Chanchal Bhati1, Neha Minocha2 , Deepika Purohit3
, Deepika Purohit3 , Sunil Kumar3, Manish Makhija3, Sapna Saini4
, Sunil Kumar3, Manish Makhija3, Sapna Saini4 , Deepak Kaushik5
, Deepak Kaushik5 and Parijat Pandey1*
and Parijat Pandey1*
1Department of Pharmaceutical Sciences, Gurugram University, Gurugram – 122018, Haryana, India.
2School of Medical and Allied Sciences, K. R. Mangalam University, Gurugram – 122013, Haryana, India.
3Department of Pharmaceutical Sciences, Indira Gandhi University, Meerpur, Rewari – 123401, Haryana, India.
4PDM School of Pharmacy, Karsindhu, Safidon, Jind - 126112, Haryana, India.
5Department of Pharmaceutical Sciences, Maharshi Dayanand University, Rohtak – 124001, Haryana, India
Corresponding Author E-mail: parijatpandey98@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2411
Abstract
Background: Chromatography is defined as a set of techniques that are used for the separation of constituents in a mixture. Introduction: High-Pressure Liquid Chromatography or High-Performance Liquid Chromatography (HPLC) is known as a specialized technique in which columns as well as liquid chromatography are used in the separation, characterizationand investigation of the active moieties existing in the mixture. Objective: Current review focuses on the HPLC technique, including its principles, instrumentation, types, applications, advancements, and patents. Result: HPLC technique is important both for quantitative as well as qualitative analysis and is used for the evaluation of biological and pharmaceutical samples. It is the safest, most versatile, and fastest technique for chromatographic analysis in the field of quality control of drug components. In this review, the authors have also tried to summarize some of the advancements and recent patents in which the HPLC technique was used for the analysis. Conclusion: The article will help in understanding the role and importance of this analytical technique in the quality control of drugs and biologicals.
Keywords
Applications; Advancements; Chromatography; Detectors; High Performance Liquid Chromatography; Patents; Pumps
Download this article as:| Copy the following to cite this article: Bhati C, Minocha N, Purohit D, Kumar S, Makhija M, Saini S, Kaushik D, Pandey P. High Performance Liquid Chromatography: Recent Patents and Advancement. Biomed Pharmacol J 2022;15(2). |
| Copy the following to cite this URL: Bhati C, Minocha N, Purohit D, Kumar S, Makhija M, Saini S, Kaushik D, Pandey P. High Performance Liquid Chromatography: Recent Patents and Advancement. Biomed Pharmacol J 2022;15(2). Available from: https://bit.ly/3wnXdXN |
Introduction
Chromatography
Nowadays, chromatography is known as the mainstay of separation science and is widely applied within different pharmaceutical industries and research organizations throughout the world 1. Chromatography is defined as the separation of a combination of compounds into specific entities by using two phases; one is mobile and the other one is stationary 2,3. This technique was first invented in 1903 by Mikhail Semyorivich Tswett, an Italian born Russian botanist, and was later considered the ‘Father of Chromatography’ 4,5. Chromatography combines two Greek words, i.e., chromo means ‘color’ and graphene means ‘to write 6. Generally, the separation method in chromatography involves the principal steps starting from retention or adsorption of a substance(s) in the stationary phase then the separation of the adsorbed substances with the help of the mobile phase. Followed by retrieval of the separated substance by a continuous flow of the mobile phase called elution; preceded by quantitative and qualitative analysis of the eluted substances 7,8.
Types of Chromatography
There are different types of chromatography can be on the basis of nature of both the phases, modes of chromatography run, based on separation, based on elution technique.
Nature of the Mobile Phase and Stationary Phase [2].
Various types of chromatographic techniques are available depending upon the type of the phases used like phases used like Gas – Liquid Chromatography, Gas – Solid Chromatography, Liquid-Liquid Chromatography: further divided into column partition chromatography and paper partition chromatography, and Solid-Liquid Chromatography includes Thin Layer Chromatography (TLC), High Performance Liquid Chromatography (HPLC) and Column Chromatography 2,9,10.
Modes of Chromatography
There are two types of chromatography, which totally depends on the polarity of both phases used for separation i.e., interaction between the solute mobile phase and the stationary phase. When both phases are polar, their affinity is greater; the same applies when both the phases used are nonpolar; their affinity is greater; but when one solvent phase is polar and the other one is nonpolar, the interaction or the affinity is less 11,12.
Normal Phase Mode Chromatography
This technique uses a polar solvent for the stationary phase and a nonpolar solvent for the mobile phase. In this, the polar compound gets engaged for a long duration in the column compared to nonpolar compounds, which travel faster because of their affinity towards the stationary phase. For pharmaceutical analysis where normal phase chromatography is instructed, columns made up of silica gels are used 13,14.
Reverse Phase Mode Chromatography
This is just a reversal of normal phase chromatography, as the stationary phase used here is nonpolar and the mobile phase is of polar nature. Therefore, polar compounds, i.e., mobile phase, get eluted first and stationary, which is a nonpolar compound, gets retained for a long time. Therefore, reverse phase chromatography is considered the most extensively used technique in pharmaceutical industries as most of the components have polar natures that don’t get retained in the column for an extended period. Bonded hydro carbons like C8 (Carbon 8), C18, C4, or octadecyl silane (ODS) are used as a stationary phase in pharmaceutical analysis 15,16.
Separation Principle
Adsorption Chromatography
This technique based upon the principle of adsorption of separation is known as absorption chromatography. Separation of a mixture of compounds occurs due to the difference in affinity towards the stationary phase. Components having high affinity towards the stationary phase travel slowly rather than compounds having less affinity towards the stationary phase, which travel at a faster rate. Examples include column chromatography, thin layer chromatography, and high-performance liquid chromatography 17,18.
Partition Chromatography
When two immiscible liquids are present, a solute mixture should be circulated through the column according to their partition coefficient. The procedure involves passing the dissolved mixture in the mobile phase through a column of stationary phase. The component that is less soluble in the mobile phase travels faster and vice versa, which leads to the separation of compounds on the basis of their partition coefficients. Example: Gas – Liquid Chromatography, Column Chromatography, etc 19,20.
Elution Technique
On the basis of elution technique, two methods are commonly used namely isocratic elution and gradient elution. The method involving constant and uniform circulation of the mobile solvent throughout the separation process is called isocratic elution. During the process, elution or polarity strength is sustained 21,22; While if a mobile phase having low polarity or elution strength is used, with a constant increase in the polarity or elution strength throughout the process then the technique is called gradient elution 23.
Analysis Type
Qualitative Analysis identifies the nature of the compound, the impurities present, and the existence of active ingredients in the mixture of the compounds, which can be identified by using the values of retention time 24. While quantitative analysis determines the number of different components present in the mixture and is identified by comparison of the sample peak area with that of the standard peak area 25.
Other Chromatography methods
Ion Exchange Chromatography
It is one of the most powerful techniques for the separation of charged particles which is capable of separating almost all charged molecules including nucleotides small amino acids, large proteins, etc. With ion-exchange chromatography, the inorganic ions can also be separated and the method can be used for both preparative and analytical purposes. The major disadvantage associated with ion exchange chromatography is the requirement of buffer. The working cost is also high due to the buffer used for the separating the components 25.
Size Exclusion Chromatography or Gel Permeation Chromatography
In this technique, the separation of particles occurs in accordance with the molecular size by using gels. The method is suitable for determining the quaternary and tertiary structures of amino acids and also determining the molecular weight of polysaccharides. For separation purposes, gels that are soft in nature like polyacrylic amide, agarose gels, and dextran are used for the separation purpose. Apart from soft gels, some semi rigid gels like alkyl, dextran and polystyrene dispersed in non-aqueous medium are also used for analysis 28,29.
Chiral Phase Chromatography
By using a chiral stationary phase, the separation of optical isomers, i.e., dextro and levo form, is achieved. Silica gel is used as the most suitable stationary medium. A chiral stationary phase is much more expensive than a chiral stationary phase like carbon 8 30,31.
Bio-Affinity Chromatography
This method is generally used in various fields such as microbiology, biochemistry, and biotechnology, where protein-ligand specific reversible interactions are totally dependent on separation. Solid supports with a bio affinity matrix are covalently attached with ligands, which interact with the column bound ligands to retain proteins 32.
High Performance Liquid Chromatography
High-Pressure Liquid Chromatography or High-Performance Liquid Chromatography (HPLC) is known as a specialized technique in which columns as well as liquid chromatography are used in the separation, characterization, amount and investigation of the active moieties existing in the mixture. When compared to other traditional techniques, HPLC has significantly improved performance 33. The expansion of HPLC has evolved from conventional column chromatography and its performance (efficiency, resolution), which was enhanced using a stationary phase composed of spherical particles of a diameter range of 2 µm to 5 µm. Therefore, owing to the small particles, the head pressure forces the solvent or mobile liquid to pass through the column at high pressure. That’s why it is known as high-pressure liquid chromatography 34,35.
HPLC technique is applied for the separation of not only volatile compounds but also ionic species, polymeric materials, labile natural products, and macromolecules as well as high molecular weight functional groups 36.
The pump is used in HPLC to allow a sample mixture dissolved in liquid solvent to pass through a solid adsorbent material filled in the column. Individual constituents in the sample relate in a different way to the adsorbing substances, letting diverse rates of flow for the different elements and foremost to the separation of the components as they flow out of the columns 2,37.
HPLC works on the adsorption-separation principle. In HPLC column, compounds that are supposed to be separated are introduced in a mixture form, but various components travel at a different rate, as per their relative affinity towards the stationary phase 38. Elements having more affinity toward the stationary phase travel at a slower rate, while elements having less affinity towards the stationary phase move at a hasty rate. Since no two compounds have the same affinity towards the stationary phase, the components get separated 39. The major components of HPLC are shown in Fig. (1) 40.
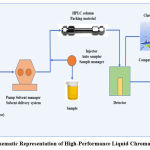 |
Figure 1: Schematic Representation of High-Performance Liquid Chromatography [15] |
Solvent Reservoir (Mobile Phase)
The contents of the mobile phase(s) are to be placed in a glass reservoir.
The concentration of the mobile phase varies and it depends upon the sample’s composition. Therefore, the mobile solvent is regularly a blend of nonpolar and polar liquid solvents. Now, generally there are four bottles used as mobile reservoirs during operating the HPLC, which contain different solvents. The holding capacity of each bottle of glass reservoir is up to 1 litre. Selection of the mobile phase used for separation purposes depends on the type of separation to be involved, i.e., isocratic separation and gradient separation 38,41.
For isocratic separation, solvents having the same polar figures or eluting power are used to prepare the mobile phase (if used in a fixed proportion) or, in general, this can be a pure solvent 42. But, in gradient separation, solvent’s polarity is progressively improved. Thereafter, the composition of the solvent has to be changed 43,44.
Mixing Chamber
Individual pressure pumps in mixing systems are generally used for the inlet of individual liquids. Each pump’s outlet is connected with mixing connectors (one outlet line and two inlets) to a mixing chamber. It is used to mix the solvent in different proportions 45.
Degasser Cabinet
Solvent reservoir systems are equipped with a means of removing dissolved gases such as O2 and N2 from the solvent as these gases interfere in the separation process by forming bubbles in the column or detector system which chokes the column, so degassing is done 46. Degassing (removal of dissolved gases) can be carried out in several ways; i.e., by warming up the solvent system, stirring with a magnetic stirrer, using ultrasonic vibrations or by bubbling helium gas through a solvent reservoir 47.
The different methods of removing dissolved gases from the mobile phase are as follows:
Ultrafiltration
It uses a membrane filter of 0.29 micrometres and the solvent gets filtered under pressure. This method allows the removal of both dissolved gases and small particulate matter 48.
Ultrasonication
In an ultrasonicator, electro frequency radiation is allowed to pass through the solvent, which takes the gases along with it. The ultrasonicator can be directly attached to the solvent, which may be advantageous, but it can only remove 30% of the dissolved gases used in conjugation with helium sparging or ultrafiltration 49.
Helium Sparging
A very efficient and effective technique of degassing with the use of more than 80% of the dissolved gases can be removed. In this method, the dissolved gases are removed from the mobile phase by passing or running it through some inert gases of low solubility. Helium is the most commonly used inert gas for the removal of dissolved gases. One of the main disadvantages is that, due to the continuous use of inert gas (Helium), it is quite expensive 50,51.
Refluxing
It is the most effective but difficult method of degassing and the least practically used as it involves boiling the solvent and distillation of the gas. This method is rarely used because of its impracticality, although it yields near perfect results 52,53.
High Pressure Pump/ Pumping System
HPLC system has many important features, and the pump that creates pressure is one of them. The mobile phase is taken up from the reservoir by this pressure pump and then the liquid phase passes through the column and then passes out (elution). The pump provides an output of at least 3.4 ×107 Pascal (psi) of solvent through the system, which is known for its good pumping system. High pressure pumps are required to achieve a satisfactory flow rate because the adsorbent is very small (5 to 10 micro) and tightly packed inside the column. This offers a higher resistance to the flow of solvent due to the slender column. The retention time of a molecule, reproducibility and detector sensibility of the column are directly dependent on the performance of the pump 54, 55.
Requirements of an Ideal HPLC Pump
While operating HPLC system there are certain requirements from pump like generation of pressure up to 5000 to 6000 psi without fluctuations. Pump should be suitable to a wide range of solvents providing a constant flow rate in the range of 0.1 – 10 ml per minute. Pumps should be made up of corrosion resistance material. Generally, the material of choice for the construction of the pump is Teflon 56, 57.
Type of Pumps used in HPLC
Reciprocating Pump
Screw Driven Syringe Pump or Displacement Pump
Pneumatic Pump or Constant Pressure Pump 35,56,57
Reciprocating Pump
It is the most prevalent and currently used pumping system on a commercial scale. It consists of a hydraulic chamber with a capacity varying from 35 µL to 400 µL. The chamber is connected to a motor-driven piston, which pumps the solvent in a back-and-forth motion. Check valves are present in a number of two, to regulate the solvent’s flow into and out of the column by alternatively opening and closing, respectively. A flexible diaphragm can also be used in a hydraulic fluid chamber to transmit the pumping action to the solvent. The use of a flexible diaphragm minimises the adulteration and erosion problems in the parts of the pump. In this, the movement of the piston causes the suction of the solvent from the mobile reservoir during the backward stroke and by the pressure of the pump, the solvent comes out of the column during the forward stroke and the inlet to the reservoir is closed. The main advantage of the reciprocating pump is that pressure up to 10000 psi can be applied, and some of the limitations are that it has a high maintenance cost and low discharging capacity 57,58.
Displacement Pump or Syringe Pump
These pumps are attached to a digital stepping motor which works on the principle of a screw driven mechanism. The piston is triggered by a screw – feed drive through which solvent flows from the solvent chamber of finite capacity (250-500 ml). That is why the displacement pump is also known as the screw driven syringe type pump. This pump is capable of generating high pressure of 200-475 atm with the main advantage being that pulse loss flow is achieved but exhibits considerable inconvenience when the solvent composition is changed from isocratic to gradient flow 59,60.
Pneumatic Pump or Constant Pressure Pump
In a pneumatic pump, a gas cylinder is fitted, which creates the pressure through which the mobile phase is compelled through the column with the help of a large piston. The compression observed by the solvent is proportional to the ratio of the area of the two pistons, usually between 30:1 and 50:1, and a high pressure (1-400 atm) is generated with a low-pressure gas source of 1-10 atm. The arrangement of the valve permits the hasty refilling of the solvent chamber with a capacity of 70 ml. This system provides pulse loss and continuous pumping, having a high flow rate, so it is called a constant pressure pump 60,61.
Sample Injector System
Liquid chromatography’s precision and accuracy of measurements are proportionate to the reproducibility with which a sample is injected into the column. The sample inclusion into the pressurised column should be like a slender plug so that the problem of peak bordening is negligible. Liquid samples having a range of 0.1-100ml volume are injected under high pressure of up to 5000 psi into the HPLC system with high reproducibility. Injecting the sample can be done either in a manual or in an automatic way 33,62.
Several Devices used in Sample Injector System
Septum Injector
It is not a commonly used device for injecting samples. A rubber septum withstanding high pressure of up to 1500 psi is used for injecting the sample. The injection of syringes used in this injector is called micro syringes 33,63.
Stopped Flow (online) Injector
In this, the pump is turned off so that the mobile phase cannot travel until the inlet pressure of the column becomes atmospheric. Then, the syringe with the sample is injected through a valve device, and then the pump is turned on. This method can be used up to a very high pressure 64,65.
Rheodyne Injector or Loop Valve Injector
Due to their high precision and high accuracy, loop valve injectors are the most widely used in HPLC systems. The volume of the loop is fixed (generally 10 micro litres or 50 micro litres). Samples can be injected via any of the two modes, i.e., inject mode or load position 66.
Load Position Mode allows loading of the sample in to the loop with the help of a syringe, which is commonly used to load samples into the injector 67. While Inject Mode allows the flow of the sample onto the column after loading the sample 39.
Column
The columns used for HPLC are the straight tube-like structures and they are manufactured of stainless steel and glass- lined metal so that they can resist high pressure of up to 5.5 × 107 Pa (8000 psi) for a length of 20-50 cm and 1-4 mm in diameter. For retention of packing material in the column, porous plugs made of stainless steel or teflon were used. These plugs must be similar to confirm the uniform flow of solvent through the column and the stationary phase is held in the column with the help of porous plugs 68,69.
Types of Columns used in HPLC
Guard Column
They possess very small amount of adsorbent and, as their name suggests, they protect and prolong the shelf life of an analytical column. It actually acts as a prefilter as it removes the particulate matter and contamination that arises from a contaminated mobile phase or from degrading sample- injection valves. It also helps to remove the contaminants bound irreversibly to the stationary phase. These can be used to separate the mobile phase from the stationary phase so that there is a minimal loss of the solvent from that of the analytical column 38,70.
Derivatising Column
It comprises a chemical transformation between the reagents and an analyte in which the physical and chemical properties of an analyte are changed. In this, the derivatising reagents are pumped with the flow of the mobile phase into either one or two outer parts of the reaction flow column. It stabilises a sensitive analyte, improves detection, and involves acetylation and hydrolysis of the analyte 71.
Capillary Column or Micro Column
HPLC involves the use of a small analytical column known as micro columns or capillary columns, which has a diameter of less than 1mm. A column is made up of depositing a permeable layer of adsorbent material (having a particle size of microns) on the inner wall of a capillary column and then coating it with a thin film of liquid phase. The sample used in this column is of nanolitre volume, which decreases the flow rate as well as solvent volume used, which leads to cost effectiveness 72,73.
Narrow-Bore Column
These columns are used for the assay of small volumes of samples. The diameter of these columns is 1-2 mm. In this, a number of short columns are joined together to build up a long column without loss of plate count and when the plate count is above 30000, long columns are used. With this analyst, we can analyze high purity solvents, which are cost effective due to the use of a minimum amount of mobile phase 74,75.
Short Fast Column
These columns are short in length (3-6 cm) and packed with 3microparticles. They can reduce solvent costs, increase the sample throughout, and deliver higher sensitivity than other columns. Analysis time is 15-120 sec for isocratic elution and 1-4 min for gradient elution. These columns are essential where analytical speed is necessary, as in quality control work 76.
Detector
Devices that are used to sense the presence of a solute present in the eluent are known as detectors. Different types of detectors are available, but their selection is of supreme importance as it is a valuable part of detecting the solute. They are designed and used according to the nature of the components to be separated. The utmost requirement is that the detecting element should be highly sensitive and steady because the application of material to the column is minute. The detector employed for HPLC should have good properties for producing efficient results, such as nse 77,78.
Detectors used in HPLC Column
Tof detectors are used in HPLC column and are described below:
Bulk Property Detectors
Bulk property detectors are also known as universal detectors. In the mobile phase, it measures the variance in the physical properties of the solute related to the mobile phase alone. Generally, the applications of these types of detectors are found in abundance, but they have a limited range of detection and poor sensitivity. These detecting systems are easily exaggerated by small variations in the conformation of the mobile phase, preventing the use of techniques such as concentration gradient. For example, evaporative light scattering detector and refractive index detector 78,79.
Solute Property Detectors
Solute property detectors are also known as sensitive detectors. They are idyllically independent of the mobile phase, and counter to a specific chemical or physical characteristic of the solute. These generally show a wide linear response range and high sensitivity, but due to the fact that they are specific in nature and to meet the demands of an analytical column, more than one detector is required. For example, absorbance detector, fluorescence detector, electrochemical detector, and mass spectrometric detector 77.
Various types of detectors used in HPLC systems are described below:
UV/Visible Detector
Its principle behind detection is the absorption of light characteristics of the sample. It includes two types of detectors. One is a fixed wavelength detector and operates at a frequency of 254 nm, where most drugs comprising composites get absorbed, and the other one is a variable wavelength detector, operating at a frequency range of 190 to 600 nm. They provide good stability and are easy to operate. It detects or visualises the obtained results in two dimensions 80,81.
Photodiode Array Detector
This is also used in UV spectrophotometer, and it works on the principle of light absorption; its operational frequency range falls between the wavelengths of 190 to 600nm. Simultaneously, radiation of all wavelengths falls on the detecting system. The analysis yields spectra with a three-dimensional plot of response vs. time vs. wavelength 82,83.
Fluorescence Detector
This detector works on fluorescent radiation emitted by compounds. Analyte atoms get excited by using a specific wavelength followed by the emission of fluorescence (which is considered a light signal). This detector has greater sensitivity and specificity, which is 10-1000 times greater than the UV detector. Even a single analyte fragment in the flow cell can be detected by using a fluorescence detector 84.
Conductivity Detector
It is based upon the electrical conductivity that is being consumed when the sample contains anions and captions as conducting ions. Therefore, detection is done on the basis of conducting ions in the sample and the response is recorded. Electronic resistance is measured and its value has a direct relationship with the number of ions present in the sample; if the presence of ions is high, they have a large value of electronic resistance and vice versa. Therefore, this can also be used for ion- exchange chromatography 39,79.
Amperometric Detector
When the potential is applied, it detects the compound based on its oxidation or reduction, followed by recording a diffusion current that is proportional to the concentration of the element eluted. This is related to the composites having functional groups that can be either oxidised or reduced. The sensitivity of this detector is very high 85.
Mass Spectrometry Detector
In this detector, a sample, analyte is detected on the basis of their molecular weight. Detecting system here is a mass analyser which detects the ions with respect to their mass to charge ratio, i.e., m/z ratio & amplifier, ions emerged from the mass analyser and record the responses. The analysis is useful for the identification of structural compounds. It can also be used for quantification of molecular and elemental compounds at very low detection limits 86,87.
Refractive Index Detector
It is the only universal detector in HPLC. The detector works by measuring the variation in the refractive index of the column effluent passing through the flow cell. The refractive index difference between the mobile phase and the sample is directly proportional to the imbalance between them. Thus, the sensitivity is higher for a greater difference in refractive index. Due to its poor specificity and sensitivity, it is not much used in analytical applications 88.
Evaporative Light Scattering Detector
Less volatile compounds (semi-volatile compounds and non-volatile) in comparison to the mobile phase are easy to detect in an evaporative light scattering detector. It is mostly used for the analysis of compounds where UV detection must be restricted, or we can say, used for compounds which do not absorb UV radiation, such as antivirals, antibiotics, etc 89.
Recorder and Integrator
After amplification, the responses obtained from the detector are recorded for analysis. They are capable to record all peaks time to time with an additional baseline. Integrators can process data and know to be a better version of recorders. They are capable of recording discrete peaks, mentioning the height and width of peaks, the area covered by a single peak, retention time, percentage of area, etc. Integrators deliver additional information on peaks than recorders 99.
Applications of High-Performance Liquid Chromatography
HPLC has several applications in pharmaceutical field, forensics, and chemical, environmental, and clinical sciences. It is also used in biotechnology, chemical separation, and food analysis, etc. which includes resolution, identification, and quantification of a compound. It is an adaptable and delicate method which can be used in several ways in different areas of work and day-to–to-day life 91,92.
In Pharmaceutical Industry
HPLC can be used to detect impurities present in pharmaceutical products and ultimately to check the quality of product. HPLC can be used for quantification of pharmaceutical products, to study the biopharmaceutical & pharmacokinetics properties of dosage forms of drugs, to study the stability of dosage form of drugs, determining the dissolution of tablet and ensuring the proper production and purity of drugs 93,95.
In Environmental Sciences
HPLC can be used to detect the presence of phenolic compounds present in drinking water, for biomonitoring the pollutants present in the environment and to detect the presence of insecticides and pesticides in water 96,97.
In Food and Flavour
HPLC is used in food industry to determine the presence of sugar present in fruit juices and to test the excellence of water and soft drinks. This can also be used for the investigation of polycyclic compounds present in vegetables, for the examination of preservatives in food products and to check the pH of the carbohydrates and oligosaccharides in anion exchange chromatography 98,99.
In Forensic Sciences
To determine the presence of cocaine, heroin, or any other abused drug in the blood, urine, etc, and to identify the presence of steroids in the blood, urine, etc. HPLC helps in the forensic analysis of textile dyes and to determine the unknown elements present in the mixture of compounds 100,101.
In Clinical and Health Related Disorders
HPLC helps in the analysis of urine and blood samples, in the analysis of bilirubin, a, bilverdin in hepatic disorders, in the detection of purines or pyrimidines present in the DNA sample and helps in diagnosing and monitoring diabetes 102,103.
In Research and Development
HPLC can be used for screening and extracting the elements present in the sample and recognising the components present in the unknown tester 56,104.
Other Applications
HPLC can be used in purification of natural and synthetic compounds, pre-concentration of trace components, Ligand-exchange chromatography and Ion-exchange chromatography of proteins 56,101,104.
New Amendments in High-Performance Liquid Chromatography Technique
Several advancements have been made in the classical HPLC technique and these newer techniques are Rapid Resolution Liquid Chromatography (RRLC), Ultra-Performance Liquid Chromatography (UPLC), Ultra-Fast Liquid Chromatography (UFLC) and Nano Liquid Chromatography (Nano LC). The description of these chromatographic techniques and their comparison with HPLC are summarized in Table 1 and discussed in the following sections 105,106.
Ultra-Performance Liquid Chromatography (UPLC)
With UPLC method, the sensitivity, resolution, and speed of the analytical process are improved. In this method, fine particles are used which helps in reducing the consumption of solvent and makes the method cost-effective. Ultra-Performance Liquid Chromatography is an evolved version of HPLC [107]. As in HPLC, packing materials have evolved which further influences the separation. It has been observed that the particle’s size present in the column directly affects the column’s efficiency; as the particle size reduces, it ultimately increases its resolution. According to the famous Van Demeter equation, if the particle size decreases to <2.5µm, a significant increase in efficiency is observed. This increase in peak capacity (number of peaks resolved per unit time) and speed of analysis when smaller particles are used is called Ultra Performance. UPLC has several advantages over HPLC that including maintained resolution performance, decreased run time with increased sensitivity using UPLC. It is more selective, more sensitive with low operational cost. It has vibrant range of liquid chromatographic analysis. Process cycle times get reduced which further helps in producing more products using existing resources and solvent consumption is also less with UPLC 108,109.
Applications of UPLC
UPLC finds application in drug discovery due to its high throughput quantitative analysis. It helps in the investigation of amino acids, determination of pesticides, testing of herbal products and traditional remedies. It helps in the identification of metabolites, ADME (Absorption, Distribution, Metabolism, Excretion) Screening, Bio-analysis / Bioequivalence Studies and dissolution testing 110,111.
Rapid Resolution Liquid Chromatography (RRLC)
RRLC system was designed to provide the maximum resolution and testing promptness and has become a method being commonly used in the pharmaceutical industry. With RRLC method, an excellent shape of the peak is observed during analysis along with increased reproducibility and sensitivity. The high speed of detection and reduced cost of analysis make this method a valuable one in the field of quality control of herbal medicines. With improved resolution and reduced analysis time, the HPLC method has also been improved and the use of smaller particles in HPLC became more popular. This relationship between the factors like efficiency of separation, the linear velocity of mobile phase, and size of the particles was studied deeply in the early 1970s, which has resulted in high resolution and high throughput HPLC of the present day. Using a shorter column can help in reducing the time of analysis, but at the same time leads to a decrease in theoretical plates and thus reduces the chromatographic resolution which is mainly required for the separation of complex mixtures of compounds. To maintain efficiency and to avoid the loss of theoretical plates, the use of smaller size particles has been found to be more effective. Therefore, longer columns and smaller particle sized packing material as used in RRLC, produce higher resolution and efficiency, with reduced analysis time without loss of chromatographic resolution 112,114. RRLC possess advantage over HPLC that it is the faster chromatography, having high resolution power 113,114.
Applications of RRLC
Finds its use in testing the quality of Rhodiola rosea roots and commercial standardized products. Fast analysis of phenolic antioxidants and erucamide slip additives in polypropylene homopolymer formulations using 1200 RRLC with RRHT columns and method translator 114,115.
Nano Liquid Chromatography (NLC)
Nano liquid chromatography can be defined in several ways based on the rate of flow of the mobile phase and column diameter. In some literatures, NLC is defined as a chromatographic technique in which the flow rate of the mobile phase is nano ML/minute. The detection aspect of NLC was considered after 2009, when the scientist Ali and his co-workers gave a scientific definition of NLC, which is an appropriate one. They defined NLC as a chromatographic method that involves samples in nanolitre quantities with flow rates of mobile phase of nano ml/min and a detection limit of nano grams/ml 116,117. NLC has several advantages like: NLC is a quicker and cheaper technique compared to HPLC. It produces less waste due to reduction in consumption of mobile phase. It helps in increasing the sensitivity along with reducing the sample requirement. Resolution power for analysing the complex sample has been found to be increased. Also, it has possessed higher efficiency 118,119. Nano LC is capable of separating sulphonamides, peptides. They are responsible for the discovery of glycomics towards biomarker using nano-LC and also used for glycobio-analysis 116,117,119.
Ultra-Fast Liquid Chromatography (UFLC)
UFLC has been found to have about ten times higher speed than traditional liquid chromatographic methods with approx. three times better separation even at normal levels of pressure. By maximizing the column performance, the UFLC method has been reported to minimize the deviation of the technique from the van Demeter theory. The technique ensures ultrafast analysis without affecting analytical reliability and precision 120,121. UFLC possess advantage over HPLC is that it reduces the testing duration by 75 % compared to a regular Liquid Chromatographic system and it also increases the separation performance 120. UFLC finds applications in many areas like identification of iodiconazole in micro-dialysis samples, analysis of podophyllotoxin in dermal and blood micro-dialysis samples of rats, simultaneous analysis of fluoroquinolones and xanthenes derivatives in serum, testing of isoflavones in soy and study of catechins in green tea 120,121.
Table 1 shows the comparison between HPLC RRLC, UPLC, UFLC, AND Nano LC, whereas Table 2 discusses the recent patents related to HPLC for the treatment of various diseases.
Table 1: Comparison between HPLC, RRLC, UPLC, UFLC AND Nano LC [105].
| Characteristics | HPLC | RRLC | UPLC | UFLC | Nano LC |
| Particle size | 3 to 10 µ | 1.8 µ | Less than 2 µ | 1.7 – 2.2 µ | 1.7 – 3 µ |
| Flow rate | 0.01-5 mL/min | 0.2-20 µL/min | 0.6 mL/min | 3.7 nL/min | 20-200 nL/min |
| Injection volume | 5 µL | 1.5 µL | 2 µL | 0.1-100 µL | 10 nL-125 µL |
| Column temperature | 30° C | Up to 100° C | 65° C | 40° C | 25-35° C |
| Analytical column
|
XTerraC18, Alltima C18 | ZORBAX Eclipse XDB−C18 RRHT | Acquity UPLCbeh C18, C8 | Shim-pack XR-ODS column | Capillary HPLC, Micro HPLC |
| Column dimensions (length x I.D) | 150 × 3.2 mm | 2.1-4.6mm | 150 × 2.1 mm | 75mm × 3.0 mm | 125 mm × 0.05mm – 4.6mm |
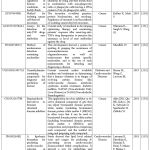 |
Table 2: Recent Patents related to HPLC for the Treatment of various Diseases. |
Conclusion
High Performance Liquid Chromatography is one of the most widely used analytical techniques. Using this technique, it is possible to produce very pure compounds. HPLC is useful both at the laboratory as well as clinical level and provides accurate, precise results with increased specificity. In this paper, the authors have tried to conclude that HPLC is a reproducible and versatile chromatographic method for analysing drug products, having a wide range of applications in both qualitative and quantitative estimation of various biological and drug molecules. Along with this, a number of patents and research have also proved the applicability of the HPLC technique in the healthcare sector in various areas, which paves the path for many successful prospects of this analysis technique.
Acknowledgement
None
Conflict of Interest
The author declares no conflict of interest, financial or otherwise.
Funding Sources
There is no funding source.
References
- Balammal, G.; Saravana, K.A. A review on basic chromatographic techniques. Indian J. Sci. Res., 2014, 4(4), 221-238.
- Coskun, O. Separation techniques: Chromatography. Northern Clin. Istanbul, 2016, 3(2), 156-160.
CrossRef - Patil, A.R.; Ghagare, P.M.; Deshmane, B.J.; Kondawar, M.S. Review on chromatography principal types and its application. J. Pharm. Dosage Forms Technol., 2020, 12(1), 1-5.
CrossRef - Ettre, L.S. 75 years of chromatography: A glimpse behind the scenes. Separation Sci., 1979; 2(8), 500-506.
CrossRef - Enyoh, C.E.; Isiuku, B.O.; Verla, A.W. Applications of column, paper, thin layer and ion exchange chromatography in purifying samples: Mini review. SF J. Pharm. Anal. Chem., 2019; 2(2), 1018-1022.
- Law, K-Y. Definitions for hydrophilicity, hydrophobicity, and superhydrophobicity: Getting the basics right. The J. Phy. Chem. Lett., 2014, 5(4), 686-688.
CrossRef - Zhang, Q.; Lin, L.; Ye, W. Techniques for extraction and isolation of natural products: A comprehensive review. Chinese Med., 2018, 13, 1-26.
CrossRef - Dubey, R.; Verma, N.K.; Kumar, S. A brief study on analytical method development: A Asian J, Res. Biol. Pharm. Sci., 2019, 7(2), 44-55.
- Priyadarshini, R.; Raj, G.M.; Shewade, D.G. Chromatography – The essence of Eur. J. Biomed. Pharm. Sci., 2016, 3(1), 366-377.
- Kumar, V.; Bharadwaj, R.; Gupta, G.; Kumar, S. An overview on HPLC method development, optimization and validation process for drug analysis. The Pharm. Chem. J., 2015, 2(2), 30-40.
- Patel, M. Chromatography principle and applications. J. Pharm. Pharm. Res., 2018, 13(4), 1-6.
- Sandron, S.; Rojas, A.; Wilson, R.; Davies, N.W.; Haddad, P.R.; Shellie, R.A.; Nesterenko, N., Kelleherc, B.P.; Paull, B. Chromatographic methods for the isolation, separation and characterisation of dissolved organic matter. Environ. Sci. Processes Impacts, 2015, 17, 1531-1567.
CrossRef - Jandera, P.; Hajek, T. Dual-mode hydrophilic interaction normal phase and reversed phase liquid chromatography of polar compounds on a single column. Separation Sci., 2020, 43, 70-86.
CrossRef - Jandera, P. Comparison of reversed-phase and normal-phase column liquid chromatographic techniques for the separation of low and high molecular weight J. Liquid Chromatogr. Relat. Technol., 2002, 25(9), 2901-2931.
CrossRef - Sneha Lakshmi, RP. A review on chromatography with high performance liquid chromatography (HPLC) and its functions. Rev.: J. Pharm. Anal., 2015, 4(1), 1-15.
- Bocian, S.; Krzemińska, K. The separations using pure water as a mobile phase in liquid chromatography using polar- embedded stationary phases. Green Chem. Lett. Rev., 2019, 12(1), 69-78.
CrossRef - Ding, P.; Liao, X.; Shi, B. Adsorption chromatography separation of the flavonols kaempferol, quercetin and myricetin using cross‐linked collagen fibre as the stationary J. Sci. Food Agriculture, 2013, 93(7), 1575-1583.
CrossRef - Lodha, L.; Sharma, N.; Viswas, A.; Khinchi, M.P. A review on chromatography techniques. Asian J. Pharm. Res. Devel., 2017, 5(2), 1-8.
- Jones, H.L.; Wadman, W. Application of paper partition chromatography to the separation of the sugars and their methylated derivatives on a column of powdered cellulose. , 1948, 162, 448-459.
CrossRef - Arige, S.S.; Arige, S.D.; Rao, L. A review on high performance centrifugal partition Int. J. Innov. Pharm. Sci. Res., 2017, 5(5), 91-108.
CrossRef - Amanolahi, F.; Mohammadi, A.; Oskuee, R.K.; Nassirli, H.; Malaekeh-Nikouei, B. A simple, sensitive and rapid isocratic reversed-phase high-performance liquid chromatography method for determination and stability study of curcumin in pharmaceutical samples. Avicenna J. Phytomed., 2017, 7(5), 444-453.
CrossRef - Kamau, F.N.; Chepkwony, H.K.; Ngugi, J.K.; Debremaeker, D.; Roets, E.; Hoogmartens, Isocratic liquid chromatographic method for the analysis of azithromycin and its structurally related substances in bulk samples. J. Chromatogr. Sci., 2002, 40, 529-533.
CrossRef - Schellinger, A.P.; Carr, P.W. Isocratic and gradient elution chromatography: A comparison in terms of speed, retention reproducibility and quantitation. Chromatogr. A., 2006, 1109(2), 253-266.
CrossRef - Hema ; Reddy, G.S. A review on new analytical method development and validation by RP-HPLC. Int. Res. J. Pharm. Biosci., 2017, 4(3), 41-50.
CrossRef - Khan, I.; Mulpuri, K.; Das, B.; Mohiuddin, M.D.; Rahman, M.H.U. Analytical techniques (Chromatography, Spectroscopy, Electrophoresis) in pharmaceutical analysis: A review. J. Res. Pharm. Nano Sci., 2015, 4(1), 19-27.
- Cummins, P.M.; Dowling, O.; O’Connor, B.F. Ion-exchange chromatography: Basic principles and application to the partial purification of soluble mammalian prolyl Protein Chromatogr., 2011, 681, 215-228.
CrossRef - Mohammed, J.S. A brief review on ion exchange chromatography. Tutor, 2017, 5(2), 30-38.
- Burgess, R.R. A brief practical review of size exclusion chromatography: Rules of thumb, limitations, and troubleshooting. Protein Exp. Purif., 2018, 150, 180-185.
CrossRef - Hong, P.; Koza, S.; Bouvier, E.S.P. A review size- exclusion chromatography for the analysis of protein biotherapeutics and their aggregates. Liq. Chromatogr. Relat Technol.,2012, 35(20), 2923-2950.
CrossRef - Nie, Y.; Liu, X.; Yang, X.; Zhao, Z. Recent application of chiral liquid chromatography– tandem mass spectrometric methods for enantiomeric pharmaceutical and biomedical J Chromatogr. Sci., 2013, 51, 753-763.
CrossRef - Teixeira, J.; Tiritan, M.E.; Pinto, M.M.M.; Fernandes, C. Chiral stationary phases for liquid chromatography: Recent developments. , 2019, 24, 865-903.
CrossRef - Anusha; Sirisha, S.P. An overview on affinity chromatography: A review. Indo Am. J. Pharm. Res., 2018, 8(7), 1462-1472.
- Malviya, R.; Bansal, V.; Pal, O.P.; Sharma, P.K. High performance liquid chromatography: A short review. Global Pharm. Technol., 2010, 2(5), 22-26.
- Yugandharudu, T.; Surendra, M.; Viswasanthi, T. A review on analytical method development and method validation. J. Pharm. Res. Anal., 2012, 2(1), 32-48.
- Hayes, R.; Ahmed, A.; Edge, T.; Zhang, H. Core-shell particles: Preparation, fundamentals and applications in high performance liquid chromatography. Chromatogr. A., 2014, 1357, 36-52.
CrossRef - Spoorthy, N. A review on high performance liquid chromatography. Rev.: J. Pharm. Nanotechnol., 2016, 4(2), 1-10.
- Thammana, M. A review on high performance liquid chromatography (HPLC). Rev.:J. Pharm. Anal., 2016, 5(2), 22-28.
- Vare, S.R.; Shelke, M.M.; Bidkar, J.S.; Dama, G.Y. HPLC: A simple and advance methods of separation and validation. World J. Pharm. Res., 2019, 8(4), 478-496.
- Yadav, V.; Bharkatiya, M. A review on HPLC method development and validation. J. Life Sci. Bioinformatics Pharm. Chem. Sci., 2017, 2(6), 166-178.
- Prathap, B.; Dey, A.; Rao, G.H.S.; Johnson, P.; Arthanariswaran, P. A review – Importance of RP-HPLC in analytical method development. J. Novel Trends Pharm. Sci., 2013, 3(1), 15-23.
- Agrahari, V.; Bajpai, M.; Nanda, S. Essential concepts of mobile phase selection for reversed phase HPLC. J. Pharm. Tech., 2013, 6(5), 459-464.
- Friesen, J.B.; McAlpine, J.B.; Chen, S-N.; Pauli, G.F. Counter current separation of natural products: An update. Nat. Prod., 2015, 78, 1765-1796.
CrossRef - Matysova, L.; Zahalkova, O.; Klovrzova, S.; Sklubalova, Z.; Solich, P.; Zahalka, L. Development of a gradient HPLC method for the simultaneous determination of sotalol and sorbate in oral liquid preparations using solid core stationary phase. Anal. Methods Chem., 2015, 1, 1-6.
CrossRef - Zhang, K.; Ma, P.; Jing, W.; Zhang, X. A developed HPLC method for the determination of Alogliptin Benzoate and its potential impurities in bulk drug and tablets. Asian J. Pharm. Sci., 2015, 10, 152-158.
CrossRef - Koli, R.S.; Patel, A.S.; Chaudhari, K.N.; Patil, K.R. A review on HPLC and its new trends. Asian J. Pharm. Anal., 2018, 8(4), 233-236.
CrossRef - Zotou, A. An overview of recent advances in HPLC instrumentation. Eur. J. Chem., 2012, 10(3), 554-569.
CrossRef - Thete, P.G.; Saudagar, R.B. Review on analytical method development and validation by RP-HPLC. Indo Am. J. Pharm. Sci., 2018, 5(5), 4897-4907.
- Wang, N.; Wang, T.; Wang, M.; Hao, A.; Li, T. Using ultrafiltration to facilitate simultaneous quantification of 5-fluorouracil in mouse plasma and tissues by HPLC. Liq. Chromatogr. Rel. Technol., 2011, 34, 2033-2047.
CrossRef - Sargenti, S.R.; Vichnewski, W. Sonication and liquid chromatography as a rapid technique for extraction and fractionation of plant material. Anal., 2000, 11(2), 69-73.
CrossRef - Farthing, C.A.; Farthing, D.E.; Koka, S.; Larus, T.; Fakhry, I.; Xi, L.; Kukreja, R.C.; Sica, ; Gehr, T.W.B. A simple and sensitive HPLC fluorescence method for determination of tadalafil in mouse plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci., 2010, 878(28), 2891–2895.
CrossRef - Bartosova, Z.; Riman, D.; Jakubec, P.; Halouzka, V.; Hrbac, J.; Jirovsky, D. Electrochemically pretreated carbon microfiber electrodes as sensitive HPLC-EC The Scient. World J., 2012, 1: 1-6.
CrossRef - Sule, S.; Ambadekar, S.; Nikam, D.; Sule, A.; Bhure, S. A practical approach to RP-HPLC analytical method development. World J. Pharm. Res., 2014, 3(9), 258-279.
- Mohammed, N.M.S.; Flowers, T.H.; Duncan, H.J. Development and validation of an HPLC method for the analysis of Chlorpropham and 3-chloroaniline in potato extracts. Res. Int., 2014; 2014, 1-6.
CrossRef - Kumar, Y.; Mumtaz, S.M.; Ahmad, M. HPLC: Principle and maintenance with application. J. Trend Sci. Res. Develop., 2018, 2(5), 1620-1626.
CrossRef - Saudagar, R.B.; Mahale, M.M. Stability indicating HPLC method development: A review. Drug Deliv. Ther., 2019, 9(3), 1103-1114.
- Azim, M.S.; Mitra, M.; Bhasin, P.S.HPLC method development and validation: A review. Res. J. Pharm., 2013, 4(4), 39-46.
CrossRef - Patil, J.R.; Joshi, D.G. Various criteria in development and validation of HPLC analytical methods – Review. J. Pharm. Qual. Assurance, 2015, 6(4), 91-99.
- Vare, S.R.; Shelke, M.M.; Gholap, S.M.; Bidkar, J.S.; Dama, G.Y. A review: Development and validation of RP-HPLC method for quantitative analysis of pharmaceutical dosage form. World J. Pharm. Res., 2019, 8(6), 502-532.
- Martin, M.; Blu, G.; Eon, C.; Guiochon, G. The use of syringe-type pumps in liquid chromatography in order to achieve a constant flow-rate. Chromatogr. A, 1975, 112, 399-414.
CrossRef - Sudev, S.; Janardhanan, S.V. Review on HPLC method development validation and Int. J. Pharm. Sci. Rev. Res., 2019, 56(2), 28-43.
- Chatzimichail, S.; Casey, D.; Salehi-Reyhani, A. Zero electrical power pump for portable high-performance liquid chromatography. Analyst, 2019, 144 6207-6213.
CrossRef - Kupiec, T. Quality-control analytical methods: High-Performance Liquid Int. J. Pharm. Compounding, 2004, 8(3), 223-227.
- Murugan, S.; Thejaswini, N.; Zeenathaman, S.; Vijayakar, S.P.; Varma, S.S.; Babu, N.M. An overview on high performance liquid chromatography. J. Biol. Pharm. Res., 2014, 1(4), 156-159.
- Dugo, P.; Fawzy, N.; Cichello, F.; Donato, P.; Mondello, L. Stop-flow comprehensive two-dimensional liquid chromatography combined with mass spectrometric detection for phospholipid analysis. Chromatogr. A, 2012, 1, 1278-1286.
CrossRef - Kulkarni, A.A.; Vaidya, I.S. Flow injection analysis: An overview. Crit. Rev., 2015, 2(4), 19-24.
- Cappielloa, A.; Famiglinia, G.; Mangania, F.; Palmaa, P. A simple approach for coupling liquid chromatography and electron ionization mass spectrometry. Am. Soc. Mass Spectrom., 2002, 13(3), 265-273.
CrossRef - Sidana, J.; Joshi, L.K. Recycle HPLC: A powerful tool for the purification of natural Chromatogr Res. Int., 2013, 2013, 1-7.
CrossRef - Gupta, V.; Jain, A.K.; Gill, N.S.; Gupta, K. Development and validation of HPLC method – A review. Res. J. Pharm. Appl. Sci., 2012, 2(4), 17-25.
- Taleuzzaman, M.; Ahmed, M.; Chattopadhyay, M. Particle size role, importance and strategy of HPLC analysis – An update. Arch. Biomed. Clin. Res., 2016, 2, 5-11.
CrossRef - Lundanes, E.; Døhl, J.; Greibrokk, T. Guard columns in HPLC: An examination of the effect of MPLC™ cartridge guard columns on column efficiencies and some theoretical aspects of the use of guard columns. Chromatogr. Sci., 1983, 21(5), 235-240.
CrossRef - Rigas, P.G. Liquid Chromatography-Post-column derivatization for amino acid analysis: strategies, instrumentation, and applications. Sci. Tech., 2012, 2(3), 161-193.
CrossRef - Novotny, M.V.; Development of capillary liquid chromatography: A personal perspective mini review. Chromatogr. A, 2017, 1523, 3-16.
CrossRef - Aulakh, J.S.; Malik, A.K.; Kaur, V.; Schmitt-Kopplin, P. A review on solid phase micro extraction-High performance liquid chromatography (SPME-HPLC) analysis of pesticides. Rev. Anal. Chem., 2005, 35(1): 71-85.
CrossRef - Ghodsia, R.; Kobarfardb, F.; Tabatabai, S.A. Application of narrow-bore HPLC columns in rapid determination of sildenafil citrate in its pharmaceutical dosage forms. Iranian J. Res.,2012, 11(1), 123-127.
- Ye, L.; Landen, W.O.; Eitenmiller, R.R. Comparison of the column performance of narrow-bore and standard-bore columns for the chromatographic determination of α-, β-, γ-, and δ-tocopherol. Chromatogr. Sci., 2001, 39: 1-6.
CrossRef - Zuvela, P.; Skoczylas, M.; Liu, J.J.; Baczek, T.; Kaliszan, R.; Wong, M.W.; Buszewski, B. Column characterization and selection systems in reversed-phase high-performance liquid Chem. Rev., 2019, 119(6), 3674-3729.
CrossRef - Swartz, M. HPLC detectors: A brief review. Liq. Chromatogr. Rel. Technol., 2010, 33(9-12), 1130-1150.
CrossRef - Sagliano, N.; Hartwick, R.A. Micro-HPLC detectors: A review. Chromatogr. Sci., 1986, 24(11), 506-512.
CrossRef - Kaushal, R.; Kaur, N.; Upadhyay, A.; Suri, O.P.; Thakkar, A. High performance liquid chromatography detectors – A review. Res. J. Pharm., 2011, 2(5), 1-7.
- Dhole, S.M.; Khedekar, P.B.; Amnerkar, N.D. Comparison of UV spectrophotometry and high-performance liquid chromatography methods for the determination of repaglinide in Pharm. Methods, 2012, 3(2), 68‐72.
CrossRef - Outuki, P.M.; Lazzeri, N.S.; de Francisco, L.M.; Bersani-Amado, C.A.; Ferreira, I.C.; Cardoso, M.L. A high-performance liquid chromatography with ultraviolet method for Eschweilera nana leaves and their anti-inflammatory and antioxidant activities. Mag., 2015, 11(43), 619‐626.
CrossRef - Zhao, L.; Wen, E.; Upur, H.; Tian, S. High performance liquid chromatography-diode array detector method for the simultaneous determination of five compounds in the pulp and seed of sea buckthorn. Mag., 2017, 13(49), 136‐140.
- Martono, S.; Febriani, I.; Rohman, A. Application of liquid chromatography-photodiode array detector for analysis of whitening agents in cream cosmetics. Appl. Pharm. Sci., 2018, 8(5), 143-147.
CrossRef - Lingeman, H.; Underberg, W.J.M.; Takadate, A.; Hulshoff, A. Fluorescence detection in high performance liquid chromatography. Liq. Chromatogr., 1985, 8(5), 789-874.
CrossRef - Honeychurch, K. Review: The application of liquid chromatography electrochemical detection for the determination of drugs of abuse. , 2016, 3, 28-37.
CrossRef - Pratima, N.A.; Gadikar, R. Liquid chromatography-Mass spectrometry and its applications: A brief review. Org. Inorg. Chem. Sci., 2018, 1(1), 26-34.
CrossRef - Korecka, M.; Shaw, L.M. Review of the newest HPLC methods with mass spectrometry detection for determination of immunosuppressive drugs in clinical practice. Transplant, 2009, 14(2), 61-72.
- Wade, J.H.; Bailey, R.C. Refractive index-based detection of gradient elution liquid chromatography using chip-integrated microring resonator arrays. Chem., 2014, 86(1), 913‐919.
CrossRef - Megoulas, N.C.; Koupparis, M.A. Twenty years of evaporative light scattering detection. Rev. Anal. Chem., 2005, 35(4), 301-316.
CrossRef - Roebuck, A.; Monasterio, V.; Gederi, E. A review of signals used in sleep analysis. Meas., 2014, 35(1), R1‐R57.
CrossRef - Lade, B.D.; Patil, A.S.; Paikrao, H.M.; Kale, A.S.; Hire, K.K. A comprehensive working, principles and applications of thin layer chromatography. J. Pharm. Biol. Chem. Sci., 2014, 5(4), 486-503.
- Modi, V.; Bhatkar, S.; Prajapati, P.; Basuri, T. A review on various applications of high-performance thin layer chromatography on pharmaceuticals. J. Innov. Pharm. Sci. Res.,2016, 4(3), 384-407.
- Sankar, P.R.; Snehalatha, K.S.; Firdose, S.T.; Babu, P.S. Applications of HPLC in pharmaceutical analysis. J. Pharm. Sci. Rev. Res., 2019, 59(1), 117-124.
- Bird, I.M. High-performance liquid chromatography: Principles and clinical applications. Med. J., 1989, 299, 783-787.
CrossRef - Bailey, F.; Applications of high-performance liquid chromatography in the pharmaceutical industry. Chromatogr., 1976, 7, 73-84.
CrossRef - Afghan, B.I.; Wolkoff, A.W. High performance liquid chromatography in environmental analysis: Present and future applications. Liq. Chromatogr., 1981, 4, 99-139.
CrossRef - Boukhobza, I.; Crans, D.C. Application of HPLC to measure vanadium in environmental, biological and clinical matrices. Arabian J. Chem., 2020, 13, 1198-1228.
CrossRef - Macrae, R. Applications of high-pressure liquid chromatography to food analysis. J. Food Sci. Technol., 2007, 16(1), 1-11.
CrossRef - Yashin, Y.I.; Yashin, Y.A. Analysis of food products and beverages using high-performance liquid chromatography and ion chromatography with electrochemical detectors. Anal. Chem., 2004, 59(12), 1121-1127.
CrossRef - Jaiswal, A.K.; Millo, T.; Gupta, M.; Teotia, A.K.; Tanwar, T.C.; Gupta, S. High Performance Liquid Chromatography (HPLC) and its forensic applications – A review. Forensic Med. Toxicol., 2008, 25(2), 19-31.
- McCulloch, G.; Morgan, R.M.; Bull, P.A. High Performance Liquid Chromatography as a valuable tool for geoforensic soil analysis. Australian J. Forensic Sci., 2017; 49(4), 421-448
CrossRef - George, E.; Jamal, A.R.; Khalid, F.; Osman, K.A. High performance liquid chromatography (HPLC) as a screening tool for classical Beta-thalassemia trait in Malays. J. Med. Sci., 2001, 8(2), 40‐46.
- Baranowska, I.; Bajkacz, S.; Baranowska, J. Clinical applications of fast liquid chromatography: A review on the analysis of cardiovascular drugs and their metabolites. Chromatogr. B, 2013, 1, 927-935.
CrossRef - Bhardwaj, S.K.; Dwivedi, K.; Agarwal, D.D. A review: HPLC method development and Int. J. Anal. Bioanal. Chem., 2015, 5(4), 76-81.
- Yandamuri, N.; Nagabattula, K.R.S.; Kurra, S.S.; Batthula, S.; Allada, L.P.S.N.; Bandam A Review: HPLC method development and validation. Int. J. Anal. Bioanal. Chem., 2015, 5(4), 76-81.
- Rao, B.V.; Sowjanya, G.N.; Ajitha, A.; Rao, V.U.M. Review on stability indicating HPLC method development. World J. Pharm. Pharm. Sci., 2015, 4(8), 405-423.
- Meenakshi, D.; Meenaxi, M. A review on ultra-performance liquid chromatography. J. Drug Devel. Res., 2013, 5, 29-34.
- Reddy, S.K.; Balammal, G.; Kumar, S. Ultra-performance liquid chromatography: An introduction and review. J. Pharm. Res. Anal., 2012, 2, 24-31.
- Taleuzzaman, M.; Ali, S.; Gilani, S.J.; Imam, S.S.; Hafeez, A. Ultra-performance liquid chromatography (UPLC) – A review. Austin J. Anal. Pharm. Chem., 2015, 2(6), 1056-1
- Chandraman, K. An updated review on ultra-performance liquid chromatography. Chem. Ind. J., 2016, 16(15), 114-122.
- Denoroy, L.; Zimmera, L.; Renaud, B.; Parrota, S. Ultra-high-performance liquid chromatography as a tool for the discovery and the analysis of biomarkers of diseases: A J. Chromatogr. B, 2017, 927, 37-53.
CrossRef - Maa, Y.C.; Wanga, X.Q.; Houa, F.F.; Ma, J.; Luoa, M.; Chena, A.; Jin, P.; Lua, S.; Xuaa, I. Rapid resolution liquid chromatography (RRLC) analysis and studies on the stability of preparations. Pharm. Biomed. Anal., 2011, 54, 265-272.
CrossRef - Xiaoying, Z.; Xiao-Ming, C. Rapid determination of paeoniflorin from Paeonia by rapid resolution liquid chromatography. Mag., 2010, 6, 98-101.
CrossRef - Zhang, X.; Zhao, T.; Cheng, T.; Liu, X.; Zhang, H. Rapid resolution liquid chromatography (RRLC) analysis of amino acids using pre-column derivatization. Chromatogr. B, 2012, 906, 91-95.
CrossRef - Ma, Y.; Wang, X.; Hou, F.F.; Ma, J.; Luo, M.; Lu, S.; Jin, P.; Terevsky, N.; Chen, A.; Xu, I.; Patel, A.V.; Górecki, D.C. Rapid resolution liquid chromatography (RRLC) analysis for quality control of rhodiola rosea roots and commercial standardized products. Prod. Commun., 2011, 6, 1-12.
CrossRef - Chervet, J.P.; Ursem, M.; Salzmann, J.P. Instrumental requirements for nano-scale liquid Anal. Chem., 1996, 68, 1507-1512.
CrossRef - Dams, M.; Dores-Sousa, J.L.; Lamers, R. High-resolution nano-liquid chromatography with tandem mass spectrometric detection for the bottom-up analysis of complex proteomic samples. Chromatogr., 2019, 82, 101-110.
CrossRef - Gama, M.R.; Collins, C.H.; Bottoli, C.B.G. Nano-liquid chromatography in pharmaceutical and biomedical research. Chromatogr. Sci., 2013, 51(7): 694-703.
CrossRef - Ladd MP, Giannone RJ, Abraham PE, Wullschleger SD, Hettich RL. Evaluation of an untargeted nanoliquid chromatography-mass spectrometry approach to expand coverage of low molecular weight dissolved organic matter in Arctic soil. Sci Rep., 2019, 9, 5810-5822.
CrossRef - Kaplitz, A.S.; Kresge, G.A.; Selover, B.; Horvat, L.; Franklin, E.G.; Godinho, J.M.; Grinias, K.M.; Foster, S.M.; Davis, J.J.; Grinias, J.P. High-throughput and ultrafast liquid Anal. Chem., 2020, 92(1), 67-84.
CrossRef - Nguyen, D.T.; Guillarme, D.; Rudaz, S.; Veuthey, J.L. Fast analysis in liquid chromatography using small particle size and high pressure. Sep. Sci., 2006, 29(12), 1836-1848.
CrossRef - Fay, W.; Woo, C-I.; Ma, CM. Compositions and methods relating to universal glycoforms for enhanced antibody efficacy. JP2020041003A, 2020.
- Kobayashi, H.; Choyke, P. Photosensitizing antibody-fluorophore conjugates. US20200085950A1, 2020.
- Fay, W.; Woo, C-I.; Ma, C.M. Compositions and methods relating to universal glycoforms for enhanced antibody efficacy. JP2020041003A, 2020.
- Gregory, S.; Tito, E.; Jay, P.; Hazel, T.; Hitchcock, A.; Gee, A. Compositions and methods for treating collagen-mediated diseases. JP2020039371A, 2020.
- Gordon, C.W.; Freeman, J. Receptors of PD-1, B7-4 and uses thereof. JP2020028302A,
- Cohen, D.; Chumakov, I.; Nabirochkin, S.; Vial, E.; Guedj, M. Baclofen and acamprosate based therapy of neurological disorders. US20200085790A1, 2020.
- Riley, E.B.; Sea, A.; Lorenzo, P.; Fritz, B.; Jonathan, G.B.; Sheige, A.M.M.; Perez, J. Anti-EGFR antibody and antibody drug conjugate. JP2020039360A, 2020.
- Cassis, A.E., Amin, K. Methods for detecting signatures of disease or conditions in bodily JP2019068804A, 2019.
- Kalluri, R.; Melo, S. Method for isolating cancer cell-derived exoxomes. EP3076949B1,
- Ohrmund, L.; Singh, S.; Wang, S.L. Assays for the detection of anti-TNF drugs and AU2017213584A1, 2019.
- Micallife, J.V. Method of nucleosomes detection containing nucleotides. RU2687483C2,
- Hazen, S.L., Wang, Z., Levison, B.L. Trimethylamine-containing compounds for diagnosis and prediction of disease. US20190234917A1, 2019.
- Akto, D.I.G.; Qin, B.K.; Keki, J.; Du, K.; Schwick, D.; Sperlandio, H.; Zabloki, Y.J. Benzimidizole derivatives as bromine structural domain inhibitor. CN105324379B, 2018.
- Laxonen, R.; Ekroskim, K.; Reimini, E.; Reini, F.; Minna, F.; Minna, J.; Riykka, J.; Riyka, ; Kiril, K.; Tarasov, T.K. A lipidomic biomarker for predicting cardiovascular outcome in patients with coronary artery disease receiving statin therapy. JP6388284B2, 2018.
- Laaksonen, R.; Ekroos, K.; Hurme, R.; Katainen, R. Lipidomic biomarkers for the prediction of cardiovascular outcomes in coronary artery disease patients not undergoing statin treatment. US9863965B2, 2018.
- Laaksonen, R.; Ekroos, K.; Hurme, R.; Jänis, M.; Katainen, R.; Tarasov, K. Lipidomic biomarkers for atherosclerosis and cardiovascular disease. DK2567240T3, 2018.
- Cassis, A.E.; Amin, K. Method for detecting diseases or conditions using phagocytic cells. JP2017051193A, 2017.
- Laaksonen, R.; Ekroos, K.; Hurme, R.; Jänis, M.; Katainen, R.; Tarasov, K. Lipidomic biomarkers for the prediction of cardiovascular outcomes in coronary artery disease patients undergoing statin treatment. EP2776842B1, 2017.
- Maggio, E.T. Compositions for drug administration. US9283280B2, 2016.
- Sprogeefelix, K.; Herzelsilvia, K.; Lessmannharald, K-F.; Wegge, R. Long-acting insulin RU2556340C2, 2016.
- Chen, P.; Wang, Y. Composition, function and use of xanthoceras sorbifolia extract and compound isolated from same, method for preparing same. CN101242850B, 2013.








