Manuscript accepted on :29-06-2022
Published online on: 30-06-2022
Plagiarism Check: Yes
Reviewed by: Dr. Niharika Kondepudi
Second Review by: Dr. Pankaj Singh
Final Approval by: Dr. H Fai Poon
Abdel-Nasser El-Shorbagi1* , Sachin Chaudhary1
, Sachin Chaudhary1 , Anurag Chaudhary2
, Anurag Chaudhary2 , Garima Agarwal2
, Garima Agarwal2 , Prabhash Nath Tripathi2
, Prabhash Nath Tripathi2 , Shweta Dumoga2
, Shweta Dumoga2 , Alaa Ali Aljarad1
, Alaa Ali Aljarad1 , Esraa Omer1
, Esraa Omer1 , Fatma Mahmoud1, Ramesh Kumar Gupta3
, Fatma Mahmoud1, Ramesh Kumar Gupta3 and Mahmoud Hamed Mohamed4
and Mahmoud Hamed Mohamed4
1Department of Medicinal Chemistry, College of Pharmacy, University of Sharjah, Sharjah, United Arab Emirates.
2Department of Pharmaceutical Technology, Meerut Institute of Engineering and Technology, NH-58, Baghpat Road Crossing, Bypass Road, Meerut, India.
3Department of Pharmacology, Hygia Institute of Pharmaceutical Education and Research Lucknow, Uttar Pradesh, India.
4Department of Pharmacognosy, College of Clinical Pharmacy, Albaha University, Saudi Arabia.
Corresponding Author E-mail: aelshorbagi@sharjah.ac.ae
DOI : https://dx.doi.org/10.13005/bpj/2398
Abstract
Aquatic environment is one of the important sources of active agents that own diverse biological properties. Metabolites from these sources are considered as alternate source to meet the mandate for effective medicines. Despite notable developments in cancer managing and/or treatment in the past years, there remains a vital requirement for innovative agents and/or innovating approaches to treat resistant and solid tumours. However, in the recent era there are new technological innovations in the elucidation of the structures, the semi-synthetic and synthetic approaches of the new antineoplastic compounds. Biological assays enable isolation and clinical evaluation of numerous scaffolds from the marine environment. This review gives a general summary of some anti-cancer agents with a brief description of their mechanisms of action. It sheds a view to the approved drugs, the potent scaffolds that newly modulated as antibody-drug conjugates, and the drug-candidates under clinical phases (I-III) with their status.
Keywords
Antibody Drug Conjugate; Anticancer Agents; Clinical Phase; Marine Drugs; Metabolites
Download this article as:| Copy the following to cite this article: El-Shorbagi A. N, Chaudhary S, Chaudhary A, Agarwal A, Tripathi P. N, Dumoga S, Aljarad A. A, Omer E, Mahmoud F, Gupta R. K, Mohamed M. H. Marine Antineoplastic Templates: Clinical trials (I-III) and Motifs Carried via Antibodies to Target Specific Cancerous Tissues. Biomed Pharmacol J 2022;15(2). |
| Copy the following to cite this URL: El-Shorbagi A. N, Chaudhary S, Chaudhary A, Agarwal A, Tripathi P. N, Dumoga S, Aljarad A. A, Omer E, Mahmoud F, Gupta R. K, Mohamed M. H. Marine Antineoplastic Templates: Clinical trials (I-III) and Motifs Carried via Antibodies to Target Specific Cancerous Tissues. Biomed Pharmacol J 2022;15(2). Available from: https://bit.ly/3uACtuT |
Introduction
Cancer conclusively, is the disease resulted as a reflection of cell’s overgrowth and uncontrollable proliferation. It is among the pre-eminent cause of morbidity and mortality in the world.1-2 Cancer has risk factors that predispose patients to it and these factors could be either internal or external. Internal factors are those related to patient immunity or genetic mutations. External factors include aging, obesity, smoking, lack of physical activity and exercise and exposure to infecting substances or hazardous substances.4 Cancer can be more complicated when cancer-cells start invading to the surrounding local environment in the body by metastasis that is responsible for 90% of deaths in cancer patients.3-5
Cancers can be detected early or later and sometimes accidentally with patients being treated or diagnosed for other types of diseases. 6-8 The diagnosis of cancer can be carried out based on the symptoms, tumour markers or histopathological tests that can be combined with other approaches like imaging techniques.9
Cancer management may be approached by one of the three methods such as surgery, radiation and chemotherapy or a combination of two of them.10 The problems associated with these approaches include the side effects that will affect the normal cells and lead to a decreased patients life quality.11 Other problem is that the treatment may not be useful as a result of tumour metastasis or drug resistance.12
Cancer is still a major public health problem that requires establishment of new strategies to discover new drugs that are able to fight it.1 The drugs obtained from natural origin can be formulated in three structural forms: original, semisynthetic or analogue structures. Recently, the interest has been driven towards marine as a source of natural products mainly due to the structural diversity and availability of multiple scaffolds that can serve in the field of therapeutics as lead compounds, in particular, secondary metabolites that are produced by different aquatic organisms.1,13
Metabolites produced during the life of organisms are assumed as a defensive mechanism for survival and adaptation with life surroundings. Beside the so many scaffolds reported for anticancer activity, marine-secondary metabolites representing an important addition. In this review, the light is focused on the diverse secondary metabolites, and it will provide an explanation of their value as anticancer compounds. Previously, we reported several frames including natural, semisynthetic, and synthetic anticancer agents.14-20 This review is a valuable contribution to look for other structural templates of promising new antineoplastic agents to treat resistant and solid cancers.21-27
Aquatic Sources as Anticancer Agent
Thousands of secondary metabolites had been isolated from different sea creatures such as plants, macroalgae (seaweed), microalgae, bacteria, actinomycetes, fungi, sponges and soft corals all are viewed in the area of medicinal field.4 Aquatic plants live in a harsh climate. One of the most recognized plants providing anticancer agents is mangrove that produces bioactive compounds and secondary metabolites like hormones and anticancer agents.28 Table 1, illustrated the summary of approved anticancer agents and drugs under clinical trials.
Table 1: Summary of approved anticancer agents and drugs under clinical trials.
| Compound | Approval year or clinical trial phase | Chemical class | Marine origin | Cancer type targeted | Mechanism of action |
| Cytarabine | Approved by FDA in 1969 | Nucleoside | Sponge | Leukemia | Anti-metabolite |
| Trabectedin | Approved by EMA and FDA in 2007 and 2015 respectively | alkaloid | Tunicate | Ovarian cancer | Growth inhibition |
| Eribulin mesylate | Approved by FDA in 2016 | Macrocyclic ketone | Sponge | Lymphoma, breast, and pancreatic cancers | Antimitotic |
| Brentuximab Vedotin | Approved by FDA and EMA in 2011 | Antibody-drug conjugate (ADC) | Mollusk | Hodgkin and large cell lymphoma | CD30 directed ADC |
| Bryostatin 1 | Phase I clinical trials | Macro-cyclic lactone | Bryozoa, Sponge, Tunicate | Lymphoma | PCK inhibitor |
| Hemiasterlin (E7974) and Taltobulin (HTI-286) | Phase I clinical trials | Tripeptide | Sponge | Colon, breast, ovary, and lung cancers | Antimitotic
(Micro-tubules de-polymer-isation) |
| Pinatuzumab Vedotin | Phase I clinical trials | ADC | Mollusk, cyano-bacteria | Non-Hodgkin lymphoma and chronic lymphocytic leukemia | Microtubules de-polymerisation |
| Tisotumab Vedotin | Phase I clinical trials | ADC | Mollusk, cyano-bacteria | Prostate, pancreatic, and cervical cancers | Microtubules de-polymerisation |
| LAF389 | Phase I clinical trials | Peptide | Sponge | Various types of cancer | Methionine aminopeptidase inhibitor |
| Tasidotin | Phase II clinical trials | Peptide | Cyano-bacteria | Various types of cancer | Micro-tubules depolymerisation |
| Glembatumumab Vedotin | Phase II clinical trials | ADC | Mollusk | Melanoma, osteosarcoma, and breast cancer | Inhibition of transmembrane GPNMB |
| Discodermolide | Phase I/II clinical trials | Polyketide | Sponge | Breast, ovarian and lung cancers | Microtubules polymerisation |
| Soblidotin | Phase I/II clinical trials | Peptide | Bacteria | Advanced and metastatic non-small cell lung cancer | Microtubules depolymerisation |
| Plitidepsin | Phase III clinical trials | Depsi-peptide | Tunicate | Various types of cancer | Cell cycle arrest and induction of apoptosis |
| Gemcitabine | Phase III clinical trials | nucleoside | Sponge | Various types of cancer | Antimetabolite |
Aquatic Bacterial Metabolites
Pseudomonas provided metabolites that are active against microbes while others bearing cytotoxic activity e.g. dibutyl-phthalate and di-(2-ethylhexyl)phthalate (compound 1, 2, Table 2) are reported as Cathepsin B inhibitors.29-30 The other types of aquatic bacterial-metabolites from Lactobacilli and Noctiluca scintillans represented anti-proliferative effects toward colon and melanoma cancer.31-33
 |
Table 2: The historical sea creature’s metabolites of potent anticancer agents. |
Gram-Positive Mycelial Bacteria
Actinomycetes produce metabolites to highest extent that is why they account about 45% of total marine metabolites. The highly potent thicoraline and BE-22179 (Compounds 3, 4, Table 2), representing a large 26-membered polypeptide ring with SS-bond and hydroxyl-quinolone moieties, and enterochelin (Compound 5, Table 2) as three 2,3-dihydroxybenzamides in 12-memered cyclic structure,34 isolated from actinomycin called Micromonospora marina. They are potent cytotoxic compounds that are effective against colon cancer.
Sponges Provided Pentacyclic-22-Membered-Ring Scaffold
These are of high contribution by ~ 30% of all-natural products of marine origin and of important biological activities.35 The moderately active cytotoxins spongo-thymidine and spongo-uridine were isolated from Caribbean sponge tethya crypta.4 Eribulin (Compound 6, Table 3) which is a semisynthetic analogue of halichondrin B (Compound 7, Table 2) of IC50 = 7 uM had been isolated from sponge Halichondria okadai. Compound 6; a perhydro-pentacyclic-structure in a 22-membered-ring in the smaller frame of macrocycle. It provided potent IC50 value of 0.27 nM, was developed as mesylate (methansulfonate) salt. It stopped and emphatically hindered the development rate of microtubules.36-39 had been obtained from sponge Halichondria okadai. It also, provided anticancer activity against breast cancer.39 Eribulin has antimitotic and non-mitotic effects. It hinders microtubule polymerization interfering with microtubule-dynamic-stability as an antimitotic. While the non-mitotic action on tumour biology have moreover been built up. It counts for tumour vasculature remodelling, expanded vascular perfusion, decreased hypoxia, and phenotypic changes.40 Tubulin displayed antiproliferative action against many diverse human cancer cell lines. Eribulin decreased both the rate and degree of tubulin polymerization, also it affects more than one stage in cell-cycle like G2 and M in lymphoma and pancreatic cancer cells and also it leads to initiation to apoptosis after 8-10 hrs.40-41 Eribulin has high affinity to microtubules. In non-mitotic mechanism Eribulin has three different mechanisms, one is through its impact on tumor vascular remodelling and perfusion, another is through its effects on EMT (epithelial mesenchymal transition) process, and finally its effects on the relocation, assault and metamorphosis of cancer cells.42
Fungal Sources Provided Indole-Bearing Pentacyclic Angular Scaffold
Some of fungi secondary metabolites have antioxidant activity toward free radical. Fungal xanthone derivatives produced by several numbers of plant families and fungi, Penicillium raistrickii, many Phomopsis spp. Alkaloids separated from Penicillium spp., found in deep ocean showed antitumor activity. These alkaloids are meleagrin analogues, meleagrin D (Compound 8, Table 2) and diketo-piperazine, roquefortine (Compound 9, Table 2) induced HL-60 cell death or detained the cellular process through G2/M phase, and bearing mild cytotoxic activity (against A-549, IC50 of 32.2 and 55.9 µM, respectively.4, 43.
Soft Corals Provided 14-Membered-Carbocyclic Scaffold
Sarcophyton is widely spread in tropical and sub-tropical oceans. One of the important metabolites produced by soft coral is cembranoids like cembranoid diterpine (Compound 10, Table 2). Cembranoids have a verity of activities, like cytotoxic and anti-inflammatory. It is reported that furano-cembranoids from Nephthea spp, and Sarcophyton cherbonnieri are effective against breast cancer and liver cancer.1, 4 The secondary metabolites are structurally variable and possessing valuable biological activities.44 The utmost familiar domains are carbohydrates (glycosides, polysaccharides), peptides, alkaloids, polyketides, terpenes, and polyphenols.1, 4
The followings are brief explanations of each class:
Polyketides Scaffolds are large groups of secondary metabolites that contain alternating carbonyl and methylene groups, they include macrolides, polyethers, polyols and aromatics.1
Polyphenols scaffolds, also named polyhydroxyphenols.45 are natural products that have phenolic skeleton and they are divided into subgroups according to either their structure, function or origin. These subgroups are: phenolic acids, poly phenolic flavonoids, tannins, catechin, anthocyanidins, epigallocatechin, lignin, epigallocatechin gallate (Compound 11, Table 2), 46 and gallic acid (Compound 12, Table 2).47-49
Terpenes scaffolds are secondary metabolites of isoprene units. Terpenes of different scaffolds of different numbers of carbons as C-10, C-15, C-20, C-25, C-30 and C-4058 have been reported as more powerful antibacterial than being cytoxic.59-60
Alkaloids from sea sources as nitrogen-containing natural scaffolds are many subclasses such as indole, pyrrole, pyridoacrine, isoquinoline, guanidine or amino-imidazole.58-60
Peptides Scaffolds having many physiological effects in the cells obtained by extraction from sea sources. Peptides are isolated by enzymatic hydrolysis of organism-proteins.61
Carbohydrates (CH2O)n of sea sources are involved in glycoproteins, lipids and with RNA and DNA. Mono-saccharides, di-saccharides and poly-saccharides were identified.47 The most common class of carbohydrates are the polysaccharides. Some of the polysaccharides have potent anticancer effects via interfering with DNA synthesis, induction of apoptosis and other mechanisms of actions.1, 47, 61
All of the aforementioned bioactive classes had been obtained from different sea-sources like: sponges, molluscs, tunicates, corals, ascidians, bacteria, fungi, sea weed. 1, 48 They are active against many types of cancer and exhibited anti-cancer activity via cell growth inhibition, disruption of the mitotic spindle, induction of apoptosis and inhibition of invasion or metastasis.1
Approved Anticancer Marine Drugs
Secondary metabolites were studied broadly throughout the last 30 years for their biological activities.62 Cytarabine, brentuximab vedotin, eribulin mesylate, and trabectedin are some of the approved anticancer agents.
The followings are the approved anticancer agents.
Cytarabine
Food and drug administration (FDA) approved cytarabine in 1969. It is now a synthetic nucleoside anticancer drug that has been originally obtained from sea Cryptotheca crypta sponge.1 There are other trivial names for cytarabine including chemical name, Ara-C and cytosine arabinoside.63 Cytarabine (Compound 16 , Table 3) acts in phase S-specific manner to induce cytotoxicity and kill cancer cells through inhibition of DNA polymerase and being incorporated into DNA instead of the normal DNA building blocks.63 Cytarabine is indicated for the treatment of leukaemia, 1, 63–64 described by the reproduction of immature leukemic blasts and their invasion to the bone marrow. It can be caused by intrinsic (genetic) or extrinsic (inflammation and release of cytokines and chemokines) factors.66 It is administrated by intravenous infusion, intramuscular, intrathecal or subcutaneous injections.67 Biologically it must be activated by phosphorylation when it is up-taken by the cells to the active form cytarabine-triphosphate to be able to induce its effect.63 It is metabolized by being deactivated via deamination into the inactive and non-toxic form uracil-arabinoside and this metabolism decreases its activity 63-64.
One approach used to solve the issue of deamination and inactivation of cytarabine was to co-administer it with tetrahydro-uridine which is a potent inhibitor of cytidine deaminase so the antitumor activity of cytarabine will increase.64
Because of its short half-life it is advised to overcome this problem via prolonged infusion of cytarabine in a high-dose therapy which will lead to increased levels in the cerebrospinal fluid where cytidine enzyme is absent and the drug can remain active to induce its effect.66-67
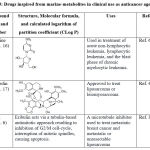 |
Table 3: Drugs inspired from marine-metabolites in clinical use as anticancer agents. |
Targeting of folate receptor by designing the prodrug of cytarabine solved the problem of its high hydrophilicity. This prodrug was assembled (Figure 1) to form spherical nano-assemblies (bare NAs) that adsorb folic acid-bovine serum albumin conjugate and (NAs/FA-BSA) form folate receptor targeting molecule. The conjugate concentrates in cancer cells that have increased expression of folate receptor therefore the antitumor activity of cytarabine will increase.68
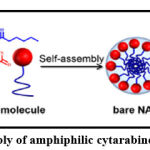 |
Figure 1: Nano-assembly of amphiphilic cytarabine prodrug molecules. |
Trabectedin
Trabectedin (Compound 17, Table 3) is an alkaloid from Ecteinascidia turbinate. It was approved by European medicines agency (EMA) in 2007 as a single medication for the treatment of rare solid tumours of mesenchymal cells.65 It is the foremost marketed natural medicine for relapsed ovarian cancer. Recently, trabectedin was accepted by FDA in 2015.69
The results of ten years of use have shown a suitable toxicity profile, without evidence of accumulative side effects. However, because of an intensive hepatic metabolism liver dysfunction, predominantly chartered by increased transaminase levels, was reported as frequent side effect.66-72
Trabectedin collaborate with DNA double helix, the structure of the compound triggers a cascade of occasions that restricted with a few translation components. It also causes inflection of cytokines and chemokines by typical cells.73
The preclinical studies on combination of trabectedin with other anticancer drugs have been currently progressing globally and one of the combinations including (trabectedin-pegylated-liposomal doxorubicin) was authorized by the European commission for treating relapsed platinum-sensitive ovarian cancer.73, 4
Antibody drug conjugates (ADC)
Brentuximab Vedotin (Adcetris™)
Brentuximab Vedotin (Compound 18, Figure 2) is one of the recent medications successfully endorsed by FDA in 2011 and received a conditional approval from EMA in 2012 for Hodgkin and non-Hodgkin lymphoma treatment. Brentuximab vedotin represents the novel strategy of targeting drugs. It is CD-30 directed antibody-drug conjugate (ADC) containing three major fragments: a chimeric human-murine IgG1 that selectively targets CD30, monomethyl auristatin E; MMAE (Compound 15, Table 2), related to dolastatins such as dolastatin 10 and 15 (Compounds 13 and 14, Table 2).74-75 MMAE is a fully synthetic analogue of dolastatin 10, isolated Dolabella auricularia. Later scientists isolated dolastatins from cyanobacteria Symploca hydnoides and Lyngbya majuscule which are a part of the sea hare’s diet. To date, dolastatin 10 is the most powerful antineoplastic substances known, with an ED50 in the picomolar range against various cancer-cell lines. Dolastatins in–vivo activity is not adequate for direct application as antineoplastic drug. Phase I and phase II clinical trials of dolastatin 10 and the water-soluble analogue auristatin PE results indicated that they were ineffective due to lack of efficacy and existence of side effects. In this strategy MMAE is linked to a protein that targets CD30 (a cell membrane protein sitting on the surface of Hodgkin’s lymphoma cells). This linkage has resulted in highly effective and well tolerated agent, Brentuximab-vedotin. It took about 40 years from the initial bioactive extract to the approved drug.76-77
Pinatuzumab Vedotin
Pinatuzumab Vedotin (Compound 19, Figure 2) is another ADC is aimed to act against surface antigens associated with cancer. It is consisting of an anti-microtubule MMAE attached to another protein anti-CD22 antibody through peptide linker that can be cleaved by protease enzyme. CD22 is one of the immunoglobulin superfamily, restricted to and expressed by mature B-lymphocytes. CD22 has a critical role in regulation of B-lymphocytes mediated signalling and it also regulates the function and survival of B-lymphocytes as well as apoptosis.74-78 The mechanism of action is when Pinatuzumab Vedotin binds to CD22, it gets into the cell then it will start releasing its cytotoxic agent MMAE that will act against microtubules in a way similar to vincristine mechanism (microtubules destabilisation).74-78 Phase I trials suggested that PV can be given alone or in combination with rituxiamb to treat refractory NHL or CLL.75-78
The advantages of using Pinatuzumab Vedotin include site specific drug delivery, increased efficacy and reduced systemic side effects. Subsequently, phase II trials started by i.v. dosage of 1.8 mg/kg, body weight or more given in a cycle of 21 days.75-79 The toxicities that were recorded with Pinatuzumab Vedotin include non-hematological toxicity (nausea, diarrhea, fatigu), grade-3 neutropenia, thrombocytopenia and peripheral neuropathy. Most side effects can be reduced by extending the cycle length to 28 days and giving growth factors that allow neutrophils to become healthy and return to the normal count more quickly 75-80.
Glembatumumab Vedotin
Glembatumumab Vedotin (Compound 20, Figure 2) is right now in clinical trial phase-2 for treating osteosarcoma, melanoma, and breast cancer. It is an antibody-drug conjugate (ADC) that targets glycoprotein GPNMB (Glycoprotein Nonmetastatic Melanoma Protein B). GPNMB is involved in multiple functions, examples are its anti-inflammatory effect, role in mineralization of bone and differentiation of osteoblasts. It also functions in cellular adhesion and gets localized exactly in plasma membrane81-82.
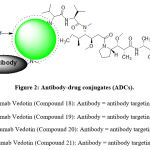 |
Figure 2: Antibody-drug conjugates (ADCs). |
GPNMB is highly expressed in a very wide group of cancers including glioblastoma, astrocytoma, breast cancer, gastric cancer, and lung cancer. GPNMB is a glycoprotein comprises a huge extracellular space with cytoplasmic tail composed of short 53 amino acids. GPNMB expression is regulated by various cytokines, growth factors, microRNAs (miRNAs) and by protein stabilization. GPNMB is involved with varied patterns in a wide range of tissues including bones, skin, liver, and immune system.83-87 The role of GPNMB in tumour formation is in melanoma, GPNMB is present in both cancer cells and normal cells but its percentage in cancer cell is higher (60%-80%) while in normal cell is (50%). For growth and metastasise, melanoma needs GPNMB, a specific phonotype of GPNMB is needed as knockdown the glycoprotein in some immune-competent mice that have melanoma studies found that no change in their growth, compared to culturing of t-cells with GPNMB. Knockdown causes the T-cell ability of functioning is impaired, it affects the production of inflammatory markers like IL-2 and IFN-γ and lose of ability to detect and kill the target cell. Glembatumumab Vedotin is a monoclonal antibody that mainly targeting GPNMB, in a recent study, the activity of Glembatumumab Vedotin was tested in number of osteosarcomas as a result, 85% of the cells responded to the drug. As shown in (Figure 2) GPNMB has multiple critical functions in cancer cells formation and if inhibited by Glembatumumab Vedotin, cancer can be treated.88-90
Tisotumab Vedotin
Tisotumab Vedotin (Compound 21, Figure 2) is also an ADC that can be isolated from marine mollusks and cyanobacteria. It is composed of an anti-microtubule MMAE linked to a monoclonal antibody specific for tissue factor (TF) through a cleavable linker 74. TF is highly expressed in many cancer types like prostate, pancreatic, lung, cervical, and breast cancers which are associated with metastasis and since it is a cause of poor prognosis of the disease, TF is considered an attractive target for anticancer agents. TF is also called thromboplastin or factor III and it is mainly expressed in three types of cells which are: perivascular cells, fibroblasts and the cells of the smooth muscles in the sub-endothelial walls of blood vessels and normally it plays a role in the extrinsic pathway of coagulation of blood through activation of factor VII.72- 91
In cancer, TF is implicated in angiogenesis (new blood vessels formation), tumor migration and advancement. The way which Tisotumab Vedotin acts by is through binding to TF and incorporation into the cell to release MMAE that then will induce its direct microtubule disrupting effect to kill the cell and its neighbouring cells through a bystander killing effect. Tisotumab Vedotin is currently under phase I clinical trials in a hope to treat different types of cancer like metastatic or relapsed cervical cancer. Cervical cancer is usually treated with first-line therapy consisting of bevacizumab along with paclitaxel and cisplatin or paclitaxel and topotecan but the problem associated with first-line therapy is the low survival and high relapse rates risk so there is a need for out of danger and powerful agent that can enhance the clinical outcomes of treatment.91
Tisotumab Vedotin was given as i.v. dosage of 2 mg/kg, body weight in a cycle of 3 weeks (to allow normalisation of neutrophils count) and it exhibited a promising antitumor effect in solid tumors especially in case of metastatic or relapsed cervical cancer 91-92. The most reported side effects were nausea, vomiting, weakness, anaemia, peripheral neuropathy, conjunctivitis, and bleeding from the nose.91-93
Anticancer Agents in Clinical Trial Phase 1
Phase I clinical trials can be divided into phase I(A) and phase I(B) trials. Dose escalation administration where a single administration of the doses in phase I(A), and multiple or daily administration in phase I(B) were done.94-95
Bryostatin 1 (Compound 22)
It is a macrocyclic lactone that is derived from marine bryozoan Bugular neritina and it can also be isolated from other organisms like sponges and tunicates. Bryostatin 1 is in clinical studies (phase I), to determine its activity against different types of cancer and it showed positive results in the management of ovarian cancer, breast cancer, lymphoma 97 and malignant melanoma.96
There are 20 different bryostatins that have a general molecular formula of C47H68O17 61, 96, 98. The mechanism of antineoplastic effect of bryostatin 1 was through binding to protein kinase C enzyme (PKC) and alteration of cellular activity. PKC is a family of serine/threonine specific kinases that have a major role in cell growth and death regulation 99-100. PKC is activated by calcium or diacyl-glycerol. PKC has a carboxyl terminal catalytic domain and an amino terminal regulatory domain with ATP and phorbol (Compound 23) binding sites respectively. Both of pryostatin 1 and phorbol ester bind to the same binding site in the regulatory domain but the difference is when bryostatin 1 binds, it induces antineoplastic effect while phorbol ester induces effect that promotes tumor formation.96-101 The cells exposed to bryostatin 1 for short period, PKC was activated resulting in three effects which are proliferation suppression, differentiation induction and promotion of apoptosis in cancer cells. Longer term exposure led to down-regulation of PKC and inhibition of its activity. Bcl-2 is implicated in human diffuse large cell lymphoma and its inhibition by bryostatin 1 has a beneficial effect in treatment.99-102
The Phase I trials suggested bryostatin 1 as a single agent will not have encouraging effect while it will have better effect when given in combination with other agents like cytarabine or paclitaxel for treating multiple cancer types. In clinical trial phase I, Bryostatin 1 is currently being tested against other diseases like HIV and Alzheimer’s disease, its side effects include lethargy, vomiting, and muscular pain.103 One of the disadvantage of bryostatin 1, is that it is only produced and obtained in small quantities from its marine source and this problem can be resolved using bacterial gene expression to produce more bryostatin 1 that can be applied in biomedical and biotechnological procedures.96-99
In phase II the efficacy and toxicity of bryostatin 1 were assessed for patients with advanced colorectal cancer who have had previous 5-fluorouracil therapy. The primary end point was tumor response. All 25 patients had disease progression within four cycles. Myalgia was the most common toxicity. Myelo-suppression was not seen. The compound as a weekly 24-hour continuous infusion lacks single-agent antitumor activity in advanced colorectal cancer.104
Hemiasterlin and Taltobulin
Hemiasterlin; E7974 (Compound 24, Table 4) and Taltobulin; HTI-286 (Compound 25, Table 4) (a synthetic analogue) are tripeptides isolated from different marine sponges like Hemiasterella minor, Siphonochalina, Cymbastela or Auletta species. E7974 and HTI-286 tested in phase I clinical trials to treat tumors that express high level of p-glycoproteins and become resistant to Paclitaxel and Vincristine. Hemiasterlin works by inducing an antimitotic action that will result in depolymerisation of microtubules and cell cycle arrest at G2/M phase.74 The in-vitro and in-vivo assessments of HTI-286 were compared with vincristine and paclitaxel and found more potent in cell growth inhibition, proliferation suppression and apoptosis induction. It was not affected by p-glycoprotein and drug efflux pump overexpression especially in case of colon cancer. It also showed a tumor suppression effect in other types of tumor cell lines like malignancy, lung, ovary, and breast cancer.105
Microtubules are important components of the cell cytoskeleton, and they have an important key aspect in maintaining the shape of the cellular components, internal movement, and cellular division through polymerisation and depolymerisation. They have their role in the formation of the mitotic spindle 106-107. Microtubules are consisting of subunits called alpha-tubulin and beta-tubulin, microtubules form cylindrical tubes.74, 105-107
 |
Table 4: The candidates of clinical trials as potent anticancer agents. |
LAF389
LAF389 (Compound 26, Table 4) is a synthetic bengamide B analogue. LAF389 in phase I clinical trial was discontinued because it exhibited cardiotoxicity. Bengamide B isolated from Jaspidae sponges 110. LAF389 possess both anti-proliferative and anti-angiogenetic properties.108-110 In addition to preclinical investigations that offer a broad antitumor activity. It is characterized as potent methionine amino-peptidase (MetAP) inhibitor, which inhibits both MetAp1 and 2 directly or indirectly. Inhibition of MetAP will cause cell cycle arrest as MetAP is responsible for DNA repair process, cell transformation, and trade in secretory vesicles and infection.111-113
Metabolites of Clinical Trial Phase 2
The studies of clinical trial phase II are performed on a group of few hundred patients and designed to determine the optimum dose of a potential drug that will induce therapeutic effect. The examples of some aquatic natural products that are in phase II clinical trials have been mentioned as below.
Tasidotin
Dolastatins are group of linear peptides originally obtained from hare Dolabella auricularia formed by cyanobacteria. Tasidotin and its C-carboxylate (Compounds 27, 28, Table 4) are synthetic third generation dolastatin-15 analogue (Compound 15). One of the major Tasidotin metabolites is C-carboxylate (Compound 28) modifies the dynamic stability of microtubules in a comparable way to Tasidotin but was 10 to 30 times more stronger 117. The hydrolytic product of Tasidotin has activity on polymerization however, it appears to give less cytotoxic activity.114-118
Anticancer Metabolites in Clinical Trial Phase 1/2
Discodermolide
Discodermolide (Compound 29, Table 4) is a polyketide obtained from sponge Discodermia dissolute. It undergoes phase I/II trials and has showed a good antitumor activity and in the treatment of several cancers like those affecting breast, ovaries, and lungs. Before being known to have a cytotoxic effect, Discodermolide was considered as an immunosuppressant as was shown during in-vitro and in-vivo testing. Discodermolide is a microtubule-stabilizing agent that promotes microtubule polymerization and prevents depolymerization resulting in cell cycle arrest at G2-M phase and inhibition of mitosis, therefore it acts in a way like that of paclitaxel or even more potent.119-120
So, it is useful as a synergistic agent as well as an alternative option to Paclitaxel for tumors that have mutated beta-tubulin and became paclitaxel-resistant as well as other multidrug resistant (MDR) tumors. The information about structure-activity-relationship (SAR) for semi-synthetic approaches to improve pharmacokinetics (PK) properties of discodermolide-analogues were reported. 74,119
Soblidotin
Soblidotin; TZT-1027 (Compound 30, Table 4) is a sea-derived peptide of an aquatic origin isolated from marine bacterium Salinispora tropica, it is in phase I and II clinical trials. Soblidotin is a newly developed dolastatin 10 analogue that has multiple activities.4, 120-121 The main difference between soblidotin and the parent drug dolastatin is the terminal dolaphenine amino-acid residue in dolastatin that is replaced in soblidotin by phenylamine group, however, the two compounds have similar activity inhibiter of polymerization of tubulin via attaching to vinca peptide site. In 2002, Soblidotin started its phase I clinical trial, and is currently in three different phases I, II and III in many companies. Soblidotin tested against lung cancer with a mechanism of tubulin inhibition and vascular disruption.121 Some researchers have found that it inhibits microtubule polymerization, hinder the microtubule assembly/disassembly balance via interaction with tubulin. It also has anti-vascular action that destroyed the new tumor vasculature. It stops the cell cycle at two phases G2 and throughout to Bcl-2 phosphorylation followed by activation of caspase-3 pathway that led to apoptosis.120-122
Anticancer Compounds in Clinical Phase III Trials
Phase III trials representing the full-scale assessment of treatment.
Plitidepsin
Plitidepsin (Compound 31, Table 4), a natural marine cyclic depsipeptide called as (Aplidin®). However, it is accessible now by chemical synthesis.74 It was fundamentally sequestered from a mediterranean tunicate Aplidium albicans. It is useful for treating breast and lung cancers and is in clinical trial phase III studies.127 Furthermore, clinical trial (Phase I and II) studies of plitidepsin showed brilliant anti-tumor property in patients with multiple myeloma, advanced medullary thyroid carcinoma, non-Hodgkin’s lymphoma, advanced melanoma and urothelium carcinoma.128
Plitidepsin aggravate cell cycle arrest dose-dependently and results to apoptosis in cultured cells obtained from solid tumors 127. These outcomes are linked to the provocation of early oxidative stress by stimulation of Rac1 GTPase and the inhibition of protein phosphatases which in concurrence cause the prolonged activation of c-Jun N-terminal kinase (JNK) and p38 MAPK.74
Gemcitabine
Gemcitabine (Compound 32, Table 4) is an anticancer nucleoside (fluorinated derivative of cytarabine) metabolic inhibitor which is a synthetic version of pyrimidine nucleoside analog acting as a prodrug in which the atoms on the 2′ carbon of deoxycytidine is substituted by fluorine atoms. Gemcitabine is presently in phase II & III clinical studies.129 It has been used for the management of numerous carcinomas of pancreas, bladder, breast, and lung cancer (non-small cell). It is used as a primary treatment for pancreatic cancer alone and in conjunction with cisplatin for the management of late-stage or metastatic bladder and lung cancer (non-small cell).74
When transported into the cell, gemcitabine is transformed into the functional form which is difluorodeoxycytidine diphosphate (dFdCDP). Then it is subsequently changed into difluorodeoxycytidine triphosphate (dFdCTP) via deoxycytidine kinase and both forms inhibit the progression of DNA synthesis.74
Limitations of Aquatic Metabolites and Sources as a Basis of Anticancer Agents
It is confirmed that using the bioactive agents from aquatic sources lead to the invention of new active drugs that made a big difference and would be attractive to most drug inventors worldwide. Nonetheless, taking into consideration some restrictions that may hinder the study of aquatic compounds which is as followings: the low amounts of bioactive agents from the organisms, some inorganic salts in addition to some toxins may be present due to the environment or even the organisms themselves.1
Hence, an exertion should be done to identify the potential adulterant to make marine extracts suitable with in vitro testing. Numerous diagnostic procedures are now accessible for the identification, and isolation of active components in marine extracts.130-131
Recommendations to Overcome Supply Problems
The controlled aquaculture techniques are the best option to solve the dependency of the variety of chemical compounds (chemo-type) produced by an organism on environmental conditions. These techniques might fend off the issue of exhausting the aquatic resources but also could be a practical option to produce the desired biomass for the scaled-up production needed in a drug discovery pipeline. Furthermore, improvements in chemical synthesis techniques and combinatorial chemistry provided satisfactory solutions for appropriate sourcing.132
At last, the cellular markers for most of the newly found marine natural products are undiscovered; therefore, various screening techniques along with proteomics and metabolomics should be mingled to blown away the constraints of in-vitro techniques.
A significant concern for aqua-derived drug discovery is the permanent accessibility of adequate quantity of these organisms and their metabolites without causing injury to environment. If obtaining from natural sources cannot be achieved in a sustainable way, alternative approaches exist to solve the issue of marine supply, like:
Biotechnological Cultivation
Most aquatic organisms cannot be easily cultured in an artificial environment, so co-cultivation process (mixed fermentation of two organisms or more to make the environment like their environment) is used and it will significantly increase chemical diversity and improve the yield of marine-derived compounds production.
Genetic Engineering
It is the transfer of a compound genetic materials and information to the inside of a host cell to produce the desired compound in a sustainable manner, but the exact genetic information must be known to avoid wrong genes insertion.114
Synthetic or Semi-Synthetic Adjustments
Both techniques can be used to transform a readily available compound into a final desired product, and these are economic alternatives for total synthesis to have variable structures and enhance the product characteristics.133
Conclusion
Secondary metabolites obtained from aquatic sources represent a wonderful area of underexplored but magic weapons that can be used against cancer. The currently available therapeutic approaches have faced limitations on their way for cancer treatment in terms of either side effects or emergence of strains resistant to treatment. Cytarabine, Trabectedin, Eribulin Mesylate and Brentuximab Vedotin are anticancer drugs that have gained the approval from FDA and EMA to be used clinically for cancer treatment. Other compounds are still undergoing different clinical trials phases for their safety and efficacy. Marine is a treasure that must be researched well and exploited.
Improvement of chemical synthesis and aquaculture or isolation processes can be utilized to overcome the issues mentioned. Other approaches like biotechnological cultivation, genetic engineering and synthetic or semi-synthetic modifications are to be used to solve supply problems and obtain aqua-derived compounds in high yield to guarantee a sustainable and effective production.
Acknowledgments
The work on this review represents graduation project at College of Pharmacy, University of Sharjah, United Arab Emirates.
Conflict of interest
The authors certify that there is no conflict of interest in this review.
Funding Sources
There is no funding source.
References
- Ruiz-Torres V, Encinar, JA, Herranz-López M, Pérez-Sánchez A, Galiano V, Barrajón-Catalán E, Micol V. An updated review on marine anticancer compounds: The use of virtual screening for the discovery of small-molecule cancer drugs. Molecules, 2017; 22(7): 1037.
CrossRef - Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends an update. Cancer Epidemiol. Biomarkers Prev., 2016; 25(1): 16-27.
CrossRef - Teixeira TR,Santos GSD, Armstrong L, Colepicolo P, Debonsi HM. Antitumor potential of seaweed derived-endophytic fungi. Antibiotic., 2019; 8(4): 205.
CrossRef - Khalifa SA, Elias N, Farag, MA, Chen L, Saeed A, Hegazy MEF, Moustafa MS, El-Wahed A, Al-Mousawi SM, Musharraf SG. Marine natural products: A source of novel anticancer drugs. Mar. Drugs., 2019; 17(9): 491.
CrossRef - Zeeshan R, Mutahir Z. Cancer metastasis-tricks of the trade. Bosn. J. Basic Med. Sci., 2017; 17(3): 172.
CrossRef - Revia RA, Zhang M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: recent advances. Materials Today., 2016; 19(3): 157-168.
CrossRef - Latimer K, Mott T. Lung cancer: diagnosis, treatment principles, and screening. Am. Fam. Physician., 2015; 91(4): 250-256.
- Miller KD, Nogueira L, Mariotto A B, Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL, Siegel RL. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin., 2019; 69(5): 363-385.
CrossRef - Hassan EM, DeRosa MC. Recent advances in cancer early detection and diagnosis: Role of nucleic acid based aptasensors. TrAC Trends Anal. Chem., 2020; 124: 115806.
CrossRef - Reddy AT, Janss AJ, Phillips PC, Weiss HL, Packer RJ. Outcome for children with supratentorial primitive neuroectodermal tumors treated with surgery, radiation, and chemotherapy. Cancer, 2000; 88(9): 2189-2193.
CrossRef - Ahles TA, Root JC. Cognitive effects of cancer and cancer treatments. Ann. Rev. Clic. Psychol., 2018; 14: 425-451.
CrossRef - Sleeman J, Steeg PS. Cancer metastasis as a therapeutic target. Eur. J. Cancer., 2010; 46 (7): 1177-1180.
CrossRef - Liang X, Luo D, Luesch H. Advances in exploring the therapeutic potential of marine natural products. Pharmacol. Res., 2019; 147: 104373.
CrossRef - Abdel-Moty SG, Sakai S, Aimi N, Takayama H, Kitajima M, El-Shorbagi A, Ahmed A N, Omar NM. Synthesis of cytotoxic 1-polyhydroxyalkyl-β-carboline derivatives. Eur. J. Med. Chem., 1998; 32(12): 1009-1017.
CrossRef - Abdu-Allah HHM, Abdel-Moty SG, El-Awady R, El-Shorbagi ANA. Design and synthesis of novel 5-aminosalicylate (5-ASA)–4-thiazolinone hybrid derivatives with promising antiproliferative activity. Bioorg. Med. Chem. Lett., 2016; 26(7): 1647-1650.
CrossRef - Aboul-Fadl T, El-Shorbagi AN, Hozien ZA, Sarhan AWAO. Investigation of alkylating, antineoplastic and anti-HIV potentials of the chalcones: 2-(3-arylpropenoyl)benzimidazole and their corresponding N1-methyl derivatives. Boll. Chim. Farm., 2000; 139(5): 228-234.
- El-Shorbagi AA, Abdel-Moty SG, Ahmed AN, Takayama H, Kitajima M, Aimi N, Sakai S. The antiviral (RNA & DNA) profile of some incomplete C-nucleosides inspired from natural ß-carboline (pyrido [3,4-b] indole) scaffold; pharmacology of the intermediates in the total synthesis. Der Pharma Chem., 2015; 7(11): 87-92.
- El-Shorbagi AN, El-Naggar M, Tarazi H, Chaudhary S, Abdu-Allah H, Hersi F, Omar H. Bis-(5-substituted-2-thiono-1,3,5-thiadiazinan-3-yl) butane as a scaffold of anti-proliferative activity, blended by a multicomponent process. Med. Chem. Res., 2018; 27(4): 1103-1110.
CrossRef - Emara S, Razee S, El-Shorbagi AN, Masujima T. Flow injection method for the determination of methotrexate with a column-packed oxidizing agent. Analyst., 1996; 121 (2): 183-188.
CrossRef - Soliman S, Hamoda AM, El-Shorbagi ANA, El-Keblawy AA. Novel betulin derivative is responsible for the anticancer folk use of Ziziphus spina-christi from the hot environmental habitat of UAE. J. Ethnopharmacol., 2019; 231: 403-408.
CrossRef - Abd-Elrahman MI, Ahmed MO, Ahmed SM, Aboul-Fadl T, El-Shorbagi A. Kinetics of solid state stability of glycine derivatives as a model for peptides using differential scanning calorimetry. Biophy. Chem., 2002; 97(2-3); 113-120.
CrossRef - Aboul-Fadl T, El-Shorbagi AN. New carriers for representative peptides and peptide drugs. Arch. Pharm., 1997; 330(11): 327-332.
CrossRef - Amin EN, Abdel-Alim AAM, Abdel-Moty SG, El-Shorbagi ANA, Abdel-Rahman MS. Synthesis of new 4,5-3(2H)pyridazinone derivatives and their cardiotonic, hypotensive, and platelet aggregation inhibition activities. Pharm. Res., 2010; 33(1): 25-46.
CrossRef - El-Shorbagi AN, Chaudhary S. Monobactams A unique natural scaffold of four-membered ring skeleton, recent development to clinically overcome infections by multidrugresistant microbes. Lett. Drug Des. Discov., 2019; 16(12): 1305-1320.
CrossRef - El-Shorbagi AN, Sakai SI, El-Gendy MA, Omar N, Farag HH. Imidazo[2,1-b]benzothiazoles. I. Chem. Pharm. Bull., 1988; 36(12): 4760-4768.
CrossRef - El-Shorbagi ANA, Husein MA. Synthesis and investigation of antihypertensive activity using anaesthetizednormotensive nonhuman primates of some 2-aryl-4-(substituted) pyrimido [1,2-a] benzimidazoles. Der Pharma Chem., 2015; 7(4): 190-200.
- Emara S, El-Gindy A, El-Shorbagi AN, Hadad G. Utility of copper(II) oxide as a packed reactor in flow injection assembly for rapid analysis of some angiotensin converting enzyme inhibitors. Anal. Chim. Acta., 2003; 489(1): 115-123.
CrossRef - Wang E, Sorolla MA, Krishnan PDG, Sorolla A. From seabed to bedside: a review on promising marine anticancer compounds. Biomol., 2020; 10(2): 248.
CrossRef - Hoang VLT, Li Y, Kim SK.Cathepsin B inhibitory activities of phthalates isolated from a marine Pseudomonas strain. Bioorg Med. Chem Lett., 2008; 18(6): 2083-2088.
CrossRef - Shi DY, Han LJ, Sun J, Wang Y, Yang YC, Shi JG, Fan X. Chemical constituents from marine alga Chaetomorpha basiretorsa. China J. Chin. Mat. Med., 2005; 30(5): 347-350.
- Isnansetyo A, Kamei Y. Bioactive substances produced by marine isolates of Pseudomonas. Ind. Microbiol. Biotechnol., 2009; 36(10): 1239-1248.
CrossRef - Geier MS, Butler RN, Howarth GS. Probiotics, prebiotics and synbiotics: a role in chemoprevention for colorectal cancer? Cancer Biol. Ther., 2006; 5(10): 1265-1269.
CrossRef - Sithranga Boopathy N, Kathiresan K. Anticancer drugs from marine flora: an overview. J. Oncol., 2010; 2010: 1-18.
CrossRef - Gademann K, Portmann C. Secondary metabolites from cyanobacteria: complex structures and powerful bioactivities. Curr. Org. Chem., 2008; 12(4): 326-341.
CrossRef - Hamoda AM, Fayed B, Ashmawy NS, El-Shorbagi ANA, Hamdy R, Soliman SSM. Marine sponge is a promising natural source of anti-SARS-CoV-2 scaffold. Front Pharmaco., 2021; 12: 666664.
CrossRef - Mayer AM, Lehmann VK. Marine pharmacology in 1999: antitumor and cytotoxic compounds. Anticancer Res., 2001; 21(4): 2489.
- Kolb EA, Gorlick R, Reynolds CP, Kang MH, Carol H, Lock R, Keir ST, Maris JM, Billups CA, DesJardins C. Initial testing (stage 1) of eribulin, a novel tubulin binding agent, by the pediatric preclinical testing program. Pediatr. Blood & Cancer., 2013; 60(8): 1325-1332.
CrossRef - Hamel E. Natural products which interact with tubulin in the vinca domain: maytansine, rhizoxin, phomopsin A, dolastatins 10 and 15 and halichondrin B. Pharmacol. Ther., 1992; 55(1): 31-51.
CrossRef - Dybdal-Hargreaves NF, Risinger AL, Mooberry SL. Eribulin mesylate: mechanism of action of a unique microtubule-targeting agent. Clin. Cancer Res., 2015; 21(11): 2445-2452.
CrossRef - Cortes J, Schöffski P, Littlefield BA. Multiple modes of action of eribulin mesylate: Emerging data and clinical implications. Cancer Treat. Rev., 2018; 70: 190-198.
CrossRef - Kuznetsov G, Towle MJ, Cheng H, Kawamura T, TenDyke K, Liu D, Kishi Y, Melvin J Y, Littlefield BA. Induction of morphological and biochemical apoptosis following prolonged mitotic blockage by halichondrin B macrocyclic ketone analog E7389. Cancer Res., 2004; 64(16): 5760-5766.
CrossRef - Funahashi Y, Okamoto K, Adachi Y, Semba T, Uesugi M, Ozawa Y, Tohyama O, Uehara T, Kimura T, Watanabe H. Eribulin mesylate reduces tumor microenvironment abnormality by vascular remodeling in preclinical human breast cancer models. Cancer Sci., 2014; 105(10): 1334-1342.
CrossRef - Daletos G, Ebrahim W, Ancheeva E, El-Neketi M, Song W, Lin W, Proksch P. Natural products from deep-sea-derived fungi a new source of novel bioactive compounds? Curr. Med. Chem., 2018; 25(2): 186-207.
CrossRef - Alves C, Silva J, Pinteus S, Gaspar H, Alpoim MC, Botana LM, Pedrosa R. From marine origin to therapeutics: The antitumor potential of marine algae-derived compounds. Front. Pharmacol., 2018; 9; 777.
CrossRef - Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxi. Med. Chem. Longev., 2009; 2(5): 270-278.
CrossRef - Hayes CJ, Whittaker BP, Watson SA, Grabowska AM. Synthesis and preliminary anticancer activity studies of C4 and C8-modified derivatives of catechin gallate (CG) and epicatechin gallate (ECG). J. Org. Chem., 2006; 71(26): 9701-9712.
CrossRef - Khowala S, Verma D, Banik SP. Biomolecules:(Introduction, Structure & Function). 2008.
- Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients, 2010; 2(12); 1231-1246.
CrossRef - Khan RA, Khan MR, Khan A. Comparative antioxidant scavenging and lipid peroxidation activity of rutin and gallic acid. Bangladesh Phamracol., 2015; 10(3): 637-638.
CrossRef - Kim SH, Kim SS, Kwon O, Sohn KH, Kwack SJ, Choi YW, Han SY, Lee MK, Park K L. Effects of dibutyl phthalate and monobutyl phthalate on cytotoxicity and differentiation in cultured rat embryonic limb bud cells; protection by antioxidants. J. Toxicol. Environ. Health Part A., 2002; 65(5-6): 461-472.
CrossRef - Miyoshi N, Kawano T, Tanaka M, Kadono T, Kosaka T, Kunimoto M, Takahashi T, Hosoya H. Use of Paramecium species in bioassays for environmental risk management: determination of IC50 values for water pollutants. J. Health Sci., 2003; 49(6): 429-435.
CrossRef - Boger DL, Ichikawa S, Tse WC, Hedrick MP, Jin Q. Total syntheses of thiocoraline and BE-22179 and assessment of their DNA binding and biological properties. J. Am. Chem Soc., 2001; 123(4): 561-568.
CrossRef - Dawson S, Malkinson JP, Paumie D, Searcey M. Bisintercalator natural products with potential therapeutic applications: isolation, structure determination, synthetic and biological studies. Nat. Prod. Rep., 2007; 24(1): 109-126.
CrossRef - Fontaine K, Mounier J, Coton E, Hymery N. Individual and combined effects of roquefortine C and mycophenolic acid on human monocytic and intestinal cells. World Mycotoxin J., 2016; 9(1): 51-62.
CrossRef - Rodrigues I, Miguel M, Mnif W. A brief review on new naturally occurring cembranoid diterpene derivatives from the soft corals of the genera sarcophyton, sinularia, and lobophytum since 2016. Molecules, 2019; 24(4): 781.
CrossRef - Turner T, Jackson WH, Pettit GR, Wells A, Kraft AS.N Treatment of human prostate cancer cells with dolastatin 10, a peptide isolated from a marine shell‐less mollusc. Prostate., 1998; 34(3): 175-181.
CrossRef - Francisco JA, Cerveny CG, Meyer DL, Mixan B J, Klussman K, Chace DF, Rejniak S X, Gordon KA, DeBlanc R, Toki BE. cAC10-vcMMAE, an anti-CD30–monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood., 2003; 102(4): 1458-1465.
CrossRef - Masyita A, Sari RM, Astuti AD, Yasir B, Rumata NR, Emran TB, Naini F, Simal-Gandara J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem., 2022; 100217.
CrossRef - Zhang Q, Mándi A, Li S, Chen Y, Zhang W, Tian X, Zhang H, Li H, Zhang W, Zhang S. N–N‐Coupled Indolo‐sesquiterpene Atropo‐Diastereomers from a Marine‐Derived Actinomycete. Eur. J. Org. Chem., 2012; 2012(27): 5256-5262.
CrossRef - Liu XH, Miao FP, Li XD, Yin XL, Jin Y. A new sesquiterpene from an endophytic Aspergillus versicolor strain. Prod. Commun., 2012; 7(7); 819-820
CrossRef - Giordano D, Costantini M, Coppola D, Lauritano C, Pons LN, Ruocco N, di Prisco G, Ianora A, Verde C. Biotechnological applications of bioactive peptides from marine sources. Microb. Physiol., 2018; 73; 171-220.
CrossRef - Mudit M, El Sayed KA. Cancer control potential of marine natural product scaffolds through inhibition of tumor cell migration and invasion. Drug Discov. Today., 2016; 21 (11): 1745-1760.
CrossRef - Murphy T, Yee KW. Cytarabine and daunorubicin for the treatment of acute myeloid leukemia. Expert Opin. Pharmacother., 2017; 18(16): 1765-1780.
CrossRef - Hamada A, Kawaguchi T, Nakano M. Clinical pharmacokinetics of cytarabine formulations. Pharmacokinet., 2002; 41(10): 705-718.
CrossRef - Pereira RB, Evdokimov NM, Lefranc F, Valentão P, Kornienko A, Pereira DM, Andrade PB, Gomes NG. Marine-derived anticancer agents: Clinical benefits, innovative mechanisms, and new targets. Drugs., 2019; 17(6): 329.
CrossRef - Litmanovich A, Khazim K, Cohen I. The role of interleukin-1 in the pathogenesis of cancer and its potential as a therapeutic target in clinical practice. Oncol. Ther., 2018; 6: 109-127.
CrossRef - Baker WJ, Royer JrGL, Weiss RB. Cytarabine and neurologic toxicity. J. Clin. Oncol., 1991; 9(4): 679-693.
CrossRef - Liu J, Zhao D, He W, Zhang H, Li Z, Luan Y. Nanoassemblies from amphiphilic cytarabine prodrug for leukemia targeted therapy. J. Colloid Interface Sci., 2017; 487: 239-249.
CrossRef - Demetri GD, Von Mehren M, Jones RL, Hensley ML, Schuetze SM, Staddon A, Milhem M, Elias A, Ganjoo K, Tawbi H. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase III randomized multicenter clinical trial. J. Clin. Oncol., 2016; 34(8): 786.
CrossRef - Germano G, Frapolli R, Simone M, Tavecchio M, Erba E, Pesce S, Pasqualini F, Grosso F, Sanfilippo R, Casali PG. Antitumor and anti-inflammatory effects of trabectedin on human myxoid liposarcoma cells. Cancer Res., 2010; 70(6); 2235-2244.
CrossRef - Dossi R, Frapolli R, Di Giandomenico S, Paracchini L, Bozzi F, Brich S, Castiglioni V, Borsotti P, Belotti D, Uboldi S. Antiangiogenic activity of trabectedin in myxoid liposarcoma: involvement of host TIMP‐1 and TIMP‐2 and tumor thrombospondin‐1. Int. J. Cancer., 2015; 136(3): 721-729.
CrossRef - Germano G, Frapolli R, Belgiovine C, Anselmo A, Pesce S, Liguori M, Erba E, Uboldi S, Zucchetti M, Pasqualini F.Role of macrophage targeting in the antitumor activity of trabectedin. Cancer cell., 2013; 23(2): 249-262.
CrossRef - David-Cordonnier MH, Gajate C, Olmea O, Laine W, de la Iglesia-Vicente J, Perez C, Cuevas C, Otero G, Manzanares I, Bailly C. DNA and non-DNA targets in the mechanism of action of the antitumor drug trabectedin. Chem. Biol., 2005; 12(11): 1201-1210.
CrossRef - Nigam M, Suleria HAR, Farzaei MH, Mishra AP. Marine anticancer drugs and their relevant targets: a treasure from the ocean. DARU J. Pharma. Sci., 2019; 27(1): 491-515.
CrossRef - Advani RH, Lebovic D, Chen A, Brunvand M, Goy A, Chang JE, Hochberg E, Yalamanchili S, Kahn R, Lu D. Phase I study of the anti-CD22 antibody–drug conjugate pinatuzumab vedotin with/without rituximab in patients with relapsed/refractory B-cell non-Hodgkin lymphoma. Clin. Cancer Res., 2017; 23(5): 1167-1176.
CrossRef - Gomes NG, Dasari R, Chandra S, Kiss R, Kornienko A. Marine invertebrate metabolites with anticancer activities: Solutions to the “supply problem”. Mar. Drugs., 2016; 14(5): 98.
CrossRef - Martins A, Vieira H, Gaspar H, Santos S. Marketed marine natural products in the pharmaceutical and cosmeceutical industries: Tips for success. Mar. Drugs., 2014; 12(2): 1066-1101.
CrossRef - MoyrOn‐QuirOz J, Partida‐Sánchez S, Donís‐Hernández R, Sandoval‐Montes C, Santos‐Argumedo L. Expression and Function of CD22, a B‐cell Restricted Molecule. Scad. J. Immunol., 2002; 55(4): 343-351.
CrossRef - Mehta A, Forero-Torres A. Development and integration of antibody-drug conjugate in non-Hodgkin lymphoma. Curr. Oncol. Rep., 2015; 17(9): 1-10.
CrossRef - Morschhauser F, Flinn IW, Advani R, Sehn LH, Diefenbach C, Kolibaba K, Press OW, Salles G, Tilly H, Chen AI. Polatuzumab vedotin or pinatuzumab vedotin plus rituximab in patients with relapsed or refractory non-Hodgkin lymphoma: final results from a phase 2 randomised study (ROMULUS). Lancet Haematol., 2019; 6(5): e254-e265.
CrossRef - Violanti SS, Bononi I, Gallenga CE, Martini F, Tognon M, Perri P. New insights into molecular oncogenesis and therapy of uveal melanoma. Cancers., 2019; 11 (5): 694.
CrossRef - Munzenrider JE. Uveal melanomas. Conservation treatment. Hematol. Oncol. Clin. North Am., 2001; 15(2): 389-402.
CrossRef - Tomihari M, Chung JS, Akiyoshi H, Cruz PD, Ariizumi K. DC-HIL/glycoprotein Nmb promotes growth of melanoma in mice by inhibiting the activation of tumor-reactive T cells. Cancer Res., 2010; 70 (14): 5778-5787.
CrossRef - Moussa FM, Hisijara IA, Sondag GR, Scott EM, Frara N, Abdelmagid SM, Safadi FF. Osteoactivin promotes osteoblast adhesion through HSPG and αvβ1 integrin. J. Cell. Biochem., 2014; 115(7): 1243-1253.
CrossRef - Narasaraju T, Shukla D, More S, Huang C, Zhang L, Xiao X, Liu L. Role of microRNA-150 and glycoprotein nonmetastatic melanoma protein B in angiogenesis during hyperoxia-induced neonatal lung injury. Am. J. Respir. Cell. Mol. Biol., 2015; 52(2): 253-261.
CrossRef - Bhaskaran M, Xi D, Wang Y, Huang C, Narasaraju T, Shu W, Zhao C, Xiao X, More S, Breshears M. Identification of microRNAs changed in the neonatal lungs in response to hyperoxia exposure. Physiol. Genomics., 2012; 44(20): 970-980.
CrossRef - Ripoll VM, Meadows NA, Raggatt LJ, Chang MK, Pettit AR, Cassady AI, Hume DA. Microphthalmia transcription factor regulates the expression of the novel osteoclast factor GPNMB. Gene., 2008; 413(1-2): 32-41.
CrossRef - Rose AA, Biondini M, Curiel R, Siegel PM. Targeting GPNMB with glembatumumab vedotin: current developments and future opportunities for the treatment of cancer. Pharmacol. Ther., 2017; 179: 127-141.
CrossRef - Turrentine J, Chung JS, Nezafati K, Tamura K, Harker-Murray A, Huth J, Sharma RR, Harker DB, Ariizumi K, Cruz JRP. DC-HIL+ CD14+ HLA-DRno/low cells are a potential blood marker and therapeutic target for melanoma. J. Invest. Dermatol., 2014; 134(11): 2839.
CrossRef - Tanaka H, Shimazawa M, Kimura M, Takata M, Tsuruma K, Yamada M, Takahashi H, Hozumi I, Niwa JI, Iguchi Y. The potential of GPNMB as novel neuroprotective factor in amyotrophic lateral sclerosis. Sci. Rep., 2012; 2(1): 1-11.
CrossRef - Hong DS, Concin N, Vergote I, De Bono JS, Slomovitz BM, Drew Y, Arkenau HT, Machiels JP, Spicer JF, Jones R, Forster MD, Cornez N, Gennigens C, Johnson ML, Thistlethwaite FC, Rangwala RA, Ghatta S, Windfeld K, Harris JR, Lassen UN, Coleman RL. Tisotumab vedotin in previously treated recurrent or metastatic cervical cancer. Clin. Cancer Res., 2020; 26(6): 1220-1228.
CrossRef - De Bono JS, Concin N, Hong DS, Thistlethwaite FC, Machiels JP, Arkenau HT, Plummer R, Jones RH, Nielsen D, Windfeld K, Ghatta S, Slomovitz BM, Spicer JF, Yachnin J, Ang JE, Mau-Sørensen PM, Forster MD, Collins D, Dean E, Rangwala RA, Lassen U. Tisotumab vedotin in patients with advanced or metastatic solid tumours (InnovaTV 201): a first-in-human, multicentre, phase 1-2 trial. Lancet Oncol., 2019; 20(3): 383-393.
CrossRef - Shimizu S, Ogawa T, Takezawa K, Tojima I, Kouzaki H, Shimizu T. Tissue factor and tissue factor pathway inhibitor in nasal mucosa and nasal secretions of chronic rhinosinusitis with nasal polyp. Am. J. Rhinol. Allergy., 2015; 29(4); 235-242.
CrossRef - Marti G, Schwarz C, Leichtle AB, Fiedler GM, Arampatzis S, Exadaktylos AK, Lindner G. Etiology and symptoms of severe hypokalemia in emergency department patients. Eur. J. Emerg. Med., 2013; 21: 46-51.
CrossRef - Wang YH, Wang DR, Guo YC, Liu JY, Pan J, The application of bone marrow mesenchymal stem cells and biomaterials in skeletal muscle regeneration. Regen. Ther., 2020; 15: 285-294.
CrossRef - Lee Y, Phat C, Hong SC. Structural diversity of marine cyclic peptides and their molecular mechanisms for anticancer, antibacterial, antifungal, and other clinical applications. Peptides, 2017; 95: 94-105.
CrossRef - Davidson S, Allen S, Lim G, Anderson C, Haygood M. Evidence for the biosynthesis of bryostatins by the bacterial symbiont “Candidatus Endobugula sertula” of the bryozoan Bugula neritina. Appl. Environ. Microbiol., 2001; 67(10); 4531-4537.
CrossRef - Trindade-Silva AE, Lim-Fong GE, Sharp KH, Haygood MG. Bryostatins: biological context and biotechnological prospects. Curr. Opin. Biotechnol., 2010; 21(6): 834-842.
CrossRef - Kortmansky J, Schwartz GK. Bryostatin-1: a novel PKC inhibitor in clinical development. Cancer Investig., 2003; 21(6): 924-936.
CrossRef - Spitaler M, Cantrell DA. Protein kinase C and beyond. Nat. Immunol., 2004; 5(8); 785-790.
CrossRef - Wender PA, Cribbs CM, Koehler KF, Sharkey NA, Herald CL, Kamano Y, Pettit GR, Blumberg PM. Modeling of the bryostatins to the phorbol ester pharmacophore on protein kinase C. Proc. Natl. Acad. Sci. USA., 1988; 85(19); 7197-7201.
CrossRef - Tsujimoto Y. Role of Bcl‐2 family proteins in apoptosis: apoptosomes or mitochondria? Genes to cells., 1998; 3(11): 697-707.
CrossRef - Plummer S, Manning T, Baker T, McGreggor T, Patel M, Wylie G, Phillips D. Isolation, analytical measurements, and cell line studies of the iron–bryostatin-1 complex. Bioorg. Med. Chem. Lett., 2016; 26(10): 2489-2497.
CrossRef - Zonder JA, Shields AF, Zalupski M, Chaplen R, Heilbrun LK, Arlauskas P, Philip PA, A phase II trial of bryostatin 1 in the treatment of metastatic colorectal cancer. Clin. Cancer Res., 2001; 7(1): 38-42.
- Loganzo F, Discafani CM, Annable T, Beyer C, Musto S, Hari M, Tan X, Hardy C, Hernandez R, Baxter M. HTI-286, a synthetic analogue of the tripeptide hemiasterlin, is a potent antimicrotubule agent that circumvents P-glycoprotein-mediated resistance in vitro and in vivo. Cancer Res., 2003; 63(8): 1838-1845.
- Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer., 2004; 4(4): 253-265.
CrossRef - Fife CM, McCarroll JA, Kavallaris M, Movers and shakers: cell cytoskeleton in cancer metastasis. Br. J. Pharmacol., 2014; 171(24): 5507-5523.
CrossRef - Towbin H, Bair KW, DeCaprio JA, Eck MJ, Kim S, Kinder FR, Morollo A, Mueller DR, Schindler P, Song HK, Van Oostrum J, Versace RW, Voshol H, Wood J, Zabludoff S, Phillips PE. Proteomics-based target identification: Bengamides as a new class of methionine aminopeptidase inhibitors. J. Biol. Chem., 2003; 278(52): 52964-52971.
CrossRef - White KN, Tenney K, Crews P. The Bengamides: A Mini-Review of Natural Sources, Analogues, Biological Properties, Biosynthetic Origins, and Future Prospects. J. Nat. Prod., 2017; 80(3); 740-755.
CrossRef - Quan DH, Nagalingam G, Luck I, Proschogo N, Pillalamarri V, Addlagatta A, Martinez E, Sintchenko V, Rutledge PJ, Triccas JA., Bengamides display potent activity against drug-resistant Mycobacterium tuberculosis. Sci. Rep., 2019; 9: 14396.
CrossRef - Dumez H, Gall H, Capdeville R, Dutreix C, Van Oosterom AT, Giaccone G. A phase I and pharmacokinetic study of LAF389 administered to patients with advanced cancer. Anti-Cancer Drugs, 2007; 18(2): 219-225.
CrossRef - Yan Y, Liu N, Tang Y, Recent developments in self-resistance genes directed natural product discovery. Nat. Prod. Res., 2020; 37(7): 879-892.
CrossRef - Nigam M, Suleria HAR, Farzaei MH, Mishra AP. Marine anticancer drugs and their relevant targets: a treasure from the ocean. DARU J. Pharm. Sci., 2019; 27(1): 491-515.
CrossRef - Frau J, Flores-Holguín N, Glossman-Mitnik D. Chemical reactivity theory and empirical bioactivity scores as computational peptidology alternative tools for the study of two anticancer peptides of marine origin. Molecules, 2019; 24 (6): 1-7.
CrossRef - Bhatnagar I, Kim SK. Immense essence of excellence: Marine microbial bioactive compounds. Mar. Drugs., 2010; 8(10): 2673-2701.
CrossRef - Lee AV, Oesterreich S, Davidson NE. MCF-7 cells- changing the course of breast cancer research and care for 45 years. J. Natl. Cancer Inst., 2015; 107 (7):
CrossRef - Ray A, Okouneva T, Manna T, Miller HP, Schmid S, Arthaud L, Luduena R, Jordan M A, Wilson L. Mechanism of action of the microtubule-targeted antimitotic depsipeptide tasidotin (Formerly ILX651) and its major metabolite tasidotin C-carboxylate. Cancer Res., 2007; 67(8): 3767-3776.
CrossRef - Bai R, Edler MC, Bonate PL, Copeland TD, Pettit GR, Ludueña RF, Hamel E. Intracellular activation and deactivation of tasidotin, an analog of dolastatin 15: Correlation with cytotoxicity. Mol. Pharmacol., 2009; 75(1): 218-226.
CrossRef - Shaw SJ. The structure activity relationship of discodermolide analogues. Mini Rev. Med. Chem., 2008, 8(3): 276-284.
CrossRef - Frau J, Flores-Holguín N, Glossman-Mitnik D. Chemical reactivity theory and empirical bioactivity scores as computational peptidology alternative tools for the study of two anticancer peptides of marine origin. Molecules., 2019; 24(6): 1115.
CrossRef - Kobayashi M, Natsume T, Tamaoki S, Watanabe JI, Asano H, Mikami T, Miyasaka K, Miyazaki K, Gondo M, Sakakibara K. Antitumor activity of TZT‐1027, a novel doiastatin 10 derivative. Jpn. J. Cancer Res., 1997; 88(3): 316-327.
CrossRef - Watanabe J, Endo Y, Shimada N, Natsume T, Sasaki T, Kobayashi M. Antiangiogenic activity of TZT-1027 (soblidotin) on chick chorioallantoic membrane and human umbilical vein endothelial cells. In Vivo., 2007; 21(2): 297-304.
- Mita AC, Hammond LA, Bonate PL, Weiss G, McCreery H, Syed S, Garrison M, Chu Q S, DeBono JS, Jones CB. Phase I and pharmacokinetic study of tasidotin hydrochloride (ILX651), a third-generation dolastatin-15 analogue, administered weekly for 3 weeks every 28 days in patients with advanced solid tumors. Clin. Cancer Res., 2006; 12(17): 5207-5215.
CrossRef - Smith III AB, Sugasawa K, Atasoylu O, Yang CPH, Horwitz SB. Design and synthesis of (+)-discodermolide–paclitaxel hybrids leading to enhanced biological activity. J. Med. Chem., 2011; 54(18): 6319-6327.
CrossRef - Riely GJ, Gadgeel S, Rothman I, Saidman B, Sabbath K, Feit K, Kris MG, Rizvi NA. A phase 2 study of TZT-1027, administered weekly to patients with advanced non-small cell lung cancer following treatment with platinum-based chemotherapy. Lung Cancer, 2007; 55(2): 181-185.
CrossRef - Alonso-Álvarez S, Pardal E, Sánchez-Nieto D, Navarro M, Caballero MD, Mateos M V, Martín A. Plitidepsin: design, development, and potential place in therapy. Drug des. Devel. Ther., 2017; 11; 253-264.
CrossRef - Muñoz-Alonso MJ, González-Santiago L, Zarich N, Martínez T, Alvarez E, Rojas JM, Muñoz A. Plitidepsin has a dual effect inhibiting cell cycle and inducing apoptosis via Rac1/c-Jun NH2-terminal kinase activation in human melanoma cells. J. Pharmacol. Exp. Ther., 2008; 324 (3): 1093-1101.
CrossRef - Cheung RCF, Ng TB, Wong JH. Marine peptides: bioactivities and applications. Mar. drugs., 2015; 13(7): 4006-4043.
CrossRef - Blunt JW, Copp BR, Keyzers RA, Munro MHG, Prinsep MR. Marine natural products. Nat. Prod. Rep., 2015; 32: 116-211.
CrossRef - Newman DJ. Developing natural product drugs: supply problems and how they have been overcome. Pharmacol. Ther., 2016; 162: 1-9.
CrossRef - Grienke U, Silke J, Tasdemir D. Bioactive compounds from marine mussels and their effects on human health. Food chem., 2014; 142: 48-60.
CrossRef - Leal MC, Calado R, Sheridan C, Alimonti A, Osinga R. Coral aquaculture to support drug discovery. Trends Biotech., 2013; 31(10); 555-561.
CrossRef - Lindequist U. Marine-derived pharmaceuticals-challenges and opportunities. Biomol. Ther., 2016; 24 (6): 561-571.
CrossRef







