Keerthana Manoharan* and P Chitra
and P Chitra
Department of Biochemistry, Sri Ramakrishna College of Arts and Science for Women, Affiliated to Bharathiar University, Coimbatore, Tamil Nadu, India.
Corresponding Author E-mail: keerthanamanoharan96@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2453
Abstract
The prevalence of diabetes is growing at an alarming rate on a global scale with approximately 536.6 million people having diabetes in 2021. Medicinal plants are gaining recognition in the treatment of diabetes owing to their safety, ability and low cost. Elaeocarpus tectorius (Lour.) Poir. is one of the least explored plants belonging to the genus Elaeocarpus. With this context, the current study was aimed to explore the antidiabetic and hypolipidemic effect of ethanolic extract of E. tectorius leaves in diabetes-induced rats. Phytochemical screening was performed and the oral acute toxicity of the plant extract was explored. Experimental diabetes was induced using streptozotocin and nicotinamide. The experimental animals were orally treated with ethanolic leaf extract of E. tectorius at doses 200 mg/kg b.w. and 400 mg/kg b.w. for 28 days after which body weight, fasting blood glucose levels, markers of serum lipid profile and histological variations in the pancreas were ascertained. Oral treatment of E. tectorius leaf extract daily for 28 days has significantly brought down (p<0.05) the fasting glucose levels in streptozotocin-nicotinamide induced diabetic rats and normalized the levels of serum lipid profile markers. The histological investigation revealed that the extract has caused significant restoration of pancreatic islet cells, and it was found that the dose of 400 mg/kg b.w. was more efficient and successful in treating hyperglycemia and circumventing related complications than 200 mg/kg b.w. The present study suggests that the leaf extract of E. tectorius possesses good antidiabetic and hypolipidemic activity and can be a useful and cheap alternative to treat diabetes.
Keywords
Diabetes; Dyslipidemia; Hyperglycemia; Medicinal plants; Streptozotocin
Download this article as:| Copy the following to cite this article: Manoharan K, Chitra P. Hypoglycemic and Hypolipidemic Activities of Ethanolic Extract of Elaeocarpus Tectorius (Lour.) Poir. Leaves in Streptozotocin- Nicotinamide Induced Diabetic Rats. Biomed Pharmacol J 2022;15(2). |
| Copy the following to cite this URL: Manoharan K, Chitra P. Hypoglycemic and Hypolipidemic Activities of Ethanolic Extract of Elaeocarpus Tectorius (Lour.) Poir. Leaves in Streptozotocin- Nicotinamide Induced Diabetic Rats. Biomed Pharmacol J 2022;15(2). Available from: https://bit.ly/3awMkuu |
Introduction
Diabetes mellitus is a metabolic disorder where individuals suffer from chronic hyperglycemia. Diabetes is one of the oldest disorders that affect millions of people worldwide.1 Diabetes is classified into two types based on the etiology, namely, type I and type II, both being characterized by hyperglycemia. Type I diabetes is triggered by the autoimmune-mediated destruction of the insulin-producing pancreatic β cells and type II results from insulin resistance, a condition that does not allow the cells to respond to higher blood glucose levels.2 The prevalence of diabetes is expected to rise to 700 million by the year 2045 as predicted and reported by the International Diabetes Federation.3
Diabetes is accompanied by various long-term life-threatening complications like retinopathy, nephropathy, neuropathy, dyslipidemia and cardiomyopathy. Dyslipidemia is one of the factors significantly contributing to the development of cardiovascular disease.4 The burden caused by cardiovascular disease is increasing every year and it is found to be one of the key reasons for mortality associated with type 2 diabetes.5 In diabetic patients, blood lipid levels are affected due to the interrelationship between carbohydrate and lipid metabolism.6 Abnormalities in lipoproteins are also commonly observed and about 30-60% of type II diabetic patients have dyslipidemia.7 The diagnosis and treatment of dyslipidemia associated with diabetes are highly essential to overcome the risk of cardiovascular diseases and associated complications.8
The current management of diabetes is mainly focused on improving glycemic control by incorporating strategies such as diet, exercise, insulin and oral hypoglycemic drugs.9 However, these oral hypoglycemic agents are associated with unpleasant side effects and high cost and they do not cure diabetes since they cannot restore the cellular glucose homeostasis.10 Herbal medications for treating diabetes are gaining fame globally due to their low cost, safety and efficacy. Medicinal plants have the potential to cure diabetes by various mechanisms including manipulation of carbohydrate metabolism through diverse mechanisms, enhancing the secretion of insulin, improving glucose uptake and utilization, regeneration of pancreatic β cells and enhancing signal transduction. Phytochemicals such as phenolics, flavonoids, alkaloids, terpenoids, steroids, saponins present in the plants possess prospective cardiovascular and metabolic effects.11
Elaeocarpus tectorius (Lour.) Poir. syn Elaeocarpus oblongus is a tree belonging to the family Elaeocarpaceae (Fig.1). The plant is distributed across the mountainous regions of South East Asia. Several species of plants belonging to the family Elaeocarpaceae are known to possess multiple health benefits including antioxidant, anti-inflammatory, antidiabetic, analgesic, anti-microbial, cytotoxic properties through pharmacological investigations.12 Although E. tectorius is reported to possess multiple medicinal properties, there is no scientific validation or documentation on the in vivo anti-diabetic or hypolipidemic activity of this plant. Hence, the present study is aimed at exploring the antidiabetic and hypolipidemic activities of the ethanolic extract of E. tectorius leaves in diabetic rats induced with streptozotocin-nicotinamide. This plant species may possibly be a safe and cheap alternative for the treatment and management of diabetes.
 |
Figure 1: Elaeocarpus tectorius (Lour.) Poir. tree. |
Methods
Plant materials
The fresh leaves of Elaeocarpus tectorius (Lour.) Poir. was collected from the Nilgiris Hills of Tamil Nadu, India. The plant material was authenticated by Dr. Arumugam, a botanist from the Botanical Survey of India, Southern Regional Centre, TNAU Campus, Coimbatore, Tamil Nadu (Reference no. BSI/SRC/5/23/2021/Tech./319). The freshly collected leaves were washed to remove dust particles, dried under shade and powdered. A sample of about 50 grams was macerated with 500 mL of ethanol and it was then incubated for 48 hours in a shaker incubator at 40ºC after which extract was filtered and the solvent was evaporated to get the dry extract.
Phytochemical screening
Preliminary phytochemical screening was performed to identify different phytochemicals present in the extracts. The leaf extract was tested for alkaloids using Meyer’s test, phenolics and tannins using ferric chloride test, flavonoids by Shinoda test, saponins using foam test, steroids and terpenoids using Liebermann-Burchard test.13-16
Acute oral toxicity studies
Acute oral toxicity of ethanolic leaf extract of E. tectorius (ETEE) was studied in healthy non-pregnant female albino Wistar rats (n= 9). The animals were kept fasting for three hours before dosing providing only water. The animals were treated with a starting dose of 200 mg/kg b.w. of leaf extract followed by 500,1000,1500,2000 mg/kg b.w. They were observed periodically for signs of toxicity, behavioral changes and mortality for about 14 days.
Animals
Healthy adult male albino Wistar rats (6 weeks old, 150-200g) were used for the study. The animals were housed in clean cages and were fed with a standard diet and clean drinking water. The animal study was performed after approval from the Institutional Animal Ethical Committee, Nanda College of Pharmacy, Erode (Approval No: NCP/IAEC/2021-22/20). All procedures were performed under the suggestions for the appropriate care and usage of laboratory animals.
Induction of Diabetes Mellitus
Animals were maintained on fasting condition all night providing only water and their initial blood glucose levels were checked. Diabetes Mellitus was then induced by a single intraperitoneal injection of 120 mg/kg of nicotinamide followed by the injection of 60 mg/kg streptozotocin and the onset of hyperglycemia was validated after 72 hours.
Experimental Design
Thirty rats were split into 5 groups (A-E) consisting of about 6 rats per group (n = 6). Group A represented the healthy normal control (NC) group, which received saline. Group B represented the diabetic control (DC) group that did not receive any oral hypoglycemic drug or plant extracts. Animals belonging to the experimental groups- Group C (ETEE 1) and D (ETEE 2) received 200 mg/kg b.w. and 400 mg/kg b.w. of leaf extract of E. tectorius orally. Group E received about 10 mg/kg b.w of the standard hypoglycemic drug, glibenclamide (Glib). These treatments were administered daily for a total of 28 days.
Evaluation of the effect of E. tectorius leaf extract on fasting blood glucose levels
Fasting blood glucose levels were measured in overnight fasting rats before treatment (0th day) and then weekly during the treatment.17
Evaluation of the effect of E. tectorius leaf extract on lipid profile
The lipid profile of experimental rats was determined on the 28th day of treatment by collecting the blood from the retro-orbital route. The serum was analyzed for total cholesterol (TC) using the method of Allain et al18, Serum triglyceride (TG) using the method of Schettler and Nussel19, High-density lipoprotein (HDL) level using the method of Grove.20 Low-density lipoprotein and very-low-density lipoprotein (VLDL) cholesterol was determined using the Friedewald formula.21 The cardiac risk index (CR) and Atherogenic risk index (AI) was also determined using the following formula:22
Histological investigation of the pancreas
At the end of the treatment period, the pancreatic sections of the animals were removed and processed for histological examination.23
Statistical analysis
All values are represented as mean ± SEM (n = 6). Statistical significance was determined by using a one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. A p-value of 0.05 or less was considered significant. These statistical analyses were performed using SPSS version 28.0.0. for Windows.
Results
Preliminary phytochemical screening
Preliminary phytochemical screening indicated that an array of phytochemicals including alkaloids, phenolics, flavonoids, tannins, terpenoids, saponins and steroids were present in the ethanolic leaf extracts of Elaeocarpus tectorius (Table-1).
Table 1: Phytochemical screening of leaves of Elaeocarpus tectorius (Lour.) Poir.
| Phytochemical | ETEE |
| Phenolics | + |
| Flavonoids | + |
| Terpenoids | + |
| Tannins | + |
| Alkaloids | + |
| Glycosides | + |
| Steroids | + |
| Saponins | + |
‘+’ indicates Present
Acute toxicity studies
The results of the acute toxicity test of Elaeocarpus tectorius leaf extract revealed that the extract was safe and non-toxic up to the tested dose of 2000 mg/kg b.w. No changes were observed in the normal behavioral pattern and there were no signs of toxicity and mortality observed as indicated by Organisation for Economic Co-operation and Development (OECD) animal welfare guidelines.
Effect of E. tectorius leaf extract on body weight
The effect of ETEE and glibenclamide on the bodyweight of the rats was measured on weeks 1, 2, 3 and 4 (Table 2). A significant reduction (p<0.05) was noticed in the mean body weight of animals after induction of diabetes except in the normal control group. The diabetic control group exhibited a significant body weight reduction (p<0.05) throughout the treatment period. Treatment of diabetic rats with ETEE and glibenclamide has improved the mean bodyweight of the rats to near that of the normal rats of Group A at the end of the experiment.
Table 2: Effect of E. tectorius leaf extract on body weight.
| Groups | Bodyweight during the treatment period* | |||
| Week 1 | Week 2 | Week 3 | Week 4 | |
| Normal healthy control | 122.2 ± 1.45a | 137.5 ± 2.42b | 139.3 ± 4.71c | 147.8 ± 5.11b |
| Diabetic control | 138.3 ± 6.34b | 122.67 ± 3.68ab | 103.7 ± 1.76a | 97.83 ± 2.23a |
| Diabetic + ETEE 1 | 135 ± 2.28ab | 114 ± 4.23a | 116.63 ± 3.31ab | 136.3 ± 6.93b |
| Diabetic + ETEE 2 | 133.7 ± 2.6ab | 120.2 ± 7.9ab | 103.3 ± 6.6bc | 139.8 ± 5.64b |
| Diabetic + glibenclamide | 128.5 ± 1.08ab | 133.3 ± 3.85ab | 137.8 ± 4.94c | 144 ±7.51b |
*Values are mean ± SEM (n=6); Values in the same column having different letters of alphabets differ significantly at p<0.05 (One-way ANOVA followed Tukey’s multiple comparison test)
Effect of E. tectorius leaf extract on fasting blood glucose levels
The effect of leaf extract of E. tectorius on the fasting blood glucose levels of experimental animals was recorded weekly during the course of treatment and the results are presented in Table 3. Initially, during treatment, the diabetic rats exhibited a significant increase (p<0.05) in fasting glucose levels when compared to the healthy normal control group. The blood glucose levels of the groups treated with the plant extract were lower than the group treated with glibenclamide at the end of the experimental period. In addition, after 28 days of oral treatment, it can be observed that a dose of 400 mg/kg b.w of ETEE was very much effective than a dose of 200 mg/kg b.w of ETEE where the blood glucose levels decreased significantly (p<0.05) from 361.67 ± 29.6 to 144.3 ± 48 mg/dL. The results show that administration of ethanolic leaf extract of E. tectorius has slightly normalized the glycemic control of diabetic rats.
Table 3: Effect of E. tectorius leaf extract on fasting blood glucose levels.
| Groups | Fasting blood glucose levels (mg/dL)* | ||||
| Day 0 | Day 7 | Day 14 | Day 21 | Day 28 | |
| Normal healthy control | 87.3 ± 3.07a | 88 ± 4.93a | 86 ± 2.77a | 83.3 ± 5.07a | 82.17 ± 3.67a |
| Diabetic control | 390 ± 32.25b | 364.17 ± 13.3c | 355± 71.4b | 386.7 ± 85.4b | 373.3 ± 75.8b |
| Diabetic + ETEE 1 | 370 ± 12.38b | 321.83 ± 10.65b | 305 ± 66.6ab | 263.3 ± 56.8ab | 158.3 ± 50.35a |
| Diabetic + ETEE 2 | 361.67 ± 29.6 b | 317.7 ± 9.5b | 301.7 ± 68.2ab | 233.3 ± 51.36 ab | 144.3 ± 48a |
| Diabetic + glibenclamide | 380 ± 19.83 b | 314.8 ± 8.3b | 270 ± 64.86 ab | 225 ± 45.8ab | 160 ± 32.86a |
*Values are mean ± SEM (n=6); Values in the same column having different letters of alphabets differ significantly at p<0.05 (One-way ANOVA followed Tukey’s multiple comparison test)
Effect of E. tectorius leaf extract on lipid profile
Diabetic control rats displayed a significant increase (p<0.05) in the total cholesterol, triglyceride, LDL and VLDL levels whereas, HDL level was significantly decreased (p<0.05) when compared with the normal healthy rats (Table 4). Oral administration of glibenclamide has significantly reduced (p<0.05) the TC, TG, LDL, VLDL levels and improved the HDL levels. Oral treatment with E. tectorius has significantly normalized (p<0.05) the lipid profile of rats where the total cholesterol, triglyceride, LDL and VLDL values were lessened and HDL levels were improved. Cardiac disease risk and atherogenic risk indices were also higher in untreated diabetic rats when compared with the normal healthy control, plant extract and glibenclamide treated groups (Table 5).
Table 4: Effect of E. tectorius leaf extract on serum lipid profile
| Groups | Markers of Lipid profile (mg/dL)* | ||||
| Total Cholesterol | Triglycerides | High-Density Lipoprotein (HDL) | Very Low-Density Lipoprotein (VLDL) | Low-Density Lipoprotein (LDL) | |
| Normal control | 175 ± 5.07a | 166.53 ± 7.46a | 77.49 ± 2.55c | 33.76 ± 2.14a | 52.68 ± 2.77a |
| Diabetic control | 253.35 ± 7.36c | 257.68 ± 8.26c | 39.02 ± 3.31a | 52.42 ± 3.08b | 130.75 ± 3.9d |
| Diabetic + ETEE 1 | 217.08 ± 9.26b | 226.78 ± 6.97b | 52.8 ± 2.76ab | 44.25 ± 2.6ab | 107.92 ± 2.82c |
| Diabetic + ETEE 2 | 168.09 ± 3.28a | 188.87 ± 1.95a | 57.11 ± 2.65b | 37.18 ± 2.3a | 89.13 ± 2.94b |
| Diabetic + glibenclamide | 176.67 ± 6.69a | 175.2 ± 4.66a | 59.57 ± 4.07b | 35.15 ± 2.09a | 101.77 ± 3.14bc |
*Values are mean ± SEM (n=6); Values in the same column having different letters of alphabets differ significantly at p<0.05 (One-way ANOVA followed Tukey’s multiple comparison test).
Table 5: Effect of E. tectorius leaf extract on cardiovascular disease risk indices.
| Experimental Groups | Cardiac disease risk index* | Atherogenic risk index* |
| Normal healthy control | 2.26 ± 0.05a | 1.26 ± 0.05a |
| Diabetic control | 6.75 ± 0.64c | 5.75 ± 0.64c |
| Diabetic + ETEE 1 | 4.2 ± 0.36b | 3.19 ± 0.36b |
| Diabetic + ETEE 2 | 2.98 ± 0.17ab | 1.98 ± 0.17ab |
| Diabetic + glibenclamide | 3.07 ± 0.34ab | 2.07 ± 0.34ab |
*Values are mean ± SEM (n=6); Values in the same column having different letters of alphabets differ significantly at p<0.05 (One-way ANOVA followed Tukey’s multiple comparison test).
Pancreatic histology
Histopathology study of the pancreas of the normal control, diabetic control, ETEE 1, ETEE 2 and glibenclamide groups are shown in Figs. 2, 3, 4, 5 and 6 respectively.
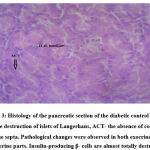
The histological examination of the pancreas of the normal rats belonging to Group A revealed normal and evenly distributed islets of Langerhans. The islets were found to be full of centrally placed β cells surrounded by acinar cells. There were no signs of atrophy in the pancreas of normal rats. The pancreas of diabetic control rats (Group B) demonstrated degenerative necrosis and destruction of islets of Langerhans containing the β cells. The acinar cells appeared swollen with increased vacuolation. The insulin-producing β cells of the rats of the untreated diabetic control group are almost entirely destroyed.
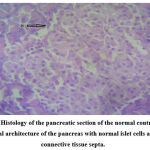 |
Figure 2: Histology of the pancreatic section of the normal control group. N- normal architecture of the pancreas with normal islet cells and intact connective tissue septa. |
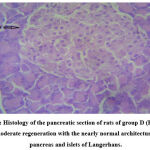 |
Figure 5: Histology of the pancreatic section of rats of group D (ETEE 2). MR- moderate regeneration with the nearly normal architecture of the pancreas and islets of Langerhans. |
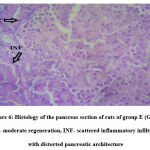 |
Figure 6: Histology of the pancreas section of rats of group E (Glib). MR- moderate regeneration, INF- scattered inflammatory infiltrates with distorted pancreatic architecture. |
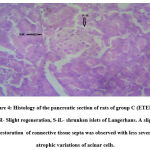
Histological examination of the pancreas of experimental animals treated with 200 mg/kg b.w of leaf extract of E. tectorius demonstrated slight restoration of the islet cells where the cells appeared less shrunken. The animals of group D treated with 400 mg/kg b.w. of E. tectorius ethanolic leaf extract presented significant regeneration and improvement in the architecture of islets of Langerhans. There was a moderate increase in the density of islet cells with reduced granulation. The pancreatic sections of diabetic rats treated with glibenclamide exhibited slight pancreatic structure reformation with scattered inflammatory infiltration.
Discussion
With the incidence of diabetes constantly rising and the side effects caused by synthetic oral- hypoglycemic agents, herbal medications are gaining attention as an alternative to synthetic drugs. This is the first study to report on the antidiabetic and hypolipidemic effects of Elaeocarpus tectorius leaf extract in diabetic rats induced with streptozotocin-nicotinamide. The outcomes of the study recommend that the leaf extract of this plant possesses good antidiabetic and hypolipidemic effects and could be beneficial in the treatment of diabetes and associated problems.
Phytochemical analysis of E. tectorius leaf extract indicated the presence of phenolics, flavonoids, tannins, terpenoids, saponins, steroids and alkaloids. The presence of these phytochemicals could account for the antidiabetic activity of this plant. Manoharan et al. had reported the presence of all these phytochemicals in the fruits of E. tectorius, similar to the outcomes of the current study.24 Plant phytochemicals possess powerful pharmacological actions. The antidiabetic effects resulting from the treatment with plant extracts are mainly attributed to the ability of phytochemicals to improve the performance of pancreatic cells by either improving insulin secretion or by reducing intestinal glucose uptake.25
The presence of flavonoids in the ethanolic extract of E. tectorius leaf is relevant since they are known to reduce intestinal glucose absorption, increase glucose uptake and use in muscle and fat tissues. They also reduce gluconeogenesis and triglyceride production resulting in the attenuation of hyperlipidemia and hyperglycemia.26 Saponins in particular are known for their capability to control hyperglycemia and prevent diabetic complications owed to their antioxidant capacity.27 They are also reported to possess antihyperlipidemic effects in the diabetic state which could help in reducing the risk of atherosclerosis and cardiovascular diseases.28 Plant tannins exert antidiabetic effects by delaying the glucose absorption in the intestinal tract and they also exert an insulin-like effect on cells sensitive to insulin. Tannins also regulate the antioxidant environment of pancreatic β-cells owing to their antioxidant properties. Tannins such as epigallocatechin-3-gallate possess antiobesity effects since they are able to interact with the adipose tissues, directly or indirectly.29
Acute toxicity studies indicated that the leaf extract had no toxic or harmful effect on healthy non-pregnant female albino rats. The present study shows that the leaf extract up to 2000 mg/kg b.w was well tolerated by the rats without any signs of toxicity and mortality during the entire observation period. Therefore, the LD50 value of the leaf extract may be greater than 2000 mg/kg b.w.
Streptozotocin is one of the cytotoxic drugs prominently used for inducing experimental diabetes in animals. Streptozotocin is a glucose analog containing a deoxyglucose molecule linked to a highly reactive methyl nitrosourea moiety.30 STZ recognizes the GLUT2 receptor abundantly present in the cell membrane of the β cells and causes specific necrosis of the pancreatic β cells by damaging the DNA.31 Nicotinamide being an active form of vitamin B3 can effectively protect the insulin-producing β cells from the cytotoxicity caused by STZ.32 While STZ destroys the pancreatic β cells selectively, nicotinamide decreases the extent of damage produced by STZ creating a condition of partial insulin deficiency and insulin resistance like in type 2 diabetes. Thus, the severity of diabetes induced by a combination of STZ-NIC is much lower than the severity of diabetes induced by STZ alone, with the rats manifesting moderate and stable hyperglycemia without needing exogenous insulin to survive.33 In this study, an injection of NIC was given to the experimental rats before the administration of STZ to partially protect the β cells and to produce a diabetic state that resembles type 2 diabetes.34
The bodyweight of the rats was documented weekly during the treatment. Diabetic control rats displayed a significant bodyweight decrease (p<0.05) throughout the experimental period. This reduction in body weight was prevented in the rats treated with Elaeocarpus tectorius leaf extract which displayed significant weight gain between weeks 2 and 3. The diabetic rats treated with ETEE have shown significant weight gain (p<0.05) at week 4 of treatment compared with the diabetic control group. Thus, it can be inferred that daily administration of E. tectorius leaf extract had improved the body weight of diabetic rats.
This study established the antidiabetic potential of different doses of ethanolic leaf extract of E. tectorius. This can be evidenced by the decrease in fasting blood glucose levels of the groups treated with ETEE across the treatment period. The leaf extract at 400mg/kg b.w. displayed potent hypoglycemic activity in STZ-NIC induced diabetic rats. Streptozotocin is known to induce diabetes by two distinct mechanisms namely alkylation of DNA by transfer of the methyl group from STZ causing DNA fragmentation and generation of free radicals leading to oxidative stress and insufficient insulin production both resulting in sequential necrotic β cells death.35 The blood-glucose-reducing activity of E. tectorius leaves could have been due to the direct scavenging of the free radicals generated, thus restoring the balance of antioxidants and pro-oxidants. Studies have shown that diabetic patients have increased ROS levels and alterations in antioxidant enzyme defense systems.36 The activities of the leaf extract could also be due to the upregulation of endogenous antioxidant enzyme systems leading to improvement of oxidative stress and could also be due to the direct influence on the pancreatic β cells, leading to enhanced insulin secretion, inhibition of intestinal glucose absorption and increased glucose utilization by adipose tissues and skeletal muscle.37,38 The other reported mechanisms by which herbal extracts exert anti-diabetic effects are through enhanced insulin sensitivity and inhibition of insulinase (insulin-degrading) enzyme.39
Dyslipidemia is one of the common diabetes-associated complications characterized by high plasma levels of TC, TG, VLDL, LDL and low levels of HDL. Diabetic patients are two to four-fold at higher risk of coronary artery disease than the general population due to insulin resistance or deficient lipid metabolism. 40,41 Insulin deficiency under diabetic conditions initiates increased free fatty acid mobilization from adipose tissues causing an increase in LDL production.42 In the current study, oral administration of E. tectorius leaf extract to experimental animals had resulted in lowered plasma total cholesterol, triglyceride, LDL and VLDL levels with an increase in HDL level. The cardiac and atherogenic risk indices of the diabetic rats treated with E. tectorius extract had also decreased. Studies have shown that diabetes is linked with an increase in the synthesis of cholesterol owing to the increased activity of the enzyme, HMG CoA reductase.43 It has been reported that hypercholesterolemia in diabetes results from increased production and intestinal absorption of cholesterol.44 Oral administration of plant extracts had normalized the lipid profile and lowered the cardiovascular disease indices of the treated groups which can be accounted for the rise in insulin secretion induced by the plant extract since the administration of insulin is known to lower plasma TG levels and increase the lipoprotein lipase activity.45
Histological investigation of the pancreas of rats belonging to Group A showed normal islet cells whereas the diabetic control Group B showed destruction of pancreatic islets caused by STZ. The treatment with E. tectorius leaf extract had produced a significant regeneration of insulin-producing pancreatic islet cells in Group C and D. This regeneration is clearer in Group D treated with 400mg/kg b.w. dose. This restoration of pancreatic architecture and islet cells could be responsible for the increased insulin secretion in the groups treated with plant extracts. Under conditions associated with type 2 diabetes, the mass and functions of β-cells are lost gradually. The histological assessment of animal groups treated indicates an improvement in the number and diameters of pancreatic islets. The groups treated with plant extracts showed stronger regeneration and healing of pancreatic architecture comparable with the glibenclamide treated group which could be responsible for its antidiabetic activity. Several plant extracts are shown to cause regeneration of insulin-producing pancreatic β cells in diabetic rats and focusing on their restoration is a potential strategy in the management of diabetes.46 The findings of the current study demonstrate the hypoglycemic and antidyslipidemic activity of E. tectorius and support the usage of this plant species for treating diabetes.
Conclusion
The current study found that ethanolic leaf extract of E. tectorius contains a variety of phytochemicals and the extract possesses excellent antidiabetic and antidyslipidemic activity. The plant extract was shown to cause regeneration of pancreatic cells, thus enhancing insulin secretion. Based on these findings, it can be concluded that the plant, E. tectorius could be employed in the treatment of diabetes and accompanying complications. Further studies should be carried out on this plant species to explore its other therapeutic activities.
Acknowledgment
The authors are thankful to Dr. G. Ariharasivakumar, Professor, Department of Pharmacology, KMCH College of Pharmacy, Coimbatore, Tamil Nadu, India for his support.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Funding Source
There is no funding sources.
References
- Emordi J.E, Agbaje E.O, Oreagba I.A, Iribhogbe O.I. Antidiabetic and hypolipidemic activities of hydroethanolic root extract of Uvaria chamae in streptozotocin-induced diabetic albino rats. BMC Complement Altern. Med., 2016; 16(1):468.
CrossRef - Öztürk E, Arslan A.K.K, Yerer M.B, Bishayee A. Resveratrol and diabetes: A critical review of clinical studies. Biomed Pharmacother., 2017; 95:230–234.
CrossRef - Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala A.A, Ogurtsova K, Shaw J.E, Bright D, Williams R. IDF Diabetes Atlas Committee (2019). Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9thDiabetes Res Clin Pract., 2019; 157:107843.
CrossRef - Ramasamy I. Update on the molecular biology of dyslipidemias. Clin Chim Acta., 2016; 454:143–185.
CrossRef - Warraich H.J, Rana J.S. Diabetic dyslipidemia: epidemiology and prevention of cardiovascular disease and implications of newer therapies. Curr Cardiol Rep., 2018; 20(12):125.
CrossRef - Dixit A.K, Dey R, Suresh A, Chaudhuri S, Panda A.K, Mitra A, Hazra J. The prevalence of dyslipidemia in patients with diabetes mellitus of Ayurveda Hospital. J Diabetes Metab Disord., 2014; 22;13:58.
CrossRef - Parasuraman S, Ching T.H, Leong C.H, BanikAntidiabetic and antihyperlipidemic effects of a methanolic extract of Mimosa pudica(Fabaceae) in diabetic rats. Egypt. J. Basic Appl. Sci., 2019; 6(1):137-148.
CrossRef - Herrera-BalandranoD, Chai Z, Hutabarat R.P, Beta T, Feng J, Ma K, Li D, Huang W. Hypoglycemic and hypolipidemic effects of blueberry anthocyanins by AMPK activation: In vitro and in vivo studies. Redox Biol., 2021; 46,102100.
CrossRef - Cefalu W.T, Dawes D.E, Gavlak G, Goldman D, Herman W.H, Van Nuys K. Insulin access and affordability working group: conclusions and recommendations. Diabetes Care., 2018; 41(6):1299–1311.
CrossRef - Moucheraud C, Lenz C, Latkovic M, Wirtz V.J. The costs of diabetes treatment in low- and middle-income countries: a systematic review. BMJ Glob Health., 2019; 4(1):e001258.
CrossRef - Alkhatib A, Tsang C, Tiss A, Bahorun T, Arefanian H, Barake R, Khadir A, Tuomilehto J. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients., 2017; 9(12):1310.
CrossRef - Dadhich A, Rishi A, Sharma G, Chandra S. Phytochemicals of Elaeocarpus with their therapeutic value: a review. Int. J. Pharma Bio Sci., 2013; 4(3):591–598.
- Gul R, Jan S.U, Faridullah S, Sherani S, Jahan N. Preliminary Phytochemical Screening, Quantitative Analysis of Alkaloids, and Antioxidant Activity of Crude Plant Extracts from Ephedra intermedia Indigenous to Balochistan. Sci. World J., 2017.
CrossRef - Harborne J.B. Phytochemical Methods A Guide To Modern Techniques Of Plant Analysis, Third Edition, Chapman Hall., 1998.
- Evans W.C. Trease and Evans Pharmacognosy, 15th edition. W.B Sauders Company Ltd, London., 2002; 137-139:230-240.
- Marka R, Talari S, Penchala S, Rudroju S, Swamy Nanna R. Preliminary phytochemical analysis of leaf, stem, root and seed extracts of Arachis Hypogaea L, Int. J. Pharm. Sci. Rev. Res., 2013; 20(1):134-139.
- Beach E.F, Turner J.J. An enzymatic method for glucose determination in the body fluids. J Clin Chem., 1958; 4(6):462–475.
CrossRef - Allain C.C, Poon L.S, Chan C.S, Richmond W, Fu P.C. Enzymatic determination of total serum cholesterol. Clin Chem., 1974; 20(4):470-475.
CrossRef - Schettler G, Nussel E. Colorimetric determination of triglycerides and Arb Med Soz Med Prav Med., 1975; 10, 25-8.
- Grove T.H. Effect of Reagent pH on the determination of high-density lipoprotein cholesterol by precipitation with sodium phosphotungstate magnesium. Clin Chem., 1979; 25(4):560-564.
CrossRef - Friedewald W.T, Levy R.I, Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without the use of the preparative ultracentrifuge. Clin Chem., 1972; 18(6):499–502.
CrossRef - Adeosun A.M, Asejeje F.O, Ighodaro O.M, Oluwole, B.A, Akinloye, O.A. Hypoglycemic, antidyslipidemic, and antioxidant activities of methanol extract of Struchium sparganophoraleaves in alloxan-induced oxidative stress-mediated diabetes in rats. Futur J Pharm Sci., 2020; 6(59).
CrossRef - Culling C.F.A. Handbook of histopathological and histochemical techniques, Third Edition, Butterworths, London., 1979.
- Manoharan A.L, Thamburaj S, Muniyandi K, Jagadeesan G, Sathyanarayanan S, Nataraj G, Thangaraj P. Antioxidant and antimicrobial investigations of Elaeocarpus tectorius (Lour.) poir. Fruits against urinary tract infection pathogens. Biocatal. Agric. Biotechnol., 2019; 101260.
CrossRef - Salehi B, Ata A, V Anil Kumar, N, Sharopov F, Ramírez-Alarcón K, Ruiz-Ortega A, Abdulmajid Ayatollahi S, Tsouh Fokou P.V, Kobarfard F, Amiruddin Zakaria Z, Iriti M, Taheri Y, Martorell M, Sureda A, Setzer W.N, Durazzo A, Lucarini M, Santini A, Capasso R, Ostrander E.A, Sharifi-Rad J. Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomolecules., 2019; 9(10);551.
CrossRef - Den Hartogh D.J, Tsiani Antidiabetic properties of naringenin: A citrus fruit polyphenol. Biomolecules., 2019; 9(3):99.
CrossRef - El Barky A.R, Hussein S.A, Alm-Eldeen A.E. Saponins and their potential role in diabetes mellitus. Diabetes Manag., 2017; 7(1):148–158.
- ElekofehintiO, Kamdem J.P, Kade I.J, Rocha J.B.T, Adanlawo I.G. Hypoglycemic, antiperoxidative and antihyperlipidemic effects of saponins from Solanum anguivi Lam. fruits in alloxan-induced diabetic rats. S. Afr. J. Bot., 2013; 88:56–61.
CrossRef - Sieniawska Activities of Tannins – from in Vitro Studies to Clinical Trials. Nat Prod Commun.. 2015; 10(11):1877-84.
CrossRef - DamascenoC, Netto A.O, Iessi I.L, Gallego F.Q, Corvino S.B, Dallaqua B, Sinzato Y.K, Bueno A, Calderon I.M.P, Rudge M.V.C Streptozotocin-Induced Diabetes Models: Pathophysiological Mechanisms and Fetal Outcomes. Biomed Res Int., 2014; 819065.
CrossRef - Wu J, Yan L. Streptozotocin-induced type 1 diabetes in rodents as a model for studying mitochondrial mechanisms of diabetic β cell glucotoxicity. Diabetes Metab Syndr Obes., 2015; 8:181-188.
CrossRef - Cruz P.L, Moraes-Silva I.C, Ribeiro A.A, Machi J.F, de Melo M.D.T, Dos Santos F, da Silva M.B, Strunz C.M.C, Caldini E.G, Irigoyen M.C. Nicotinamide attenuates streptozotocin-induced diabetes complications and increases survival rate in rats: role of autonomic nervous system. BMC Endocr Disord., 2021; 21(1):133.
CrossRef - Masiello P, Broca C, Gross R, Roye M, Manteghetti M, Hillaire-Buys D, Novelli M, Ribes G. Experimental NIDDM: development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes., 1998; 47(2):224–9.
CrossRef - Aboonabi A, Rahmat A, Othman F. Antioxidant effect of pomegranate against streptozotocin-nicotinamide generated oxidative stress-induced diabetic rats. Toxicol Rep., 2014; 1: 915-922.
CrossRef - Elamin N.M.H, Fadlalla I.M.T, Omer S.A, Ibrahim H.A.M. Histopathological Alteration in STZ-Nicotinamide Diabetic Rats, a Complication of Diabetes or a Toxicity of STZ?. Int J Diabetes Clin Res., 2018; 5:091.
CrossRef - PalekarV, Ray K.S. (2016) Oxidative stress in patients with diabetes mellitus. J Diabetes Metab Disord Control., 2016; 3(6):138-143.
CrossRef - BandeiraD.M, da Fonseca L.J, Guedes G.D.S, Rabelo L.A, Goulart M.O, Vasconcelos S.M. Oxidative stress as an underlying contributor in the development of chronic complications in diabetes mellitus. Int J Mol Sci., 2013; 14(2):3265–3284.
CrossRef - Kooti W, Farokhipour M, Asadzadeh Z, Ashtary-Larky D, Asadi-Samani M. The role of medicinal plants in the treatment of diabetes: a systematic review. Electron Physician., 2016; 8(1):1832–1842.
CrossRef - Mahdizadeh R, Moein S, Soltani N, Malekzadeh K, Moein M. Study the molecular mechanism of Salvia species in prevention of diabetes. Int J Pharm Sci Res., 2018; 9(11):4512–4521.
- SchofieldD, Liu Y, Rao-Balakrishna P, Malik RA, Soran H. Diabetes Dyslipidemia. Diabetes Ther., 2016; 7(2):203–219.
CrossRef - Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuniga F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol., 2018. 17,122.
CrossRef - Murali B, Upadhyaya U.M, Goyal R.K. (2002) Effect of chronic treatment with Enicostemma littorale in non-insulin-dependent diabetic (NIDDM) rats. J Ethnopharmacol., 2002; 81(2):199-204.
CrossRef - Goodman M.W, Michels L.D, Keane W.F. Intestinal and hepatic cholesterol synthesis in the alloxan diabetic rats. Proc Soc Exp Biol Med., 1982; 170(3):286–290.
CrossRef - Mathe D. Dyslipidemia and diabetes: animal models. Diabete Metab., 1995; 21:106–111.
- Langhi C, Cariou B. Cholesterol metabolism and beta-cell function. Med Sci (Paris)., 2010; 26(4):385-390.
CrossRef - Nurdiana S, Goh Y.M, Ahmad H, Dom S.M, Azmi N.S, Zin N.S.N.M, Ebrahimi M. Changes in pancreatic histology, insulin secretion and oxidative status in diabetic rats following treatment with Ficus deltoidea and vitexin. BMC Complement Altern Med., 2017; 17:290.
CrossRef