weeks, after which they were randomly assigned based on weights into 5 groups (A-E, n=5 each). Group A which was the control was administered distilled water (2 mL/kg) as placebo, group B was administered caffeine only (40 mg/kg), group C was administered Myristica fragrans only (200 mg/kg), group D was administered caffeine (40mg/kg) and Myristica fragrans (100mg/kg), while group E was administered caffeine (40 mg/kg) and Myristica fragrans (200 mg/kg). Caffeine and Myristica fragrans prepared solution was administered oro-gastrically with the aid of an oral cannula. Administration in control and experimental groups spanned for 21 days.
Table 1: Grouping and Experimental Design.
| GROUPS | AGENT | DOSES | ROUTE | DURATION |
| A | Distilled water | 2 mL/kg | oral | 21 days |
| B | Caffeine only | 40 mg/ kg | oral | 21 days |
| C | Myristica fragrans only | 200 mg/kg | oral | 21 days |
| D | Caffeine +Myristica fragrans | 40 mg/kg +
100 mg/kg |
oral | 21 days |
| E | Caffeine + Myristica fragrans | 40mg/kg +
200 mg/kg |
oral | 21 days |
The body weight measurements were taken weekly throughout the study period
Myristica fragrans extract preparation
The modified method of Dewi et al.,8 was followed for the preparation of Nutmeg extract. Myristica fragrans was obtained in powder form from the town of Ado-Ekiti, Ekiti State. Extract preparation was done by percolating the powder form of Myristica fragrans extract (1:10 w/v) in ethanol for 72 hours with intermittent shaking with a mechanical shaker. The solution was then filtered through Whatman filter paper No. 2 filter paper and the filtrate obtained was evaporated to dryness by slow heating and continuous stirring in a water bath at 40oC. The dark brown residue left behind was collected and used. The percentage yield of the extract was determined by W2/W1 x 100. Where W1 is the final weight of the extract after the extraction process and W1 is the initial weight. The resultant yield of extract was about 8.50g. 100 mg and 200 mg of Myristica fragrans stock solutions were prepared by dissolving 1g and 2g of the extract yield in 20 mL of distilled water.
Caffeine Solution Preparation
40 mg of caffeine solution was prepared by dissolving 0.4g of the powder in 20 mL of distilled water and then refrigerated until use.
Blood Collection and Sacrifice
Following the last administration (day 22), blood samples were collected through the intracardiac puncture method to assay for activity of LDH and cardiac troponin I level in the serum. Thereafter, animals were sacrificed via cervical dislocation. The heart tissues were fixed in 10% formol saline and processed for routine paraffin wax embedding and sectioning was at 5 microns before staining.
Body Weight Determination
Body weights of rats were done weekly with the aid of the weighing scale (Metler Toledo, P163)
Percentage weight change was calculated using:
Where W1= initial weight and W2 is the weight at sacrifice (Final weight)
The relative heart weight was estimated by the formula
Histology, Histochemical, and Histomorphometry
The excised cardiac tissues were fixed in 10% formal saline for 48 hours before being treated using the paraffin wax embedding procedure. Sections of 5 micron thickness were cut from paraffin-embedded tissues and stained in hematoxylin and eosin stains to show the overall histoarchitecture of the left ventricle. Scoring was based on the existence and severity of the following parameters: edema, leukocytic infiltration, muscular necrosis, and chronic inflammation. Each component was graded using a semi-quantitative scale, with 0 being normal and 1–7 representing mild to severe abnormalities9.10. The overall cardiac damage score for each heart was determined as the average of all component injury ratings.
Verhoeff – Van Gieson Stain
Slides were immersed in water for an hour before being stained in Verhoeff’s solution for an hour, resulting in tissue black staining. They were then washed in tap water with 2-3 changes and differentiated in a 2 percent ferric chloride solution for 1-2 minutes. As a result, slides were washed in tap water, then treated for a minute in a 5 percent sodium thiosulphate solution before being rinsed for 5 minutes in running tap water. Counterstained in Van Gieson’s solution for 3-5 minutes and immediately dehydrated via 95 percent alcohol for two changes of absolute alcohol, which was afterwards cleaned in two changes of xylene for three minutes each before being coverslipped with resinous mounting material.
Determination of Cardiac Troponin I level (cTnI) and Lactate Dehydrogenase (LDH) activities
Cardiac Troponin I Assay (cTnI) levels in serum were determined using a diagnostic kit and the technique described by Bishop et al.7 (Stanbio Laboratory, TX, USA). The rise in absorbance at 340 nm was measured spectrophotometrically to compute (cTnI) level as (U/L). LDH activity was measured using a Biogamma diagnostic kit (Rome, Italy). The rise in absorbance was measured spectrophotometrically at 340 nm at 1 minute intervals for 3 minutes. The total LDH activity in serum was estimated as (U/L)7.
Quantification of staining intensity
Image analysis and processing for Java (ImageJ) was utilized to assess and quantify Verhoeff- Van Gieson staining intensity. ImageJ was used to convert RGB pictures to grayscale ones. The program evaluates staining intensity by measuring the pixel value of each pixel in grayscale photographs following the threshold of areas of staining activity and converting the pixel value to brightness or gray value. The results were then statistically examined.
Photomicrography
The stained heart slices were examined using an Olympus binocular microscope connected to a 5.1-megapixel MV550 camera. This was linked to a PC running picture capture and analyzing software. The technology was tweaked to provide more clarity and resolution. Digital photomicrographs at (X400) magnification were saved.
Statistical Analysis
For data analysis, GraphPad Prism (Version 6.1; Graphpad Software Inc., San Diego, CA) was used. One-way ANOVA was used to analyze the data, followed by Student Newman-Keuls posthoc mean comparisons. Statistically significant differences were found at p< 0.05.
Results
Effect of caffeine and Myristica fragrans on body weight
Percentage weight change was significantly different (F = 3.256; p = 0.0373) in caffeine-only group (B) across all experimental groups. The weight loss induced by caffeine was significantly and dose-dependently reversed by Myristica fragrans administration, while the Myristica fragrans only group showed significantly increased (F = 3.256; p = 0.0373) weight gain as compared to the caffeine-only group.
Effect of caffeine and Myristica fragrans on the heart and relative heart weights body weight, cardiac Troponin I, and LDH activity
There were no significant differences in relative heart weight and cardiac Troponin I level across all experimental groups (F = 1.200; p = 0.3692) (Table 2) and (F= 0.4408, p = 0.7766) (Figure 2) respectively. Lactate dehydrogenase activity significantly increased in group B (F = 5.249; p = 0.0153) relative to control. (Fig. 3)
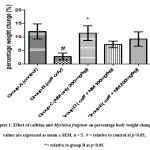 |
Figure 1: Effect of caffeine and Myristica fragrans on percentage body weight change. values are expressed as mean ± SEM. n = 5. # = relative to control at p<0.05; *= relative to group B at p<0.05. |
Table 2: The Effect of Caffeine and Myristica fragrans on Heart Weight and Relative Heart Weight.
| Groups | Absolute Heart Weight (g) | Relative Heart Weight (%) ± SEM |
| A- Distilled water (2mL/kg) | 0.83 ± 0.07 | 0.47 ± 0.03 |
| B- Caffeine only (40 mg/kg) | 0.72 ± 0.07 | 0.47 ± 0.03 |
| C- Myristica fragrans only (200 mg/kg) | 0.63 ± 0.16 | 0.47 ± 0.03 |
| D- Caffeine (40 mg/kg) + Myristica fragrans (100 mg/kg) | 0.62 ± 0.01 | 0.33 ± 0.03 |
| E- Caffeine (40 mg/kg)+ Myristica fragrans(200 mg/kg) | 0.72 ± 0.05 | 0.43 ± 0.03 |
Values are expressed as mean ± SEM.No significant difference was observed across the groups (p<0.05). n=5.
 |
Figure 2: Effect of caffeine and Myristica fragrans on cardiac troponin I concentration. Values are expressed as mean ± SEM. n = 5. No significant difference across groups at p<0.05. |
 |
Figure 3: Effect of caffeine and Myristica fragrans on serum LDH activity. Values are expressed as mean ± SEM. n = 5. # = relative to control at p<0.05; *= relative to group B at p<0.05. |
Histological findings
Assessment of the left ventricular histoarchitecture with the H and E showed regular arrangement with clear striations of myocardial fibres without histological alterations and centrally placed nuclei in the control slides. However, in the caffeine-only group (figure 4B), there was a deviation or derangement in the regular arrangement of the myofibrils, with disoriented nuclei and evidence of tissue necrosis. The histological presentations of figures 4D and E, the Myristica fragrans treated groups showed a gradual restoration of the normal myocardial histoarchitecture.
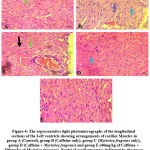 |
Figure 4: The representative light photomicrographs of the longitudinal sections of the Left ventricle showing arrangements of cardiac Muscles in group. |
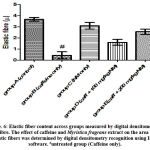
The Verhoeff- van-Gieson stain was used for the assessment of connective tissue fibres (elastic and collagen fibres). Elastic fibers (black) distribution (figure 5B) was reduced when compared to the control group (figure 5A) and 5C (Myristica fragrans only). In the Myristica fragrans treated groups (figures 5D and 5E), there was a significant improvement in the deposition of elastic fibers.
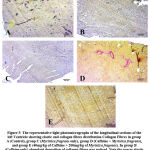 |
Figure 5: The representative light photomicrographs of the longitudinal sections of the left Ventricle showing elastic and collagen fibres distribution Collagen Fibres in group . |
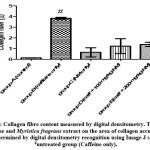
There was an increased distribution of collagen fibers in the left ventricle caffeine-only treated group (figure 5B) and group D administered 100 mg/kg of Myristica fragrans (figure 5D). Collagen and elastic fibres content measured by digital densitometry were shown as result of collagen and elastic fibres content in each specimen (figs. 6 and 7).
Discussion
Several studies have reported various effects of caffeine on the cardiovascular system, although there are inconsistencies in these findings. This study, based on these sought to investigate the role of Myristica fragrans on caffeine-induced cardiotoxicity in adult male Wistar rats.
Myristica fragrans has been shown to be preferred, helpful, and to have a wide range of pharmacological properties. Loizzo et al.11 previously demonstrated that Myristica fragrans has antioxidant, anticonvulsant, analgesic, anti-inflammatory, antidiabetic, hypolipidemic and hypocholesterolemic, antibacterial, and antifungal properties. In group B rats, there was a substantial decrease in percentage weight change.
The possible explanation for this could be due to the dosage of caffeine which is also in tandem with the study of Ogunwole et al.,12 who had earlier reported that rat birth weights were affected following administration of a high dose of caffeine13. The dose of caffeine used in their study was far lesser than in our study and this could be a pointer that weight-reducing consequences of caffeine could also be dose-dependent.
Other possible weight-reducing mechanisms of caffeine could be by its ability to increase the basal metabolism rate, increase thermogenesis, and its potentiality to inhibit cellular proliferation and differentiation of adipocytes through inhibition of adipogenic related factors14, 15. While Myristica fragrans stabilized weight in the groups co-administered with caffeine, this shows its potentiality as a weight-stabilizer and which could also be due to the myriads of phytochemicals present in this extract. While there was no significant variation in relative heart weight across groups.
LDH is a very stable enzyme that has been frequently utilized to assess the presence of tissue and cell damage and toxicity. It is an oxidoreductase that catalyzes the interconversion of pyruvate and lactate while also interconverting NADH and NAD+16. LDH has multiple isoforms; LDH1 is prominent in the heart, whereas LDH2 is present in the serum16. According to prior studies, the presence of LDH in the tissue indicates cardiac integrity, whereas its release indicates myocardial injury16. Experiments on rats revealed that an increase in blood LDH activity was associated with a decrease in cardiac muscle LDH17 activity.
The result obtained from the assay of plasma LDH activity in this study showed that the activity of LDH was significantly higher in the negative control group (B) as compared to the control (Fig 2). This is indicative of myocardial injury18. Cardiac troponin is a protein released from myocytes when myocardial damage occurs19 and is the biomarker of choice for the detection of cardiac injury. In this study, the level of cTnI was not significant across all groups.
The histology of myocardial tissues of the control and Myristica fragrans alone groups showed normal myocardial histoarchitecture with regular striations. However, the photomicrographs of the caffeine alone group showed that caffeine-induced both degenerative and inflammatory changes in the heart tissue manifested as small focal cardiomyocytes necrosis and infiltration with lymphocytes. The photomicrograph of group D and group E showed restoration of the myocardial histoarchitecture. This anti-inflammatory response may be attributed to the characteristic polyphenolic compounds present in Myristica fragrans, which have all been associated with various degrees of anti-inflammatory activities6. Verhoeff – van Gieson’s stained sections showed that caffeine significantly depleted elastic fibres deposition in the caffeine alone group relative to control. Intact elastic fibers are required for proper vascular mechanics to work, and their loss has been linked to the development of cardiovascular diseases20. According to Komolafe et al., sparsely distributed elastic fibers lower the tensile strength and elasticity of vascular systems, potentially leading to the start of cardiovascular problems21. Myristica fragrans, on the other hand, reduced the damage caused by caffeine by restoring elastic fiber deposition, as demonstrated in the Verhoeff – van Gieson’s micrographs of groups D and E.
Cardiac tissues are high collagen areas that have grown to replace the dead cells produced by tissue necrosis (figure 4B). Cardiac fibroblasts are primarily responsible for the creation of extracellular collagen I and III in the left ventricular myocardium. Cardiac fibrosis is pathophysiologically linked to hypertension and cardiac hypertrophy22, which leads in impaired left ventricular relaxation23. Myristica fragrans reduced tissue necrosis in groups D and E by restoring the antioxidant capabilities of the cells. This was consistent with Naglaa’s findings, who reported well on the antioxidant activity of Myristica fragrans6.
Conclusion
Caffeine administration resulted in alteration in cardiac histoarchitecture, elevation in markers of cardiac injury, connective tissue fibers disorientation, while 100 and 200mg/kg Myristica fragrans ameliorated these alterations. Myristica fragrans could serve as a drug lead in the management of cardiac-related conditions
Conflict of Interests
Authors have declared that no conflict of interests exists.
Funding Sources
There is no funding source.
References
- Cappelletti S, Piacentino D, Sani G, Aromatario M. Caffeine: cognitive and physical performance enhancer or psychoactive drug?. Current neuropharmacology,2015; 13(1):71-88
CrossRef - Rudolph T, Knudsen K. A case of fatal caffeine poisoning. Acta Anaesthesiol. Scand. 2010;54(4):521–523.
CrossRef - Klatsky A. L, Hasan A. S, Armstrong M. A, Udaltsova N, Morton C. Coffee, caffeine, and risk of hospitalization for arrhythmias. Perm. J. 2011;15(3):19–25.
CrossRef - Paşaoğlu H. The effect of caffeine on oxidative stress in liver and heart tissues of rats, TÜBİTAK, 2011; 41: 665-71
- Naren G, Jared B. Energy drinks: health risks and toxicity.The Medical Journal of Australia 2012; 196: 46-9
CrossRef - Naglaa H. M. Protective Effect of Myristica fragrans and Rosemary on Oxidative Stress in Hypercholesterolemic Rats. International Journal of Nutrition and Food Sciences. 2015; 4: 465-76
CrossRef - Olaleye M. T. Antioxidant properties of Myristica fragrans (Houtt) andits effect on selected organs of albino rats,African Journal of Biotechnology 2006; 5: 1274-8
- Dewi K, Widyarto B, Erawijantari P, Widowati W. In vitro study of Myristica fragrans seed (Nutmeg) ethanolic extract and quercetin compound as anti-inflammatory International Journal of Research in Medical Sciences2017; 3(9): 2303-2310.
CrossRef - Zhang J, Knapton A, Lipshultz S. E, Weaver J. L, Herman E. H. Isoproterenol-induced Cardiotoxicity in Sprague-Dawley Rats:Correlation of Reversible and Irreversible Myocardial Injury with Release of Cardiac Troponin T and Roles of iNOS in Myocardial Injury. Toxicologic Pathology 2008;36:277-88
CrossRef - Ali M. G, Abdel N. I, Badr A. A. Cardiotoxicity induced by cyclophosphamide in rats: Protective effect of curcumin. Journal of Research in Environmental Science and Toxicology 2013; 2: 87-95
- Abdel-Wahab B. A, Metwally M. E. Clozapine-induced cardiotoxicity in rats: Involvement of tumour necrosis factor alpha, NF-kβ and caspase-3Toxicology Reports 2014; 1: 1213-23
CrossRef - Loizzo M. R, Sicari V, Xiao J, Tundis R. Are Myristica fragrans (Myristicaceae) and Its Phytochemicals Useful for Human Health?. In: Mérillon JM., Ramawat K. (eds) Bioactive Molecules in Food. Reference Series in Phytochemistry. Springer, Cham. 2019; https://doi.org/10.1007/978-3-319-78030-6_23
CrossRef - Ogunwole E, Akindele O. O, Omobola F. O, Salami A, Raji Y. Effects of Oral Maternal Administration of Caffeine on Reproductive Functions of Male Offspring of Wistar Rats. Niger. J. Physiol. Sci.2015; 30:051 – 8
- Habeeb A. K, Abdullah S. A, Samia H. S, Syed S. H, Zohair A. A, Adnan A. K. Serum markers of tissue damage and oxidative stress in patients with acute myocardial infarction. Biomedical Research 2013; 24: 15-20
- Saka O. S, Komolafe O. A, Ogunlade O, Olayode A. A, Akinjisola A. A, Odukoya S. A. Biochemical Studies of Aqueous Extract of Garlic on th Myocardium of Left Ventricle of High Salt fed Adult Wistar Rats. Fiziologia-Physiology 2016; 26: 9-12
- Ayoola I. O, Komolafe O. A, Saka O. S, Odukoya S. A. Biochemical Effects of Aqueous Extract of Persea Americana (mill.) on the Myocardium of Left Ventricle of High Salt Fed Adult Wistar Rats. Journal of Evidence-Based Complementary & Alternative Medicine 2017; 22: 765-9
CrossRef - Mosaku J. T, Komolafe O. A, Owolabi A. R, Odukoya S. A, Saka O. S, Anjorin O. A. Effects of Methanolic Extract of Buchholzia Coriacea (Buchholz) Seed on the Myocardium of Left Ventricle of Zidovudine-induced Cardiotoxic Wistar Rats. European journal of anatomy 2020; 24: 193-203
- Hiroshi W. Role of Elastic Fibers on Cardiovascular Disease. Journal of Health Science. 2011; 57: 449-57.
CrossRef - Komolafe O. A, Adeyemi D. O, Adewole O. S, Obuotor E. M, Abiodun A. A. Morphological and morphometric studies of the aorta, pulmonary trunk, and heart of streptozotocin-induced diabetic Wistar rats. Folia Morphologica. 2009; 68: 207-14.
- Ichihara S, Noda A, Nagata K, Obata K, Xu J, Ichihara. Pravastatin increases survival and suppresses an increase in myocardial matrix metallo-proteinase activity in rat model of heart failure. Cardiovascular Research 2006; 726-35.
CrossRef - Cingolani O. H, Yang X. P, Cavasin M. A, Carretero O. A. Increased systolic performance with diastolic dysfunction in adult spontaneously hypertensive rats. Hypertension 2003; 41:249-54.
CrossRef - Zheng G, Sayam K, Okubo T, Juneja L. R, Oguni I. Anti-obesity effects of three major components of green tea, catechins, caffeine and theanine, in mice. In Vivo 2004; 18: 55-62
- Heckman M. A, Weil J, Gonzalez de Mejia E. Caffeine (1,3,7-trimethylxanthine) in foods: a comprehensive review on consumption, functionality, safety and regulatory matters. J Food Sci 2010; 75: R77-R87.
CrossRef